The characteristic and biomarker value of transcranial sonography in cerebellar ataxia
Abstract
Objective
Transcranial sonography (TCS) is a noninvasive neuroimaging technique, visualizing deep brain structures and the ventricular system. Although widely employed in diagnosing various movement disorders, such as Parkinson's disease and dystonia, by detecting disease-specific abnormalities, the specific characteristics of the TCS in cerebellar ataxia remain inconclusive. We aimed to assess the potential value of TCS in patients with cerebellar ataxias for disease diagnosis and severity assessment.
Methods
TCS on patients with genetic and acquired cerebellar ataxia, including 94 with spinocerebellar ataxias (SCAs) containing 10 asymptomatic carriers, 95 with cerebellar subtype of multiple system atrophy (MSA-C), and 100 healthy controls (HC), was conducted. Assessments included third ventricle width, substantia nigra (SN) and lentiform nucleus (LN) echogenicity, along with comprehensive clinical evaluations and genetic testing.
Results
The study revealed significant TCS abnormalities in patients with cerebellar ataxia, such as enlarged third ventricle widths and elevated rates of hyperechogenic SN and LN. TCS showed high accuracy in distinguishing patients with SCA or MSA-C from HC, with an AUC of 0.870 and 0.931, respectively. TCS abnormalities aided in identifying asymptomatic SCA carriers, effectively differentiating them from HC, with an AUC of 0.725. Furthermore, third ventricle width was significantly correlated with SARA and ICARS scores in patients with SCA3 and SCOPA-AUT scores in patients with MSA-C. The SN area and SARA or ICARS scores in patients with SCA3 were also positively correlated.
Interpretation
Our findings illustrate remarkable TCS abnormalities in patients with cerebellar ataxia, serving as potential biomarkers for clinical diagnosis and progression assessment.
Introduction
Cerebellar ataxia is a group of highly heterogeneous disorders with inherited and acquired patterns associated with progressive degeneration of the cerebellum. Spinocerebellar ataxia (SCAs), an autosomal dominant inherited disease, is a common type of genetic cerebellar ataxia.1, 2 The cerebellar subtype of multiple system atrophy (MSA-C), a sporadic neurodegenerative disease, should be distinguished from SCAs.3 Clinically, patients with cerebellar ataxia may exhibit pyramidal and extrapyramidal symptoms/signs, cortical impairment, and parkinsonian features besides motor incoordination.2, 4, 5 Pathologically, basal ganglia involvement has also been observed in patients with cerebellar ataxia. Postmortem studies have revealed severe neuronal loss in the basal ganglia, including the substantia nigra (SN) and globus pallidus, a part of the lentiform nucleus (LN), in polyglutamine (polyQ) SCAs, such as SCA1, SCA2, SCA3, and SCA7.5-7 Moreover, patients with MSA-C also demonstrate severe basal ganglia pathology with primary cerebellar symptoms.3, 8 Furthermore, single-photon emission computed tomography (SPECT) showed early degeneration of nigrostriatal dopaminergic neurons in both patients with SCAs and MSA-C.9-15 However, the technical complexity, high cost, and radiation hazards of SPECT limit its clinical application. Other neuroimaging technologies, such as magnetic resonance imaging (MRI), often display normal images in the early stage and may fail to detect early changes in the basal ganglia of patients.16-18
Transcranial sonography (TCS) is a noninvasive, cost-effective, and real-time imaging technology that reveals deep brain structures, including the SN, red nucleus, median raphe, and LN.19, 20 Visualization of the ventricular system further enhances the value of TCS in diagnosing diverse movement disorders.21 Currently, the TCS is a valid tool for assessing patients with Parkinson's disease (PD), with a hyperechogenic SN found in up to 90% of patients with PD.22-24 Additionally, TCS examinations can detect disease-specific abnormalities in other movement disorders, such as Huntington's disease (HD).25, 26 TCS has also demonstrated notable abnormalities, such as enlarged third ventricles and increased hyperechogenicity in the SN and LN during its research on patients with cerebellar ataxia, including a small number of patients with SCA2, SCA3, SCA6, and SCA7.10, 23, 27-34 However, a systematic evaluation of the TCS features in patients with cerebellar ataxia is lacking. The role of the TCS in distinguishing patients with symptomatic or asymptomatic cerebellar ataxia from healthy controls needs investigation. Furthermore, we raised some questions that deserve further exploration: (1) Do hereditary or acquired ataxia share common alterations reflected by TCS? (2) Are there specific TCS changes in different subtypes of hereditary ataxia? (3) Is there any correlation between TCS measurements and disease severity of patients with cerebellar ataxia?
Here, we enrolled the largest cohort of SCAs (including SCA1, 2, 3, 6, 12, 17, and SCA36) and MSA-C patients to date, aiming to reveal the TCS characteristics of both inherited and acquired cerebellar ataxia patients. We also assessed the value of TCS as a potential biomarker for diagnosing and evaluating the disease severity in cerebellar ataxia.
Methods
Subjects
This study recruited 94 patients with SCA, 95 patients with MSA-C, and 100 healthy controls (HC) from the neurology department of Xiangya Hospital, Central South University, between 2022 and 2023. All participants were adults who provided written informed consent in accordance with the Declaration of Helsinki. SCA3, the most prevalent SCA subtype in China, was present in 68 patients in our cohort. In addition, we enrolled patients with other SCAs, including 2 with SCA1, 9 with SCA2, 4 with SCA6, 3 with SCA12, 4 with SCA17, and 4 with SCA36. All cases of SCAs were confirmed using genetic tests. Among the 68 patients with SCA3, 10 were in the preclinical stage. Preclinical SCA3 was defined as an abnormal expansion of the ATXN3 gene detected through genetic tests, absence of clinical symptoms, and an Assessment and Rating of Ataxia (SARA) score below 3.0. In addition, according to existing predictive models, 80% of preclinical SCA3 patients in this cohort had not yet reached the predicted median age of onset based on CAG length35, 36 (Table S1). The clinical diagnosis of MSA-C followed the second consensus statement on the diagnosis of multiple system atrophy,37 and polyQ genetic mutations were ruled out in all patients with MSA-C. Exclusion criteria encompassed poor temporal acoustic windows, history of epilepsy, stroke, other neurological or psychiatric diseases, history of brain surgery, head trauma, history of alcohol or drug abuse, and intracranial organic lesions unrelated to SCA or MSA-C as identified using neuroimaging examination. For patients with SCA, the following scales were examined: SARA, International Cooperative Ataxia Rating Scale (ICARS), Inventory of Non-Ataxia Symptoms (INAS), Unified PD Rating Scale Part III (UPDRS-III), and Mini-Mental State Examination (MMSE). Patients with MSA-C were assessed using the Unified Multiple System Atrophy Rating Scale (UMSARS), Scale for Outcomes in PD for Autonomic Symptoms (SCOPA-AUT), Wexner, Rapid Eye Movement Sleep Behavior Disorder Screening Questionnaire (RBDSQ), MMSE, Montreal Cognitive Assessment (MoCA) and Frontal Assessment Battery (FAB).
The Ethics Committee of Xiangya Hospital of Central South University in China approved this study.
TCS
An experienced sonographer, blinded to the patients' clinical data performed the TCS examinations. To guarantee reproducibility, precision, and stability in our quantifications, TCS was conducted twice in a smaller cohort before this study. Our analysis of the disparity between the two sets of results revealed no statistically significant differences. The Philips CX50 ultrasound system (Philips Healthcare, Netherlands) equipped with a S5-1 transducer (frequency, 2.5–3.0 MHz; dynamic range, 45–55 dB; depth, 14–16 cm) was utilized. The patients were positioned supine with the temporal window fully exposed, and the ultrasound transducer was aligned parallel to the orbitomeatal line on the temporal plane. The SN at the midbrain level, third ventricle, and lenticular nucleus at the thalamic level were also detected (Fig. 1).
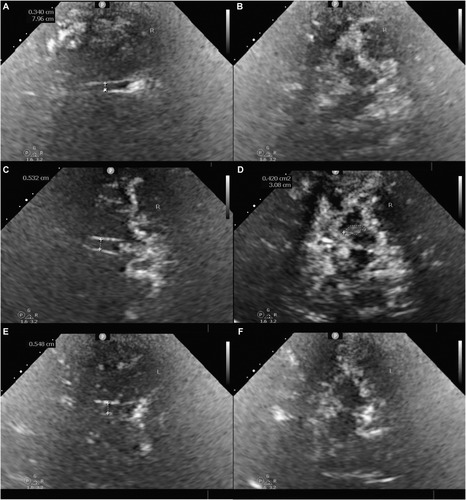
Third ventricle width was defined as the vertical distance between the ipsilateral and contralateral inner layers of the hyperechogenic ependyma. The area of SN echogenicity was manually measured in horizontal transverse sections of the midbrain, thereby identifying patients with an SN echogenic area ≥20 mm2 in at least one measurement as having SN hyperechogenicity. The largest area of echogenicity within the bilateral SN was designated as the SN area for further analysis. LN hyperechogenicity was considered when the LN exhibited a patchy echo or an echo above that of the surrounding brain parenchyma.21, 38, 39
Statistical analysis
Data analysis was performed using SPSS version 26.0. Continuous variables were presented as means (± standard deviation) or median [interquartile spacing]. Normally distributed data were assessed using ANOVA, and non-normal data were evaluated using the Kruskal–Wallis test. A multivariate logistic regression model was used to control confounding factors. Adjusted odds ratios were utilized to assess differences among groups. Numerical data, summarized as patient counts or percentages, were compared using the Chi-square test. Receiver operating characteristic (ROC) curves were used to calculate the area under the curve (AUC), sensitivity, and specificity of the differential diagnoses. Correlation analysis was performed using Spearman's correlation coefficient. p < 0.05 was defined as statistically significant. Figures are drawn using GraphPad Prism 9.5.1.
Results
Demographics and clinical data
The demographic and clinical characteristics of the participants are presented in Table 1. We recruited patients with different SCA subtypes for this study. In addition to the prevalent SCA3 subtype in China, this study included a few patients with SCA1, SCA2, SCA6, SCA12, SCA17, and SCA36. The study also included 10 patients with preclinical SCA3, each with a SARA score of less than 3.0. Table 2 summarizes the demographic and clinical data of the patients with different SCAs. Sex distribution among the groups was not different, whereas patients with MSA-C and other SCAs were older than those with SCA3 and HC (p < 0.001). The median disease duration was 1.0 year for patients with MSA-C and 3.0 years for patients with SCA3 and other SCAs (p = 0.011).
| HC (n = 100) | SCA3 (n = 68) | Other SCAs (n = 26) | MSA-C (n = 95) | p-Value | |
|---|---|---|---|---|---|
| Sex (M/F) | 45/55 | 36/32 | 13/13 | 56/39 | 0.276 |
| Age (years) | 41.5 [33.0, 48.0] | 35.0 [30.0, 51.0] | 52.5 [36.5, 57.2] | 56.0 [51.0, 59.0] | <0.001 |
| Disease duration (years) | – | 3.0 [1.0, 5.0] | 3.0 [1.0, 6.0] | 1.0 [2.0, 3.0] | 0.011 |
| Age of onset (years) | – | 36.2 ± 11.0 | 44.1 ± 12.1 | 54.0 ± 6.5 | <0.001 |
| SARA | – | 9.0 ± 5.6 | 9.8 ± 4.9 | – | 0.631 |
| ICARS | – | 23.1 ± 13.8 | 26.2 ± 12.7 | – | 0.464 |
| INAS | – | 2.0 [1.0, 3.0] | 1.5 [1.0, 2.0] | – | 0.585 |
| UPDRS-III | – | 8.6 ± 6.2 | 10.1 ± 4.2 | – | 0.507 |
| HY stage | – | – | 2.5 [2.5, 3.0] | – | |
| UMSARS-I | – | – | 16.5 ± 6.8 | – | |
| UMSARS-II | – | – | 15.6 ± 7.6 | – | |
| UMSARS-IV | – | – | 1 [2, 3] | – | |
| SCOPA-AUT | – | – | 19.5 ± 8.6 | – | |
| MMSE | – | 29 [28, 30] | 27 [25, 30] | 27 [24, 29] | <0.001 |
| 3rd ventricle width (cm) | 0.22 ± 0.10 | 0.36 ± 0.16 | 0.41 ± 0.22 | 0.46 ± 0.21 | <0.001 |
| SN area (cm2) | 0.00 [0.00, 0.18] | 0.32 [0.14, 0.37] | 0.23 [0.16, 0.29] | 0.25 [0.17, 0.30] | <0.001 |
| SN hyperechogenicity | 17.0% | 65.6% | 64.0% | 64.8% | <0.001 |
| LN hyperechogenicity | 13.0% | 23.4% | 41.7% | 37.9% | <0.001 |
- Continuous variables that follow a normal distribution are represented by the mean (±standard deviation), while those not following a normal distribution are represented by the median [interquartile spacing].
- HC, healthy controls; ICARS, International Cooperative Ataxia Rating Scale; INAS, Inventory of Non-Ataxia Symptoms; LN, lentiform nucleus; MMSE, Mini-Mental State Examination; MSA-C, cerebellar subtype of multiple system atrophy; SARA, Assessment and Rating of Ataxia; SCA, spinocerebellar ataxia; SCOPA-AUT, Scale for Outcomes in PD for Autonomic Symptoms; SN, substantia nigra; UMSARS, Unified Multiple System Atrophy Rating Scale; UPDRS, Unified PD Rating Scale.
| SCA1 (n = 2) | SCA2 (n = 9) | aSCA3 (n = 10) | SCA3 (n = 58) | SCA6 (n = 4) | SCA12 (n = 3) | SCA17 (n = 4) | SCA36 (n = 4) | SCA (n = 94) | |
|---|---|---|---|---|---|---|---|---|---|
| Sex (M/F) | 2/0 | 3/6 | 5/5 | 31/27 | 2/2 | 2/1 | 3/1 | 1/3 | 49/45 |
| Age (years) | 36.0 | 45.9 ± 13.1 | 33.5 ± 5.1 | 40.2 ± 12.5 | 45.2 ± 12.8 | 59.3 ± 5.1 | 49.8 ± 13.0 | 49.8 ± 7.0 | 41.8 ± 1.3 |
| Disease duration (years) | 4.5 | 4.7 ± 2.9 | – | 4.0 ± 2.3 | 2.5 ± 1.9 | 6.3 ± 4.0 | 2.8 ± 1.9 | 1.7 ± 0.6 | 3.5 ± 0.3 |
| Expanded repeats (n) | CAG (51.0) | CAG (38.6 ± 2.8) | CAG (69.9 ± 3.8) | CAG (71.7 ± 4.6) | CAG (21.5 ± 2.5) | CAG (44.3 ± 9.5) | CAG (42.0 ± 1.2) | GGCCTG (>14) | – |
| Extrapyramidal sings | 1/2 | 6/9 | 1/10 | 32/58 | 3/4 | 2/3 | 1/4 | 0/4 | 47/94 |
| Bradykinesia | 0/2 | 1/9 | 0/10 | 10/58 | 0/4 | 1/3 | 1/4 | 0/4 | 13/94 |
| Static tremor | 0/2 | 4/9 | 0/10 | 4/58 | 1/4 | 2/3 | 0/4 | 0/4 | 12/94 |
| Stiffness | 1/2 | 4/9 | 1/10 | 29/58 | 2/4 | 1/3 | 1/4 | 0/4 | 39/94 |
| Olfactory impairment | 0/2 | 0/9 | 0/10 | 1/58 | 0/4 | 0/3 | 0/4 | 0/4 | 1/94 |
| Autonomic dysfunctiona | 0/2 | 0/9 | 0/10 | 15/58 | 1/4 | 2/3 | 3/4 | 0/4 | 21/94 |
| SARAa | 9.5 | 10.1 ± 1.9 | 0.5 ± 0.3 | 10.4 ± 0.6 | 6.2 ± 0.9 | 11.0 | 9.0 ± 5.3 | 9.2 ± 6.2 | 9.0 ± 5.3 |
| ICARSa | 25.5 | 27.3 ± 14.4 | 2.5 ± 1.4 | 26.7 ± 11.6 | 13.3 ± 5.0 | 21 | 24.0 ± 24.0 | 21.7 ± 16.9 | 23.2 ± 13.6 |
| INASa | 2 | 1 [1, 2] | 1[0, 1] | 1 [2, 3] | 1 [2, 3] | 3 | 0 [0, 0] | 1 [1, 2] | 2 [1, 3] |
| MMSEa | 28 | 27 [25, 30] | 30 [30, 30] | 29 [27, 30] | 28 [24, 30] | 26 [25, 28] | 28 [26, 29] | 28 [27, 29] | 29 [27, 30] |
| UPDRS-III | 8.0 | 11.3 ± 4.1 | 0.7 ± 1.1 | 10.2 ± 5.5 | 6.8 ± 5.1 | 17.0 | 13.0 ± 8.9 | 5.5 ± 3.7 | 8.9 ± 6.1 |
| 3rd ventricle width (cm)a | 0.34 | 0.35 ± 0.15 | 0.29 ± 0.15 | 0.37 ± 0.16 | 0.44 ± 0.15 | 0.78 ± 0.35 | 0.36 ± 0.20 | 0.31 ± 0.16 | 0.37 ± 0.18 |
| SN area (cm2) | 0.18 | 0.18 [0.06, 0.25] | 0.20 [0.00, 0.35] | 0.35 [0.16, 0.39] | 0.42 [0.12, 0.58] | 0.22 [0.16, 0.27] | 0.29 [0.26, 0.40] | 0.24 [0.19, 0.30] | 0.28 [0.15, 0.37] |
| SN hyperechogenicity | 0 | 55.6% | 50.0% | 70.0% | 75.0% | 50.0% | 100.0% | 75.0% | 65.1% |
| LN hyperechogenicity | 50.0% | 22.2% | 30.0% | 22.2% | 66.7% | 50.0% | 25.0% | 75.0% | 28.4% |
- a Means of a statistical difference among the groups with p < 0.05.
In SCA groups, patients with SCA3 and other SCAs did not differ in clinical scores (SARA: SCA3: 9.0 ± 5.6 vs. other SCA: 9.8 ± 4.9; ICARS: SCA3: 23.1 ± 13.8 vs. other SCA: 26.2 ± 12.7). In addition to ataxia, some patients with SCA develop extra-cerebellar symptoms, including the most frequently occurring limb stiffness (39/94, 41.49%), autonomic dysfunction (21/94, 22.34%), bradykinesia (13/94, 13.83%), and static tremor (12/94, 12.77%), during the disease. However, our patients did not have other movement disorders, such as dystonia or chorea. In the MSA-C group, the median HY stage was 2.5, the UMSARS-I score was 16.5 ± 6.8, the UMSARS-II score was 15.6 ± 7.6, the median UMSARS-IV score was 1, and the SCOPA-AUT score was 19.5 ± 8.6.
TCS features in different groups
In our cohort, the third ventricle width in the HC group was 0.22 ± 0.10 cm. The median SN area was 0.00 cm2 in HC (Q1:0.00 cm2, Q3:0.18 cm2). Additionally, 17.0% and 13.0% of normal individuals exhibited hyperechogenic SN and LN, respectively (Table 1).
Patients with cerebellar ataxia had greater third ventricle width than those HC, with an average of 0.36 cm in SCA3, 0.41 cm in other SCAs, 0.46 cm in MSA-C, and 0.22 cm in HC (p < 0.001, Table 1). After adjusting for age, the disparities in third ventricle width between the SCA3 and HC groups, other SCA and HC groups, and MSA-C and HC groups were significantly retained (Fig. 2A). Despite a significant increase in the width of the third ventricle in patients with cerebellar ataxia, most patients did not meet the current diagnostic criteria for “abnormal third ventricle width.” The criteria were established based on the data from Caucasian health controls, defining abnormal third ventricle width as ≥7 mm for individuals aged 20–60 and ≥10 mm for those over 60.19, 38 According to these criteria, only six patients with SCA and seven patients with MSA-C in our cohort were diagnosed with abnormal third ventricle width. However, due to racial differences,40 we preliminarily defined the normal range of the third ventricle width in the Chinese population using data from 100 normal individuals in our cohort. Defining the normal range as a 99% confidence interval of normal observations, we found it to be <4.50 mm for individuals aged 20–60 in the HC group. As our cohort did not include normal controls aged over 60 years, the normal range of the Chinese cohort for this age group remains undefined. Based on the Chinese criteria, 23.9% of patients with SCA and 35.5% of patients with MSA aged 20–60 in our cohort exhibited abnormal third ventricle enlargement.
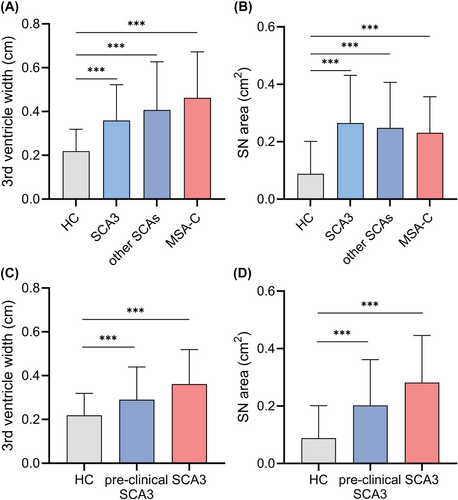
The SN areas in patients with cerebellar ataxia were larger than those in HC. The median SN area was 0.32 cm2 in the SCA3 group, 0.23 cm2 in the other SCAs group, 0.25 cm2 in the MSA-C group, and 0.00 cm2 in the HC group (p < 0.001), and the differences were remarkable after age adjustment (Fig. 2B). The prevalence of hyperechogenic SN was 65.6%, 64.0%, and 64.8% in the SCA3 group, other SCAs group, and MSA-C group, respectively, which was significantly higher than that in the HC group (17.0%; p < 0.001). In addition, patients with cerebellar ataxia had a higher rate of hyperechogenic LN than HC (p < 0.001), with a prevalence of 23.4% in SCA3, 41.7% in the other SCAs, 37.9% in the MSA-C and 13.0% in the HC group (Table 1).
Although the TCS findings in patients with cerebellar ataxia significantly differed from those in HC, no significant differences were found in third ventricle width, SN area, and rate of SN and LN hyperechogenicity, among the three disease groups (Fig. 2A,B).
The TCS features of each SCA subtype are shown in Table 2. Patients with preclinical SCA3 exhibited a greater third ventricle width than HC (mean 0.29 cm in patients with preclinical SCA3 vs. 0.22 cm in HC), and the difference was retained after adjusting for age (p = 0.022). The SN area in the preclinical SCA3 group was larger than that of the HC group, with a median of 0.20 cm2 in patients with preclinical SCA3 and a median of 0.00 cm2 in HC (p = 0.007). Moreover, 50.0% and 30.0% of patients with preclinical SCA3 presented with hyperechogenic SN and LN, respectively, which were significantly higher than the respective 17.0% and 13.0% in HC. Among these patients with preclinical SCA3, TCS abnormalities can manifest an average of 7 years before the predicted onset age based on CAG length. However, no significant differences were found between patients with preclinical SCA3 and those with SCA3 after adjusting for age (Fig. 2C,D).
Some patients with SCA in our cohort exhibited extrapyramidal symptoms. We sub-classified these patients according to the presence of extrapyramidal symptoms, including bradykinesia, static tremor, or stiffness, to investigate whether these symptoms affect the TCS features of patients with cerebellar ataxia. Patients with or without extrapyramidal symptoms exhibited significant differences in clinical scores, such as INAS and UPDRS-III in SCAs (Table S2), and HY stage, UMSARS-I, and UMSARS-II in MSA-C (Table S3). However, extrapyramidal symptoms did not influence TCS results.
TCS in differentiating patients with SCAs and MSA from HC
Given the limited number of patients with other SCA subtypes and no significant difference in TCS indicators between patients with SCA3 and other SCAs, we performed ROC analysis by pooling data from both SCA3 and other SCAs patients, collectively referred to as the SCA group. Analysis of TCS parameters among groups revealed that TCS is a reliable tool for distinguishing patients with cerebellar ataxia from HC. The combined use of third ventricle width and SN area could distinguish patients with SCAs from HC (AUC = 0.870; sensitivity, 76.2%; specificity, 87.0%). In patients with MSA-C, combining third ventricle width with SN and LN hyperechogenicity provided an accurate diagnosis (AUC = 0.931; sensitivity, 78.8%; specificity, 94.0%). TCS was also effective in identifying patients with preclinical SCA3 from HC. The SN area showed an AUC of 0.725 in patients with preclinical SCA3, with a sensitivity of 70.0% and specificity of 75.0% (Fig. 3).
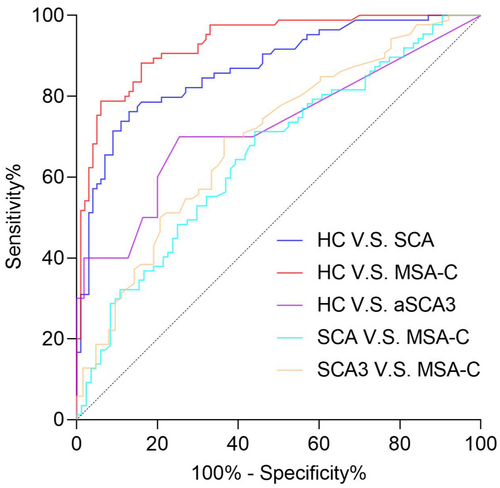
Regardless of the parameter combinations used, TCS could not effectively distinguish SCA from MSA-C. The highest AUC achieved was 0.656, which combined the third ventricle width and SN area. Similarly, to discriminate SCA3 from MSA-C, the most effective combination was third ventricle width and hyperechogenic LN, yielding an AUC of 0.694 (Fig. 3). In addition, TCS parameters were not effective in discriminating between patients with preclinical SCA3 and SCA3.
Correlation of TCS parameters with disease severity in patients with SCA3 and MSA-C
Correlation analysis was performed between third ventricle width, SN area, and clinical scale scores in patients with SCA (Table S4) and MSA-C (Table S5). Given that disease progression may not be consistent among different SCA subtypes,41 we also separately analyzed the association between TCS parameters and clinical scores in patients with SCA3 (Table S6). Similar results were found in the SCA and SCA3 groups, but analysis of patients with SCA3 alone excluded confounding factors affecting clinical scores and, thus, proved more reliable.
A positive correlation was observed between age and third ventricle width in SCA3 (r = 0.284, p = 0.020) and MSA-C (r = 0.362, p < 0.001) groups. Third ventricle width showed no correlation with disease duration in either cerebellar ataxia group. The SN areas did not correlate with age or disease duration (Tables S5 and S6).
Third ventricle width positively correlated with SARA and ICARS scores in patients with SCA3 (r = 0.320, p = 0.009, and r = 0.317, p = 0.010, respectively). After adjusting for age, the significant correlations persisted (Fig. 4A; Table S6). In the MSA-C group, a modest correlation was observed between third ventricle width and both UMSARS-I (r = 0.260, p = 0.027) and SCOPA-AUT scores (r = 0.280, p = 0.017). However, when controlling for age as a confounding factor, no significant correlation was found with the UMSARS-I (Fig. 4C; Table S5).
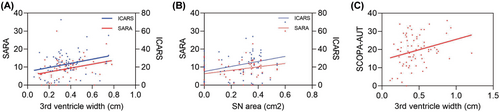
The SN area was positively correlated with SARA (r = 0.264, p = 0.045) and ICARS scores (r = 0.311, p = 0.018) in patients with SCA3 (Fig. 4B). Conversely, no significant correlation was observed between the SN area and clinical scale scores in patients with MSA-C (Table S5).
Discussion
TCS systems, known for their time and cost efficiency, provide a comparable accuracy to MRI in visualizing deep brain structures.42 This study recruited a large cohort of patients with cerebellar ataxia and included individuals at the preclinical stage for the first time to explore the applicability of TCS in cerebellar ataxia. Our study found enlarged third ventricle widths and elevated rates of hyperechogenic SN and LN in patients with cerebellar ataxia, suggesting potential roles for TCS in the diagnosis and disease severity assessment of cerebellar ataxia.
The width of the third ventricle varies among racial groups. For example, compared with Caucasians, the Chinese had relatively shorter brain length and height.40 In this study, we preliminarily established a normal range for third ventricle width in a Chinese population aged 20–60 using our control group data. Despite the limited sample size and the exclusion of individuals aged 60 and above, our exploration of the normal range of the third ventricle is valuable in uncovering brain atrophy in the younger population. This may also underpin the characteristics of TCS in the healthy Chinese population.
The third ventricle width is a reliable indicator of brain atrophy.26, 43 In our study, an enlarged third ventricle, as assessed by TCS, was observed in patients with cerebellar ataxia after age adjustment. This means that cerebellar atrophy is a common feature shared by genetic and acquired ataxia, and that these abnormalities are correlated with disease progression.
In our study, SN hyperechogenicity was identified as a prominent TCS feature in patients with SCA, aligning with and reinforcing observations from previous studies.27, 29, 30 Patients with MSA-C show significantly elevated rates of hyperechogenic LN and SN. Furthermore, our investigation revealed that the SN area was positively correlated with SARA and ICARS in patients with SCA3. Studies have suggested that hyperechogenicity in the SN and LN may arise from the accumulation of metals such as iron and copper, correlated with neuronal loss and functional impairment in the deep brain nuclei.21, 44-47 Therefore, the high prevalence of SN and LN hyperechogenicity in our study reflects the involvement of the deep brain nuclei in patients with cerebellar ataxia. SN and LN hyperechogenicity in the preclinical stage of SCA carriers indicate basal ganglia involvement in the initial stages of the disease. These findings are consistent with those of previous neuropathologic studies.3, 5-8 Moreover, recent studies have highlighted the role of the basal ganglia-cerebellar neurocircuitry in motor function, memory, and emotion.48, 49 Disruption of this neurocircuitry is one of the mechanisms underlying various neurodegenerative diseases, including PD and dystonia.50-53 Our results provide further evidence that the disruption of the basal ganglia-cerebellar neurocircuitry may represent a common pathway in neurodegenerative disorders, which are also present in cerebellar ataxia.
Although patients with cerebellar ataxia share common features with those with basal ganglia damage, their symptoms may vary.18, 54 Clinically, patients with MSA-C often manifest parkinsonian symptoms in later stages. Therefore, SN hyperechogenicity may predict parkinsonism in patients with MSA-C. However, despite the early and severe involvement of the SN in patients with SCA, parkinsonian features are rarely observed. This paradox may be because of the degeneration of the subthalamic nucleus. The involvement of the SN in patients with SCA may act as an internal compensatory mechanism that prevents the development of parkinsonism.7 Considering this perspective, we propose that TCS may play a more significant role in monitoring the severity of central nervous system degeneration than in predicting the onset of parkinsonian symptoms.29, 30
The TCS abnormalities in SCA3 carriers appear before the onset of clinical symptoms. Here, we found that an enlarged third ventricle, or SN and LN hyperechogenicity in TCS may emerge approximately 7 years before the predicted onset age, suggesting that patients in the preclinical stage presented early pathophysiological changes. PolyQ expansion-related neuron loss and brain atrophy precede the appearance of ataxia symptoms in preclinical SCA carriers, and disease-modifying therapies and neuroprotective agents are likely to be the most effective at this stage.55 However, the SARA and ICARS lack sensitivity in the early stages.56, 57 The MRI techniques, as non-invasive and quantitative methods, have gained attention for assessing structural alterations in preclinical carriers. However, no study has specifically addressed the interval between subtle abnormalities in structural and functional brain imaging before the manifestation of ataxia symptoms.16, 17, 58 TCS, as a noninvasive, time- and cost-effective method, can be used as an auxiliary diagnostic tool to assess brain atrophy and basal ganglia damage in patients with SCA after genetic testing. Further, TCS is a potential source of neuroimaging biomarkers that helps identify the potential pathogenesis underlying both manifested and preclinical SCA in patients, assess disease severity and progression, monitor therapeutic effects, and determine nuances that can be used as endpoints for future clinical trials.
This study had some limitations. First, our study predominantly included patients in the preclinical and early to-middle stages of cerebellar ataxia, with fewer patients in the advanced stage. Second, the lack of a follow-up study limits our understanding of long-term implications. Future studies should aim to establish TCS criteria for various populations, including the Chinese population. Additionally, longitudinal follow-up studies across multiple centers, including patients in later stages of cerebellar ataxia, are necessary. These endeavors will contribute to a more comprehensive clinical assessment of the value of TCS in patients with cerebellar ataxia.
In conclusion, our study revealed remarkable abnormalities in the TCS images of patients with cerebellar ataxia, including enlarged third ventricle width and SN and LN hyperechogenicity. The width of the third ventricle, which is a marker of brain atrophy, can be reliably monitored using TCS. Abnormalities in TCS images can differentiate between healthy controls and patients with ataxia, even in the preclinical stage. TCS may be a useful screening tool for early diagnosis and assessment of disease progression in patients with ataxia.
Acknowledgments
We thank all the participants for their involvement in this study.
Author Contributions
Conception of the work: YTS, SDZ, and HJ; Acquisition of the data: YTS, SDZ, FYD, YF, DJC, and XRY; Data interpretation: YTS, SDZ, and CRW; Statistical analysis: SDZ and CRW; Writing the first draft: SDZ; Critical revision and approval of the manuscript: All authors. All authors read and approved the final manuscript.
Conflict of Interest Statement
The authors declare that they have no competing interests.
Funding Information
This study was funded by the National Key Research and Development Program of China (No. 2021YFA0805200 to H Jiang), the National Natural Science Foundation of China (82171254 to H Jiang; 81901305 to C Wang; 82371272 to Z Chen; 82301628 to L Wan; 82301438 to Z Long; 82201411 to L He), the Natural Science Foundation of Hunan Province (No. 2024JJ3050 to H Jiang; 2022JJ20094 to Z Chen; 2022JJ40783 to L He; 2022JJ40703 to Z Long; 2024JJ6638 to L Wan), the Major Scientific Research Project for High-level Health Talent in Hunan Province(No. R2023047 to H Jiang), Furong Lab Research Project(No. 2023SK2084 to H Jiang), the Central South University Research Programme of Advanced Interdisciplinary Study (No. 2023QYJC010 to H Jiang), the Science and Technology Innovation Program of Hunan Province (2022RC1027 to Z Chen), Postdoctoral Fellowship Program of CPSF(GZB20230870 to L Peng).
Ethics Approval and Consent to Participate
This study was approved by the Ethics Committee of Xiangya Hospital, Central South University and informed consent according to the Declaration of Helsinki was obtained from all participants.
Consent for Publication
Not applicable.
Open Research
Data Availability Statement
Data available on request due to privacy/ethical restrictions.




