Clinical heterogeneity within the ALS-FTD spectrum in a family with a homozygous optineurin mutation
Abstract
Objective
Mutations in the gene encoding for optineurin (OPTN) have been reported in the context of different neurodegenerative diseases including the amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD) spectrum. Based on single case reports, neuropathological data in OPTN mutation carriers have revealed transactive response DNA-binding protein 43 kDa (TDP-43) pathology, in addition to accumulations of tau and alpha-synuclein. Herein, we present two siblings from a consanguineous family with a homozygous frameshift mutation in the OPTN gene and different clinical presentations.
Methods
Both affected siblings underwent (i) clinical, (ii) neurophysiological, (iii) neuropsychological, (iv) radiological, and (v) laboratory examinations, and (vi) whole-exome sequencing (WES). Postmortem histopathological examination was conducted in the index patient, who deceased at the age of 41.
Results
The index patient developed rapidly progressing clinical features of upper and lower motor neuron dysfunction as well as apathy and cognitive deterioration at the age of 41. Autopsy revealed an ALS-FTLD pattern associated with prominent neuronal and oligodendroglial TDP-43 pathology, and an atypical limbic 4-repeat tau pathology reminiscent of argyrophilic grain disease. The brother of the index patient exhibited behavioral changes and mnestic deficits at the age of 38 and was diagnosed with behavioral FTD 5 years later, without any evidence of motor neuron dysfunction. WES revealed a homozygous frameshift mutation in the OPTN gene in both siblings (NM_001008212.2: c.1078_1079del; p.Lys360ValfsTer18).
Interpretation
OPTN mutations can be associated with extensive TDP-43 pathology and limbic-predominant tauopathy and present with a heterogeneous clinical phenotype within the ALS-FTD spectrum within the same family.
Introduction
Mutations in the gene encoding optineurin (OPTN) have been associated with different neurodegenerative phenotypes including the amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD) spectrum as well as atypical parkinsonism with variable disease progression and survival.1-3 On a cellular basis, optineurin acts as an ubiquitin-binding adaptor protein and is involved in multiple biological processes including autophagy, tumor necrosis factor alpha (TNFα) signaling, mitophagy, and necroptosis.4-6 OPTN mutations can thus lead to a disruption of these cellular processes facilitating abnormal protein aggregation and promoting neurodegeneration.7 Neuropathological descriptions in OPTN mutation carriers are scarce and based on single case reports, revealing mostly an accumulation of transactive response DNA-binding protein 43 kDa (TDP-43), and in some cases associated with variable amounts of tau and/or alpha-synuclein in restricted brain areas.7-9
In this study, we present a comprehensive clinical and laboratory workup of two siblings with different clinical phenotypes in the context of a homozygous frameshift mutation in the OPTN gene, together with an in-depth neuropathological description of one of the siblings revealing predominant TDP-43-pathology combined with a prominent limbic tauopathy.
Methods
Genetic analyses
DNA was extracted from the patients' peripheral blood, and whole-exome sequencing (WES) was conducted at the Institute of Human Genetics, Technical University of Munich, Germany. WES was performed on an Illumina NovaSeq6000 system (Illumina, San Diego, California) using a SureSelect Human All Exon Kit (Agilent 60mb V6) for enrichment in the index patient and a Twist Human Exome 2.0 Plus Comprehensive Exome Spike-in and Mitochondrial Panel in the brother of the index patient. Reads were aligned to the Genome Reference Consortium Human Build 37 (GRCh37) using Burrow-Wheeler Aligner.10 Single nucleotide variants and small insertions and deletions were analyzed using the SAMtools pipeline,11 and ExomeDepth as well as Pindel were used to detect copy number variations.12 Variant calling was carried out with the exome variant analysis database (EVAdb) pipeline of the Institute of Human Genetics of the Technical University Munich as previously described.13 Apolipoprotein E (APOE) status was evaluated performing qPCR with TaqMan assays (Thermofisher) at two SNP positions of the APOE gene (rs429358 and rs7412). Allelic discrimination analysis of both positions was used to determine the APOE genotype. Chromosome 9 open reading frame 72 (C9orf72) genotyping was performed using flanking PCR and repeat primed PCR with FAM-labeled primers as previously described.14 Detection and sizing of PCR products were performed on the ABI 3500 Instrument (Applied Biosystems) using GeneScanTM LIZ600 size standard v.2.0. (Applied Biosystems).
Neuropathological examination
Postmortem histopathological brain examination was conducted in the index patient. Gross brain examination was followed by the evaluation of formalin fixed and paraffin-embedded tissue blocks representing both hemispheres that included the following anatomical regions: prefrontal cortex, motor cortex, anterior cingulate, parietal, temporal, occipital cortices, hippocampus, ento- and transentorhinal cortex, amygdala, anterior and posterior basal ganglia, thalamus, mesencephalon, pons, medulla oblongata, spinal cord, and cerebellum. In addition to hematoxylin and eosin (HE) and Luxol-fast-blue staining, the following mouse monoclonal antibodies were applied for immunohistochemistry: anti-phosphorylated tau (1:2000, clone AT8, pS202/pT205; Thermo Scientific, Rockford, IL, USA), anti-phosphorylated TDP-43 (1:20,000, clone 11–9, pS409/410; Cosmo Bio, Tokyo, Japan), anti-p62 (1:500, clone 3/p62 lck ligand; BD Transduction Laboratories, Franklin Lakes, NJ, USA), anti-ßA4-amyloid (1:100, clone 6F/3D; DAKO, Glostrup, Denmark), anti-alpha-synuclein (1:4000, clone 5G4; Roboscreen, Leipzig, Germany), and two anti-optineurin antibodies (non-C-terminal: 1:500 polyclonal Proteintech Group, Rosemont, IL, US; C-terminal: 1:800, polyclonal, Cayman Chemical, Ann Arbor, MI, US). The DAKO EnVision© detection kit, peroxidase/DAB, rabbit/mouse (Dako, Glostrup, Denmark) was used for visualization of antibody reactions.
RNA extraction and rtPCR
The RNAeasy Plus Mini Kit (QIAGEN) was used for extraction of RNA from PBMCs (brother of the index patient and control) and following the manufacturer's instructions. cDNA was then synthesized from 30 ng RNA with an iScript cDNA synthesis Kit (Bio-Rad, Cat. No 1708891). Quantitative rt-PCR was performed with the SYBR™ Green Master Mix (ThermoFisher) using the Agilent AriaMx rt-PCR system. The primers for the target gene (OPTN, forward: AAAACTGGAACTGGCAGAGAA, reverse: TGCAATGCCAGTTGCTCCTTT) and house-keeping gene (GAPDH, forward: ATGGTGAAGGTCGGAGTGAA, reverse: CGTGGGTGGAATCATACTGG) were used to analyze RNA expression. Technical triplicates were performed.
Protein extraction and western blot
For western blot analyses, protein was extracted from fresh deep frozen frontal cortex tissue (index patient and control) and PBMC lysates (brother of the index patient and control). Following digestion with up to 300 μL cold RIPA buffer (ThermoFisher) and addition of Complete™ protease inhibitor cocktail (Merck) and PhosSTOP™ phosphatase inhibitor (Merck) samples, centrifugation at 14,000 rcf (for 10 min at 4°C) was performed and supernatants were used for further analysis. Protein concentrations were quantified with a Bradford Protein Assay Kit (Bio-Rad). To achieve a final concentration of 1 μg/μL, protein samples were diluted with RIPA buffer (ThermoFisher), 2x Laemmli buffer (Bio-Rad, 1610737), and distilled water. Proteins were then denatured at 70°C for 10 min.
Brain tissue lysates and PBMC lysates were loaded onto NuPAGE™ Bis-Tris 4–12% gradient gels (ThermoFisher) at a total protein quantity of 5 μg and 30 μg per well, respectively. Proteins were separated by gel electrophoresis with NuPAGE™ MOPS SDS running buffer (ThermoFisher) at up to 90 V (brain tissue lysates) or 120 V (PBMC lysates). The Trans-Blot Turbo™ Transfer System was used for transfer of proteins onto nitrocellulose membranes at 25 V for 15 min. Membranes with loaded proteins were incubated with EveryBlot Blocking Buffer (Bio-Rad) at room temperature for 30–60 min, followed by incubation with primary antibodies at 4°C for at least 8 h. We used two OPTN polyclonal antibodies (Proteintech, Cat. No. 10837-1-AP, at a dilution of 1:10,000 & Cayman, Item No. 100000, at a dilution of 1:1000), of which one binds to the C-terminus of optineurin (Cayman), and a GAPDH antibody (Cell Signaling, Cat No. 2118, at a dilution of 1:1000). This was followed by washing membranes with TBS-T 3x for 10 min, incubation with the appropriate anti-horseradish peroxidase (HRP)-coupled secondary antibodies (mouse: Cell Signaling, Cat. No 7076; rabbit: Merck Millipore, Cat No. GENA934-1:10,000 dilution) for 30 min, and a second washing course with TBS-T as described above. Before imaging with the VILBER FUSION FX system, membranes were incubated with Clarity ECL solution (Bio-Rad) for 30–45 sec. Technical triplicates were performed.
Results
Index patient
The 41-year-old male patient developed dysphagia, cervical pain, and headache followed by rapidly progressing clinical features of upper and lower motor neuron dysfunction, starting with dysarthria and weakness of his right arm and further evolving into a right-accentuated spastic tetraparesis with muscle atrophy and fasciculations. He experienced cognitive deterioration, affecting his attention, memory, language, and executive function. He developed behavioral changes, which started with paranoid delusions and intermittent bursts of aggression and evolved into a severe depressive state with suicidal ideation and apathy. The patient did not show any extrapyramidal signs in his clinical course. He was admitted to the Department of Neurology of the Medical University of Vienna 8 months after symptom onset. At the time of hospitalization, the patient was tetraparetic and unable to walk or communicate with a score of 12 at the revised amyotrophic lateral sclerosis functional rating scale (ALSFRSR). He was diagnosed with ALS-FTD based on current diagnostic criteria.15, 16
The patient had a history of rheumatoid arthritis (RA), affecting primarily his lower extremities, although at the time of admission there were no related clinical symptoms and the patient did not receive any immunosuppressive therapy.
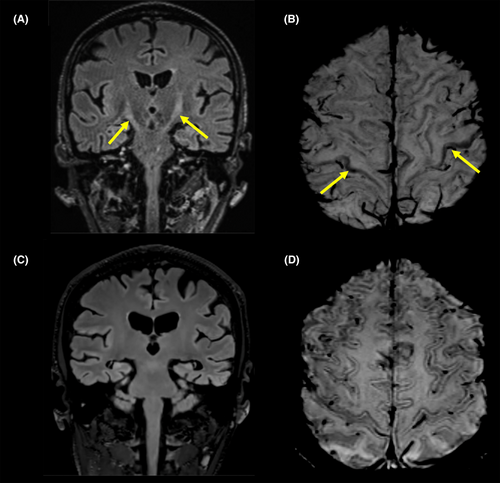
3-Tesla magnetic resonance imaging (MRI) was acquired on a Siemens Vida and showed increased symmetrical T2 signal intensities in the pyramidal tracts (Fig. 1A), band-like cortical/juxtacortical paramagnetic susceptibility artifacts in the precentral gyrus, consistent with a motor band sign in susceptibility weighted images (SWI) (Fig. 1B), and increased symmetric STIR signal intensities of the shoulder girdle musculature, consistent with denervation edema. Electromyography revealed signs of acute denervation in the bulbar, cervical, and lumbar region and showed no evidence of chronic denervation. Cerebrospinal fluid (CSF) and blood biomarker profile are summarized in Table 1. The concentration of total tau (tTau) in the CSF was increased (664 pg/mL, reference value <300 pg/mL), phosphorylated tau181 (pTau181) was normal (30 pg/mL, reference value <61 pg/mL), whereas the level of amyloid-β 42 (Aβ42) was decreased (350 pg/mL, reference value >500 pg/mL). The Aβ42/40 ratio was within the normal range (0.12 pg/mL, “in-house” reference value >0.07). Only a few days after admission, the patient developed aspiration pneumonia and died due to respiratory insufficiency 8 months after symptom onset.
| Index patient | Brother of index patient | Reference value | |
|---|---|---|---|
| CSF | |||
| tTau | 664 pg/mL | 523 pg/mL | <300 pg/mL |
| pTau181 | 30 pg/mL | 104 pg/mL | <61 pg/mL |
| Aβ42 | 350 pg/mL | 1072 pg/mL | >500 pg/mL |
| Aβ42/40 ratio | 0.12 pg/mL | 0.06 pg/mL | >0.07 |
| IATI | 0.3 pg/mL | 1.3 pg/mL | >1 pg/mL |
| 14-3-3 | pos. | pos. | |
| NfH | n.a. | 378 pg/mL | <560 pg/mL |
| Serum | |||
| NfL | n.a. | 39 pg/mL | <45 pg/mL |
- Aβ, amyloid-beta; CSF, cerebrospinal fluid; IATI, Innotest Amyloid Tau Index; n.a., not available; NfH, neurofilament heavy chain; NfL, neurofilament light chain; pTau181, phosphorylated Tau 181; tTau, total Tau.
Brother of index patient
The 44-year-old brother of the index patient started to exhibit progressive behavioral changes and mnestic deficits at the age of 38. The patient gradually isolated himself, lost all forms of social contact and neglected personal care. He was diagnosed with FTD at the age of 43 according to current diagnostic criteria.16 Follow-up examinations showed a progressing decline in memory, executive and language function as well as increasing apathy, loss of empathy and dietary changes, without any clinical evidence of extrapyramidal or motor neuron dysfunction. Neurophysiological examination did not show any abnormalities.
Cranial MRI demonstrated a global gray matter volume loss with regional and global metrics below the age- and sex-corrected tenth percentile (Siemens syngo.via) without any evidence of imaging features associated with ALS (Fig. 1C,D). PTau181 and tTau CSF levels (104 and 523 pg/mL, respectively) were elevated, while the concentration of Aβ42 was normal (Table 1). The Aβ42/40 ratio was marginally decreased (0.06 pg/mL). Fluorodeoxyglucose positron emission tomography (FDG-PET) scan depicted a frontomesial and parietal accentuated hypometabolism, whereas amyloid-PET imaging was negative. At the time of this report, the brother/patient is still alive with the clinical diagnosis of behavioral FTD.
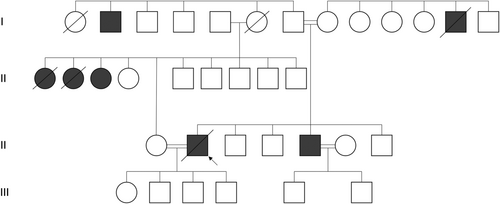
Familial pedigree
The pedigree is visualized in Figure 2. The described family is of Turkish descent, with both parents being first cousins as well as the index patient and the brother of the index patients being involved in a consanguineous marriage to their first cousins. The pedigree included three cousins on the father's side diagnosed with slow-progressing ALS as well as one uncle on the mother's side and one on the father's side who were affected by dementia at later ages.
Genetic analyses
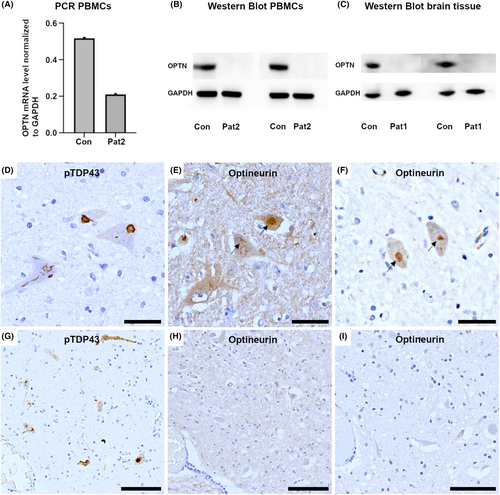
WES revealed an autosomal-recessive pathogenic homozygous frameshift mutation in the OPTN gene (NM_001008212.2: c.1078_1079del; p.Lys360ValfsTer18) in both siblings (PVS1, PM2 and PM3 according to the 2015 ACMG criteria).17 Due to the high coverage of the variant with WES in both subjects (37x and 77x coverage, respectively; Figs. S1–S3), we did not perform additional Sanger sequencing. This variant has previously been listed as a (likely) pathogenic variant in ClinVar and has not been listed in the Genome Aggregation Database (gnomAD) v2.1.1. No other candidate mutations in other FTD- and ALS-related genes were observed. The index patient had an APOE3/4 genotype, while his brother was APOE3 homozygous. Additionally, the TMEM106B rs1990622 A/A genotype was excluded in both patients. Rt-PCR revealed expression of OPTN-mRNA, but we could not detect optineurin in western blot analyses and immunohistochemistry of brain tissue from the index patient and PBMCs from his brother (Fig. 4A–C).
Neuropathological findings (index patient)
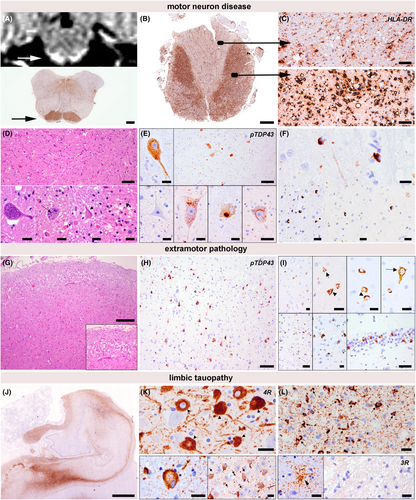
Basic histopathological examination revealed severe neurodegenerative changes. The motor cortex, the anterior horn of the spinal cord, and the motor nuclei in the brainstem showed a marked loss of motor neurons accompanied by a florid degeneration of the corticospinal tract (Fig. 3A–C). Moreover, a pattern of frontotemporal lobar degeneration was observed and characterized by superficial laminar spongiosis in frontotemporal regions and diffuse neuronal loss and gliosis (Fig. 3G). The basal ganglia and the substantia nigra were also affected by neuronal loss, gliosis, and microglial activation. Immunohistochemical examination revealed widespread TDP-43-pathology in motor and extramotor regions involving neurons and glial cells. The neuronal pathology included diffuse “dash-like” cytoplasmatic, filamentous, and compact morphologies, and affected all cortical layers, diffusely the neurons of the basal ganglia and the motor neurons (Fig. 3E,H,I); there were also fine neurites throughout the cortex. The glial pathology was prominent and characterized by a high density of comma-shaped cytoplasmic oligodendroglial inclusions localized in both the gray matter and white matter (Fig. 3F). These changes were consistent with an ALS-FTLD pattern and with Stage 4 of ALS-related TDP-43 pathology according to Brettschneider et al.18 The pattern of TDP-43 pathology in cortical and subcortical areas was consistent with a FTLD type B pattern according to Mackenzie et al.19, 20 No optineurin aggregates were detected in motor neurons containing phospho-TDP43 inclusions (Fig. 4D–I), in contrast to what we observed in a sporadic ALS case used as a positive control. In addition, there was a prominent temporo-medial tauopathy involving the amygdala and the hippocampus. These areas showed a marked neuronal and glial tau pathology with features that were partly reminiscent of argyrophilic grain type pathology including abundant ballooned neurons (Fig. 3K lower left panel, asterisk), pretangles (Fig. 3L upper panel, arrow heads), small tangles, neuropil threads, coiled bodies (Fig. 3L upper panel), single granular fuzzy astrocytes (Fig. 3L lower left panel), and grain-like structures (Fig. 3K lower right panel). These inclusions were mainly immunoreactive for 4-repeat tau. While the pathology was most prominent in mesiotemporal areas (i.e., amygdala and hippocampus), it also extended to the septal region and minimally to the pontine tegmentum and spinal cord, where isolated neuropil threads and pretangles were identified. Overall, the extent of tau pathology exceeded the range expected by the patient's age. P62-positive and TDP-43-negative inclusions in the hippocampus or cerebellum suggestive of C9orf72-repeat expansion could not be observed. (image not shown). Immunohistochemical screening for other pathological protein aggregates showed no evidence of beta-amyloid pathology in frontal, temporal, parietal, or occipital cortical areas, the hippocampus, the basal ganglia, the midbrain or in the cerebellar cortex. There was also no evidence of α-synuclein pathology in the olfactory bulb, the amygdala, or the medulla oblongata (image not shown).
Discussion
In this study, we outline the association of a homozygous frameshift mutation in the OPTN gene with phenotypically different conditions in a consanguineous family. While the index patient was diagnosed with a rapidly progressive ALS-FTD phenotype leading to death 8 months after symptom onset, his brother exhibited a pure FTD phenotype without any clinical or neurophysiological evidence of motor neuron dysfunction and was still alive 6 years after symptom onset. Three cousins of the index patient had developed pure ALS associated with an exceptionally slow progression and survival times ranging between 10 and 14 years, and two uncles suffered from dementia without any evidence of deficits suggestive of motor neuron disease. Mutations in the OPTN gene are rare and have been primarily described in patients with familial and sporadic ALS,1, 21-24 with only a few reports linking OPTN mutations to an ALS-FTD phenotype25, 26 or pure/predominant FTD-related phenotypes.2, 3, 27 Homozygous variants in the OPTN gene were already described in Turkish familial ALS patients, including the variant outlined in the current study. Similar to our index patients, the reported cases exhibited an early age of onset but were solely associated with a typical ALS phenotype only, without FTD.24 Another recent study reported on a case with parkinsonism and corticobasal syndrome (CBS) in association with a heterozygous OPTN frameshift insertion,3 and yet another study of familial ALS revealed two Tunisian siblings with a clinical phenotype of ALS-CBS and a homozygous OPTN mutation,28 further underlining the heterogeneous phenotype–genotype correlation. It could be deduced that OPTN mutations may result in different neurodegenerative conditions within the ALS-FTD-parkinsonism phenotypic spectrum, with significant variation in disease progression between affected individuals, even within the same consanguineous family. It has been hypothesized that differences in disease duration could lead to the phenotypic heterogeneity as some patients may die before developing the full disease spectrum with ALS and dementia,7 although this explanation appears unlikely to be the case in our study, considering the long survival time of the index patient's cousins with pure ALS. Alternatively, OPTN mutations could result in different levels of penetrance to various diseases7 as has been also reported for mutations in valosin-containing protein (VCP) that can lead to different phenotypes including myopathy, Paget disease, FTD, and ALS.29, 30 Moreover, mutations in the microtubule-associated protein tau (MAPT) are also associated with different clinical phenotypes, ranging from FTD to atypical parkinsonian syndrome and primary progressive aphasia. The heterogeneity is partly explained by the specific mutation carried, causing variability in penetrance, phenotype, age of onset, and disease duration.31
In TDP-43 proteinopathies, particularly the TDP-43 pathology in FTLD frequently involves the basal ganglia and substantia nigra; therefore, extrapyramidal symptoms can be also expected to be part of the clinical manifestations and mimic an atypical parkinsonian syndrome like CBS. The exact underlying molecular mechanisms linking one mutation with different phenotypes, however, are yet to be unraveled. Noteworthy, the co-existence of OPTN mutations with mutations in other disease relevant genes, as reported for ALS cases with aggressive progression and short survival,32-34 was excluded in our index patient through WES.
At the present time, there are only few neuropathological reports on OPTN mutation carriers. These have shown a predominant TDP-43 proteinopathy within the ALS-FTLD spectrum and were in some cases associated with variable amounts of co-existing tau and alpha-synuclein pathology in restricted brain areas.1, 7-9 Our neuropathological findings in the index patient are in line with these findings and included characteristic features of ALS-FTLD associated with TDP-43 proteinopathy. Similar to the case described by Nolan et al.,23 oligodendroglial TDP-43 pathology was very prominent. Inclusions were, however, negative for optineurin. In addition, we observed a prominent co-existing limbic-predominant 4R-tauopathy, reminiscent of argyrophilic grain disease (AGD).35
As the amount of 4R-tauopathy was atypical for the patient's age, we further excluded a TMEM106B polymorphism as a potential cause, as the TMEM106B rs1990622A/A genotype has been described to be associated with an atypical neuronal and glial tauopathy that is temporally predominant.36 The described cases were also similar to AGD, albeit demonstrating a different distribution with the highest burden in the inferior temporal gyrus and less affection of the hippocampus.36 Some previous studies have also reported a higher incidence of temporo-medial tauopathies, including AGD type pathology in young patients with ALS.37 The co-occurrence of tau and TDP-43 is increasingly reported in different conditions,38-41 and the potential interaction of both proteins is current topic of research. It could well be that the dysfunction of OPTN mediated cellular processes also facilitates an abnormal aggregation of various other proteins.7
Recently, OPTN has also attracted attention for its role as a modulator in autoimmune disorders.42-44 In RA, OPTN was shown to play a protective role against joint destruction by downregulation of receptor activator of nuclear factor-κB ligand (RANKL). It was hypothesized that the absence of OPTN might worsen RA by the facilitation of a joint-destructive condition.43 The absence of OPTN could thus also have contributed to the development of RA in the index patient, although this potential relationship could not be examined further in our study and therefore remains elusive. More reports on patients with OPTN mutations are required to elucidate whether autoimmune disorders are part of the OPTN-associated disease spectrum.
In conclusion, our data confirm the association between OPTN mutations and the development of a neurodegenerative pathology within the ALS-FTD spectrum, and highlight the variability of clinical phenotype with intrafamilial heterogeneity regarding disease duration and clinical symptoms. Considering the accumulation of various proteins, in our case TDP-43 and limbic neuro-glial tauopathy, further studies are required to reveal more insight into the exact role of OPTN in neurodegenerative disorders.
Acknowledgements
We would like to thank the patients and their families whose help and participation made this work possible.
Author Contributions
TP and HC collected and managed the data. SK and EG performed the neuropathological examination. OK and SI performed rt-PCR and western blot analyses. TP and SK interpreted the data and drafted the manuscript. EG and HC reviewed and edited the manuscript. TK, HC, LH, TB, MW, TTW, MB, AZ, ES, and JR provided feedback and major contribution to the manuscript. All authors read and approved the final manuscript.
Funding Information
Not applicable.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Consent for Publication
Written informed consent was obtained from the brother of the index patient to perform clinical and laboratory investigations and to publish the results.
Open Research
Data Availability Statement
All data generated or analyzed during this study are included in this published article.




