Clinical characterization of Collagen XII-related disease caused by biallelic COL12A1 variants
Abstract
Objective
While there have been several reports of patients with dominantly acting COL12A1 variants, few cases of the more severe recessive Collagen XII-related disorders have previously been documented.
Methods
We present detailed clinical, immunocytochemical, and imaging data on eight additional patients from seven families with biallelic pathogenic variants in COL12A1.
Results
All patients presented with a consistent constellation of congenital onset clinical features: hypotonia, dysmorphic features, most notably gingival hypertrophy, prominent distal joint hyperlaxity, with co-occurring contractures of large joints, and variable muscle involvement, evident both clinically and on muscle imaging. Five patients presented with a severe congenital phenotype manifesting with profound weakness, significantly delayed or minimal attainment of motor milestones, respiratory insufficiency, and feeding difficulties. Three patients presented with mild-to-moderate muscle weakness and delayed milestones but were able to achieve independent ambulation. Patients were found to have biallelic loss-of-function COL12A1 variants, except for one family (p.I1393Ffs*11/p.A1110D). Consistent with the variable clinical spectrum, in vitro immunocytochemistry analysis in fibroblasts ranged from complete absence of Collagen XII expression in a patient with severe disease, to a mild reduction in a patient with milder disease.
Interpretation
Here we characterize the clinical presentation, muscle imaging, and dermal fibroblast immunostaining findings associated with biallelic variants in COL12A1, further establishing COL12A1 as a recessive myopathic Ehlers–Danlos syndrome (mEDS) gene, and expanding the clinical spectrum to include a milder EDS phenotype.
Introduction
Collagen XII (COLXII) is the largest member of the fibril-associated collagen with interrupted triple helix family. COLXII is a homotrimer assembled from three COLXII alpha chains encoded by COL12A1. It is essential for collagen fibril organization by bridging Collagen I-containing fibrils bound at the collagenous domain with various extracellular matrix (ECM) proteins including tenascin-X.1, 2 In addition to regulating matrix formation, COLXII is thought to be important for modulating mechanical properties of collagen fibrils in connective tissues and bone.1, 3
Pathogenic variants in COL12A1 have only recently been associated with disease in mice and humans.4, 5 Both recessive variants, as well as dominantly acting variants in COL12A1 have been reported to cause myopathic Ehlers–Danlos syndrome (mEDS), an overlap disorder involving connective tissue and muscle.5, 6 In the initial report, patients with recessive biallelic loss-of-function (LoF) COL12A1 variants manifested with a severe congenital-onset disease characterized by generalized hypotonia, muscle weakness with minimal attainment of motor milestones, feeding and respiratory difficulties, and striking joint laxity with coexisting milder contractures of larger joints.5 Heterozygous dominantly acting COL12A1 variants, typically glycine substitutions or in-frame exon skipping variants, cause a dominant-negative interference of the Gly-X-Y containing triple helical (TH) domain. Patients manifest with a milder phenotype of motor delay, mild proximal or distal weakness, and joint hyperlaxity with pes planus deformities of the feet. While additional patients with dominant acting COL12A1 variants are increasingly being recognized,4, 7-9 patients with recessive biallelic variants remain rare. Recently, a single patient with a homozygous COL12A1 splice site variant was identified, presenting with congenital onset hypotonia and weakness, delayed motor milestones, progressive scoliosis, who nevertheless reported no physical limitations at age 47 years.10 The overall clinical spectrum, disease manifestation, and genotype–phenotype correlation of recessive COL12A1-related disease (COL12A1-RD) remains poorly understood.
We report eight patients from seven families with biallelic pathogenic variants in COL12A1 presenting with a consistent phenotype of myopathic EDS characterized by congenital onset hypotonia, joint hyperlaxity with concurrent joint contractures, variable degrees of muscle weakness, and involvement as evident on MRI. Taken together, this series expands the phenotypic and mutational spectrum associated with the emerging group of recessive COL12A1-RD to now also include a milder phenotype.
Methods
Patient recruitment and sample collection
All patients were identified through their local neuromuscular or genetic provider and underwent detailed clinical examinations. Samples including DNA muscle and skin biopsies were collected based on standard procedures. For research studies, written informed consent and age-appropriate assent were obtained from each participant. Ethical approval for human subject research studies was obtained from the following institutional review boards: National Institutes of Health (NIH), National Institute of Neurological Disorders and Stroke (NINDS) (Protocol 12-N-0095), Rady's Children's Hospital/UCSD (UCSD IRB# 172020, 170437), Health Research Authority, NRES Committee East of England – Hatfield (REC 13/EE/0398), and NRES Committee North East – Newcastle & North Tyneside 1 (reference 08/H0906/28). UK Regional Ethics Committee REC (reference 06/Q0406/33).
Genetic analysis
Whole exome sequencing (WES), whole genome sequencing (WGS), or next-generation sequencing-based panel testing was performed in all patients. Details of the clinical and research-based genetic testing methods are listed in Supplementary Table 1. Confirmation of variants and segregation testing was performed through Sanger Sequencing for all patients.
Imaging studies
Conventional T1-weighted spin echo and short tau inversion recovery (STIR) of the lower extremities on a 3.0-T Achieva Philips MRI system were obtained for F1P1 and F5P5. Leg muscle MRI in F4P4 was performed using 1.5 T scanner according to standard protocols axial T1-weighted and STIR sequences were acquired covering thighs and calves. For F7P8 standard T1-weighted images were acquired on a 3.0-T scanner (Philips Intera Achieva, Siemens TIM Trio). Muscle ultrasound images were obtained using a Siemens S2000 with a 15-MHz linear probe and scored based on the Heckmatt Scale.11
Fibroblast culture and immunocytochemistry analysis
Dermal fibroblasts isolated from skin biopsy were grown in Dulbecco's modified Eagle medium with 10% fetal bovine serum (Life Technologies) in 5% CO2 at 37°C. For immunofluorescence staining, cells were cultured in the presence of 50 μg/ml L-ascorbic acid phosphate (Wako) until 5 days post-confluent, fixed with 4% paraformaldehyde (PFA), incubated with primary antibodies at 4°C overnight [guinea pig anti-collagen XII (KG76, generated by Dr. Koch12) and rabbit anti fibronectin antibody (Sigma Aldrich)], the antibody labeling was detected with secondary antibodies for 1 h at room temperature [Alex 568-conjugated goat anti-guinea pig IgG and Alexa 488-conjugated goat anti-rabbit IgG (Molecular Probes)]. Immunoblotting analysis of overnight conditioned culture media from confluent fibroblast cultures were performed using rabbit anti-collagen XII (KR75, generated by Dr. Koch),12 rabbit anti fibronectin (F3648, Sigma Aldrich), and mouse anti-tubulin antibody (T5168, Sigma Aldrich) followed with appropriated infrared fluorescent dye labeled secondary antibodies (LI-COR Biosciences).
Results
Clinical findings
Detailed clinical information for all patients, (five males and three females) is summarized in Table 1 and Supplemental Results, with ages ranging from 15 months to 18 years at the time of the most recent examination. Limited clinical details for F7P8 were previously reported.13
| Family/Patient | F1/P1 | F2/P2 | F3/P3 | F4/P4 | F5/P5 | F5/P6 | F6/P7 | F7/P8 |
|---|---|---|---|---|---|---|---|---|
| COL12A1 Variants | c.5794+2T>A (p), c.5269C>T; p.R1757* (m) | c.8464C>T; p.R2822* (homozygous) | c.6340+1G>T (homozygous) | c.946_947insA; p.V316fs*6 (homozygous) | c.3329C>A; p.A1110D (p), c.4177del; p.I1393Ffs*11 (m) | c.5664+1G>A (p), c.6127delG; p.V2043* (m) | c.5230+1G>A (homozygous) | |
| Sex/Age at visit | F/15 months | F/18 years | M/4 years | M/13 years | F/3 years | M/15 months | M/3 years | M/16 years |
| Ethnicity/consanguinity | European/No | Palestinian/Yes | Afghani/Yes | Pakistani/Yes | European/No | European/No | Pakistani/Yes | |
| Onset | Congenital | Congenital | Congenital | Congenital | Congenital | Congenital | Congenital | Congenital |
| Presenting symptoms |
Global weakness Arthrogryposis |
Hypotonia Arthrogryposis DDH |
Hypotonia Arthrogryposis DDH |
Hypotonia Distal arthrogryposis DDH |
Hypotonia DDH |
Hypotonia Hip clicks at birth |
Congenital hip dysplasia Talipes equinovarus |
Hypotonia |
| Dysmorphic features | ||||||||
| Head |
Dolichocephaly Low-set ears |
Dolichocephaly | Plagiocephaly | Plagiocephaly | ||||
| Neck | Mild webbing | Mild webbing | ||||||
| Ocular |
Blueish sclera Epicanthal folds |
Bluish sclera | Blueish sclera | |||||
| Face | Myopathic | Long narrow face | Myopathic | Myopathic | ||||
| Jaw | Micrognathia | Micrognathia | Micrognathia | Micrognathia | ||||
| Palate | High arched with deep midline ridge | High arched | High arched | High arched | High arched | High arched |
High arched Severe prominence of lateral palatine ridges |
|
| Dentition |
Gingival hypertrophy Dental eruption cysts |
Gingival hypertrophy Dental malocclusion |
Gingival hypertrophy | Gingival hypertrophy | Gingival hypertrophy |
Gingival hypertrophy Dental malocclusion |
||
| Skeletal | Prominent calcanei |
Prominent calcanei Pectus excavatum Short lower limbs |
Prominent calcanei Pectus excavatum |
Prominent calcanei |
Gracile ribs Multiple fractures at birth |
Prominent calcanei | ||
| Other |
Bell-shaped chest Sacral dimple |
Soft skin Webbing Shallow palmar creases Mild thenar atrophy |
Soft skin |
Overlapping fingers Reduced LE muscle bulk |
Mild axillary pterygia | |||
| Maximum motor development |
Poor head control Unable to sit without support |
Sat with support at age 3 years | Limited head control | Able to run, jump, climb stairs | Ambulatory at age 15 months | Ambulatory at age 16 months | Cruised at 11 months, although unable to pull to stand | Never achieved independent ambulation |
| Pattern of weakness (MRC) |
UEs (3/5) LEs (2/5) |
UEs and LEs (4/5) Neck flexion and deltoid (2/5) |
Generalized weakness |
Mild proximal and axial weakness |
UEs and LEs (4+/5) Neck flexion (2/5) |
UEs and LEs (4+/5) Neck flexion (2/5) |
Proximal upper and lower extremities at least (3/5) |
Distal > proximal weakness; LE > UE weakness |
| Joints | ||||||||
| Hyperlaxity | Distal and proximal | Distal and proximal | Distal | Distal hands and knees | Distal and proximal | Distal and proximal | Distal hands | Distal and proximal |
| Contractures |
Long finger flexors Fingers Toes |
Long finger flexors Knees |
Fingers | Fingers | Hip adductors | Hip adductors | Fingers | Knees |
| Spine | Thoracic kyphoscoliosis | Progressive scoliosis | Kyphosis | Hyperlordosis | Scoliosis | Scoliosis | ||
| Cardiac involvement |
Thickened mitral valve leaflets Mild-moderate MR |
HCM MVP WPW syndrome s/p ablation |
Dysplastic mitral and tricuspid valve with redundant tissue Mild MR |
Trivial mitral regurgitation | ||||
| Respiratory insufficiency | No | Yes | Yes | Yes | No | No | Yes | Yes |
| Neonatal feeding difficulties | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes |
- DDH, developmental dysplasia of hip; EF, ejection fraction; F, female; FVC, forced vital capacity; HCM, hypertrophic cardiomyopathy; LEs, lower extremities; M, male; m, maternal; MR, mitral regurgitation; MRC, Medical Research Council; MVP, mitral valve prolapse; p, paternal; s/p, status post; UEs, upper extremities; WPW syndrome, Wolff–Parkinson–White syndrome.
All patients presented with congenital onset symptoms including reduced fetal movement, hypotonia, and hip dysplasia, with four patients (F1P1, F2P2, F3P3 and F4P4) presenting with arthrogryposis. Patient F6P7 was found to have multiple (left femur fracture and left tibia) fractures at birth. Neonatal feeding difficulties were present in seven, requiring a nasogastric or gastrostomy tube placement. Respiratory involvement was variable, ranging from none to tracheostomy and ventilatory dependency since birth (F6P7). All patients had delayed acquisition of motor milestones. Five patients presented with a severe congenital phenotype manifesting with profound weakness and joint hypermobility, minimal attainment of milestones accompanied by respiratory failure and feeding difficulties. Three patients (F4P4, F5P5, and F5P6) presented with milder disease with mild to moderate muscle weakness and were able to achieve independent ambulation at age 3 years, age 15 months, and age 16 months, respectively. None of the patients had significant worsening of motor symptoms, instead showing steady improvement over time. F2P2, F6P7 and F7P8 had progressive scoliosis requiring spinal surgery at age 10 years in F2P2. F1P1 and F3P3 were noted to have progressive thoracic kyphoscoliosis. Distal joint laxity was noted in all patients (Fig. 1A–C), typically affecting wrists and ankles. Concurrent joint contractures were present in six patients and typically affected the long finger flexors (Fig. 1D) as well as the knees.
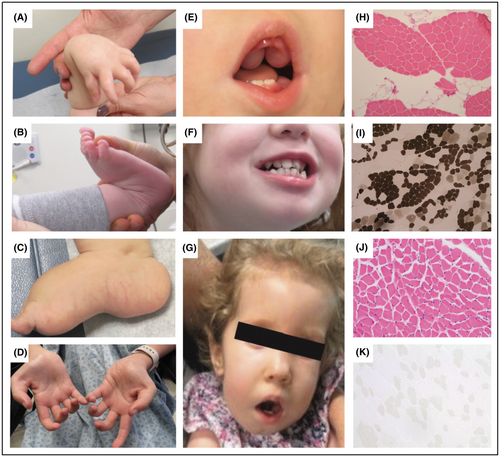
Dysmorphic features were evident in all patients. Patients F1P1 and F2P2 were found to have dolichocephaly, and F4P4 and F6P7 presented with plagiocephaly. Other common findings included micrognathia (n = 4), gingival hypertrophy (n = 6) (Fig. 1E), dental abnormalities (n = 3) (Fig. 1F), bluish hue to the sclerae (n = 3), myopathic facies (n = 4) (Fig. 1G), and webbing of joints (n = 2). Four patients also had evidence of cardiac involvement, with one (F2P2) presenting with Wolff–Parkinson–White syndrome. F1P1 and F3P3 had echocardiograms in infancy showing mild valvular anomalies with thickened mitral valve leaflets and mild-to-moderate mitral regurgitation in F1P1 and dysplastic mitral and tricuspid valves with redundant tissue and mild mitral valve regurgitation in F3P3. Creatine kinase (CK) levels were normal in F1P1, F3P3, F5P5, and F5P6, but mildly elevated in F2P2 and F6P7 (500 U/L range). Muscle biopsy in F2P2, F3P3, F4P4, and F7P8 revealed subtle variation in fiber size, consistent with a mild myopathic process, with internalized nuclei seen in F3P3 (Fig. 1H–K).
Taken together, patients presented with a constellation of symptoms including congenital onset hypotonia, dysmorphic features with notable gingival hypertrophy, prominent distal joint hyperlaxity with concurrent contractures of large joints and variable degrees of muscle involvement, cardiac involvement, respiratory insufficiency, and feeding difficulties.
Muscle imaging
Muscle MRI was available for F1P1 (age 15 months), F4P4 (age 11 years), F5P5 (age 6 years), and F7P8 (age 7 years) (Fig. 2A). F1P1 had evidence of diffuse severe muscle atrophy with fatty replacement throughout the proximal and distal lower extremity muscles bilaterally. Some asymmetry and imaging artifact related to contractures was noted in the lower leg. In contrast, MRI in F4P4 revealed mainly posterior thigh involvement with relative sparing of the semitendinosus muscle. The medial and anterior compartments of the upper leg were relatively preserved, aside from the rectus femoris, which showed mild atrophy and fatty infiltrates around the central fascia. Muscles of the distal lower extremity were normal. MRI in F5P5 was normal, demonstrating normal appearance of muscles of the thigh and distal lower extremity. MRI in F7P8 revealed relative sparing of the anterior and medial thigh compartments compared to the posterior thigh. Within the anterior thigh, the rectus femoris was most involved, with mild atrophy and some instances of fatty infiltrates along the central fascia. A lesser degree of atrophy and fatty infiltration of the vastus lateralis was seen.
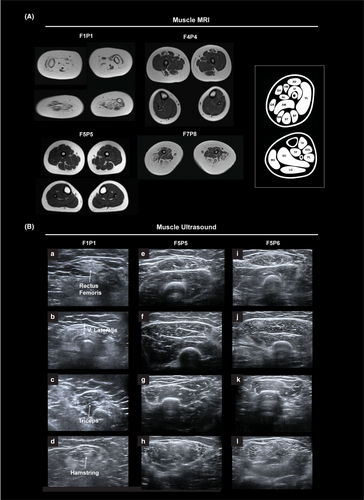
Muscle ultrasound images were obtained for F1P1 (age 15 months), F5P5 (age 6 years), and F5P6 (age 4 years) (Fig. 2B). Muscle ultrasound in F1P1 revealed diffuse muscle atrophy (lower extremities more than upper extremities) with increased echogenicity with a dense, granular appearance in the rectus femoris and vastus lateralis. Increased echogenicity with a mixed granular/streaky pattern was present in the triceps. Muscle ultrasound in F5P5 and F5P6 revealed diffuse mild granular and streaky increased echogenicity with greater posterior thigh involvement, relative sparing of the medial and anterior compartments of the upper leg, and relatively preserved appearance of the distal lower extremity muscles. In the upper extremity, increased echogenicity with a mixed granular/streaky pattern was present in the triceps. There was no muscle atrophy.
Identification of COL12A1 variants
Disease occurrence was sporadic in six families, affecting only a single family member. Family history was significant in family (F5), with two affected siblings born to unaffected parents. Four families (F2, F3, F4, and F7) reported consanguinity. The sister of F4P4 was diagnosed with congenital myotonia caused by a homozygous pathogenic variant in CLCN1 (c.1696G>A p.(Ala566Thr)). F4P4 was found to be heterozygous for this variant.
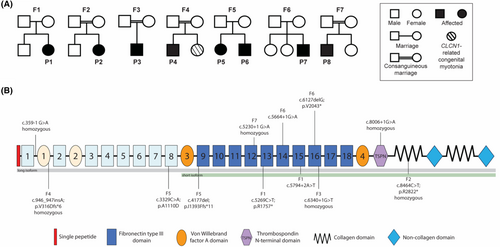
Family history was otherwise non-contributory (Fig. 3A). Next-generation-based sequencing identified rare biallelic COL12A1 (NM_004370.6) variants in all eight patients. Details of the COL12A1 variants can be found in Figure 3B and in Table S1. Four patients (F2P2, F3P3, F4P4, and F7P8) were found to be homozygous for either a splice site or a truncating variant, and two patients (F1P1 and F6P7) were compound heterozygous for different LoF alleles. Variants were either rare, or absent, from gnomAD v4.0.0.14 COL12A1 is constrained for LoF variation, with only 102 of 320.4 expected predicted LoF variants observed in gnomAD v4.0.0 (pLI score 0.97, LOEUF score 0.3814).
The two affected siblings (F5P5 and F5P6) in Family 5 were found to be compound heterozygous for a maternally inherited LoF allele c.4177del; p.I1393Ffs*11 and a paternally inherited c.3329C>A; p.A1110D missense variant. The missense variant is absent from gnomAD v4, and is highly conserved. There are three different variants at the same residue p.A1110T (listed once), p.A1110S (listed three times), and p.A111V (listed twice). The p.A1110D COL12A1 variant has inconsistent in silico computational predictions (SIFT: deleterious, polyphen-2: probably damaging) and has not yet been reported to cause disease.
Five patients (F1P1, F2P2, F3P3, F6P7, and F7P8) were found to have either homozygous or compound heterozygous LoF variants impacting both the long (NM_004370.6) and the short isoform (NM_080645.3) (Fig. 3B). F4P4 was homozygous for a LoF variant, which impacted the long isoform only, leaving the short isoform intact. F5P5 and F5P6 (Family 5) were compound heterozygous for a paternal missense variant (p.A1110D), which impact the long isoform and leaves the short isoform intact, and a maternal LoF variant that impacts both isoforms.
Immunocytochemistry
Immunofluorescence staining of fibroblasts was performed in a control and in three patients (F1P1, F4P4, and F5P5) (Fig. 4A). COLXII immunocytochemistry in control fibroblasts revealed both intracellular (Triton X100 treated culture) and extracellular (ECM) Collagen XII immunoreactivity. In comparison, no detectable intracellular or extracellular Collagen XII immunoreactivity was seen in F1P1 (c.5794+2T>A/p.R1757*), while F4P4 (homozygous p.V316fs*6) showed a reduced intracellular and extracellular Collagen XII immunoreactivity. In F5P5 (p.I1393Ffs*11/ p.A1110D), no significant Collagen XII abnormality was observed in the fibroblast cultures compared to control.
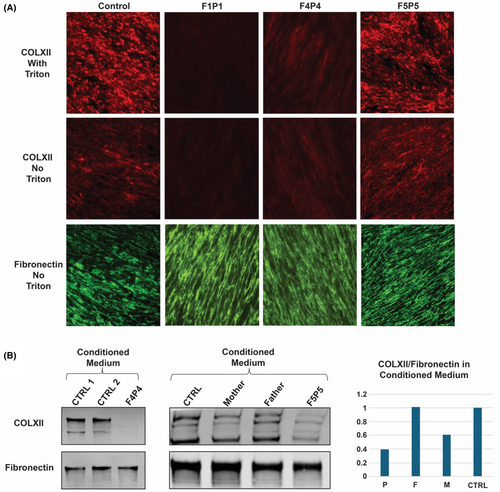
In addition, we analyzed the secreted soluble Collagen XII expression in the overnight conditioned medium of the fibroblast cultures by immunoblotting for F4P4 and F5P5 (Fig. 4B). In F4P4, the secreted soluble Collagen XII signal was barely detectable, while it was reduced in F5P5 compared to both heterozygous unaffected parents and a control sample.
Discussion
Both recessive and dominant mutational mechanisms in COL12A1 cause an emerging overlap syndrome involving muscle and connective tissue. While additional dominant cases have recently been reported, there have been fewer cases of the originally more severe recessive COL12A1-RD identified. In this series, we provide detailed clinical, imaging, and immunocytochemical data for eight patients from seven families with biallelic variants in COL12A1. All patients manifest a consistent phenotype of congenital onset hypotonia, dysmorphic features, including most notably gingival hypertrophy, prominent widespread but distally more pronounced joint laxity with variable concurrent joint contractures, and variable degrees of muscle weakness, cardiac involvement, respiratory insufficiency, and feeding difficulties. Muscle involvement ranged from profound weakness with significantly delayed or minimal attainment of motor milestones, to isolated hip flexor weakness without functional limitation. Five patients presented with a severe congenital phenotype, while three patients (F4P4, F5P5, and F5P6) presented with milder disease, and were able to achieve independent ambulation. All patients demonstrated slow steady improvements in motor function over time without clinical regression.
The clinical diagnosis of EDS is based on the presence of clinical hallmarks of EDS including joint hypermobility, skin hyperextensibility, and tissue fragility, with further delineation of the specific EDS subtypes relying on characteristic phenotypic findings such as neuromuscular manifestations, with subsequent confirmatory genetic testing.15 Within the EDS spectrum, there can be substantial overlap with primary EDS, those with myopathic or kyphoscoliotic EDS, and myopathies with connective tissue involvement.16 COL12A1-RD, emerges as a clinical overlap syndrome involving muscle and connective tissue, alternatively also classified as myopathic EDS. The spectrum of underlying neuromuscular manifestations of COL12A1-RD remain insufficiently characterized given the limited number of patients identified and reported thus far, in particular for the more severe recessive form of the disease, which, based on the number of identified patients, appears to be extremely rare. These patients present with congenital hypotonia and often initially profound, albeit nonprogressive, muscle weakness, but in time may improve somewhat from a functional motor standpoint. While clinical aspects of a hereditary disorder of connective tissues are prominent, the changes identified on muscle imaging in combination with muscle biopsy in four patients demonstrating mild myopathic findings without dystrophic features support a concomitant myopathic process.
While clinically distinct, findings of distal joint laxity with coexisting contractures in the setting of muscle weakness in patients with COL12A1-RD, partially resemble patients with Collagen VI-related dystrophy (COL6-RD).17 COL6-RD encompasses a spectrum of disease ranging from the severe Ullrich congenital muscular dystrophy, to intermediate and Bethlem myopathy on the milder end, and is therefore a relevant consideration in the differential diagnosis of the COL12A1-RD spectrum. However, the current OMIM classification for COL12A1 that includes Ullrich congenital muscular dystrophy 2 and Bethlem myopathy (OMIM: 120320) should be reconsidered as there are distinct phenotypic differences. The Collagen XII disease spectrum might be better viewed as a form of myopathic EDS. In fact, this is the designation applied in the latest EDS nomenclature.15, 18
Patients with COL6-RD typically present with progressive muscle weakness and joint contractures, and also have distally predominant joint laxity, which is less widespread than is seen in COL12A1-RD where it also affects some of the larger joints. Therefore, striking more widely distributed joint laxity, less prominent contractures, and nonprogressive muscle weakness allowing for some clinical improvement of motor function (in particular in patients with less severe involvement at the outset), are important features to help distinguish patients with COL12A1 myopathic EDS from those with COL6-RD in whom the muscle involvement can be quite progressive and dystrophic in nature, including progressive respiratory failure. In addition, specific oral phenotypic features, namely gingival and dental abnormalities are atypical for COL6-RD. Interestingly, while both disease groups present with velvety skin of palms and feet, patients in this series do not display other skin findings including keratosis pilaris and keloid scarring which are often seen in COL6-RD.19 Early kyphosis and progressive scoliosis are a common finding in COL12A1-RD5, 10 and can also be seen in COL6-RD as well as other forms of kyphoscoliotic EDS including FKBP14 and PLOD1-related disease, with hearing loss being an important differential seen in FKBP14-related disease.20, 21
Muscle imaging can serve as useful noninvasive diagnostic tool to aid in the clinical differential diagnosis, with COL6-RD often characterized by increased signal along fascial planes and, giving rise to a so-called “central cloud” along the central fascia of the rectus femoris, and a characteristic rimmed appearance of the vastus lateralis. This typical COL6-RD-associated pattern is different from our observations in patients with COL12A1-RD, in whom a different pattern of muscle involvement may be emerging. Among the three patients presenting with milder disease (F4P4, F5P5, and F5P6), the hamstrings appear most affected, with some involvement of the rectus femoris, and lesser involvement of the vastus lateralis. While imaging reveals diffuse muscle atrophy and fatty replacement in severely affected F1P1, imaging of F7P8 in comparison with the milder patients confirms the predominant hamstring, and lesser anterior thigh muscle pattern of involvement. Interestingly, the relative more prominent involvement of the rectus femoris among the muscles of the quadriceps has also been noted in an older patient with dominant COL12A1-RD and in patients with FKBP14-RD as another myomatrix disease.6, 20
In addition to the initially reported severe congenital onset phenotype associated with biallelic null variants in COL12A1, this series of eight additional patients both confirms the severe end, but expands the clinical spectrum, to now include a milder connective tissue muscle overlap phenotype. This milder phenotype also presents with a congenital onset, but demonstrates subsequent steady improvement in motor function allowing for the acquisition of milestones such as independent ambulation. All five (P1, P2, P3, P7, and P8) patients presenting with severe disease had biallelic LoF variants affecting both the long (NM_004370.6) and the short (NM_080645.3) isoforms (Fig. 3B). These variants are expected to cause nonsense-mediated mRNA decay (NMD) of both isoforms, consistent with the absent COL12 IF signal as shown in P1's fibroblast culture. The homozygous truncating variant (p.V316Dfs*6) in P4 is expected to cause NMD of the long isoform only and leaves the short isoform intact, resulting in a significantly reduced COL12 signal in both IF and conditioned medium. This result also implies that long isoform might be the major expression pattern of dermal fibroblasts in culture condition.
Recently, a homozygous COL12A1 splice variant was identified in a patient with congenital hypotonia, weakness, joint laxity, and progressive scoliosis who was able to achieve independent ambulation and who did not report physical limitations in childhood.10 In our series we report three patients (P4, P5, and P6) with biallelic COL12A1 variants who present with a similar milder disease, further establishing COL12A1-related myopathy and expanding the phenotypic spectrum. The previously reported patient, in addition to the patients with milder clinical phenotypes in our series (F4P4, F5P5, and F5P6), had either one, or both variants impacting the COL12A1 long isoform (Collagen XIIA1). In F5, the maternal variant (p.I1393Ffs*11) is expected to cause NMD of both the short and long isoform; while the paternal missense variant (p.A1110D) is expected to produce a mutant long isoform and intact short isoform. As a result, only a mildly reduced COL12 expression in the overnight conditioned culture medium was observed. In the extended culture condition, such as the deposited ECM for 5 days, however, the COL12 IF signal is similar to the normal control, but its function may be affected due to the substitution.
In mice, the long isoform is predominately expressed in early prenatal development, while postnatally it is mostly restricted to dense connective tissues, with the short isoform becoming more predominantly expressed.22-24 In chicken, however, it has been shown that the embryonic expression of the long isoform is more restricted in tissue compared to the short isoform.23 Taken together, the congenital presentation of patients with biallelic COL12A1 variants with subsequent clinical improvement, albeit variable, perhaps points toward a previously underrecognized developmental function for both isoforms. In addition, we can see a genotype–phenotype correlation trend emerging with homozygous LoF variants impacting the short isoform having a more severe congenital phenotype with profound weakness, respiratory insufficiency and feeding difficulties and minimal attainment of motor milestones compared to patients with variants impacting the long isoform (Fig. 3B).
This series characterizes the clinical presentation, immunostaining, and muscle imaging findings associated with recessive biallelic variants in COL12A1 and further establishes COL12A1 myopathic EDS as a connective tissue/muscle overlap syndrome, with an expanding clinical spectrum from severe to milder phenotypes. Identification of additional variants in COL12A1 and the characterization of the spectrum of associated clinical, histological, and imaging findings, specifically in older patients, will lead to further insight in the disease manifestations and emerging genotype–phenotype correlations associated with pathogenic biallelic COL12A1 variants.
Author Contributions
Study concept, design, and supervision: SD and CGB. Data acquisition: DS, PM, CGL, HP, YZ, MM, CT, YH, LF, RP, SC, PM, AJS, JRF, SRB, SN, DMM, MNN, SN, DPD, SFK, TEL, ARF, and VS. Data analysis and interpretation: RMM, PY, FM, and VS. Drafting the manuscript: SD, RMM, and DS. Critical review of the manuscript: All authors.
Acknowledgments
We would like to thank the patients and their families for participating in our research study. We would also like to thank Gilberto (“Mike”) Averion, Christine Jones, Kia Brooks, and Christopher Mendoza for their help in the clinic. We also thank Dr. Manuel Koch for his expertise. The work in C.G. Bönnemann's laboratory is supported by intramural funds from the NIH National Institute of Neurological Disorders and Stroke. MYO-SEQ was funded by Sanofi Genzyme, Ultragenyx, LGMD2I Research Fund, Samantha J. Brazzo Foundation, LGMD2D Foundation and Kurt+Peter Foundation, Muscular Dystrophy UK, and Coalition to Cure Calpain 3. V. Straub is supported by the NIHR Newcastle Biomedical Research Centre (BRC).
Conflict of Interest
The authors have no conflict of interest to report.
Open Research
Data Availability Statement
Data available on request from the authors.




