Alzheimer's Disease and Small Vessel Disease Differentially Affect White Matter Microstructure
Abstract
Objective
Alzheimer's disease (AD) and cerebral small vessel disease (cSVD), the two most common causes of dementia, are characterized by white matter (WM) alterations diverging from the physiological changes occurring in healthy aging. Diffusion tensor imaging (DTI) is a valuable tool to quantify WM integrity non-invasively and identify the determinants of such alterations. Here, we investigated main effects and interactions of AD pathology, APOE-ε4, cSVD, and cardiovascular risk on spatial patterns of WM alterations in non-demented older adults.
Methods
Within the prospective European Prevention of Alzheimer's Dementia study, we selected 606 participants (64.9 ± 7.2 years, 376 females) with baseline cerebrospinal fluid samples of amyloid β1-42 and p-Tau181 and MRI scans, including DTI scans. Longitudinal scans (mean follow-up time = 1.3 ± 0.5 years) were obtained in a subset (n = 223). WM integrity was assessed by extracting fractional anisotropy and mean diffusivity in relevant tracts. To identify the determinants of WM disruption, we performed a multimodel inference to identify the best linear mixed-effects model for each tract.
Results
AD pathology, APOE-ε4, cSVD burden, and cardiovascular risk were all associated with WM integrity within several tracts. While limbic tracts were mainly impacted by AD pathology and APOE-ε4, commissural, associative, and projection tract integrity was more related to cSVD burden and cardiovascular risk. AD pathology and cSVD did not show any significant interaction effect.
Interpretation
Our results suggest that AD pathology and cSVD exert independent and spatially different effects on WM microstructure, supporting the role of DTI in disease monitoring and suggesting independent targets for preventive medicine approaches.
Introduction
While physiological changes in white matter (WM) microstructural integrity can be observed in healthy aging, neurodegeneration due to Alzheimer's disease (AD)1 and cerebral small vessel disease (cSVD)2 are characterized by accelerated myelin disruption and axonal damage and loss. Both AD and cSVD affect oligodendrocyte function, acting through iron overload, oxidative stress, and endothelial dysfunction pathways, ultimately resulting in demyelination and axonal loss.3, 4 Previous studies have investigated the interplay between AD and cSVD on white matter microstructural integrity, observing variable degrees of association when looking at different imaging biomarkers of cSVD, such as white matter hyperintensities (WMHs),5 and cerebral microbleeds (CMBs).6 Conflicting evidence has emerged regarding their possible interactive effects on neurodegeneration and white matter disruption, with studies supporting both a synergistic effect7 and the independence8 of AD from cSVD and cardiovascular risk factors. Therefore, it is still unclear whether these associations are related to shared risk factors or to the mechanistic interplay between the underlying pathological alterations.
Diffusion tensor imaging (DTI) is an MRI technique sensitive to white matter (WM) microstructural changes.9 By measuring the diffusion properties of water molecules, it is possible to assess metrics of WM integrity such as fractional anisotropy (FA – the fraction of diffusion along one preferential direction) and mean diffusivity (MD – the average of diffusion along three main axes).10 Decrease in FA and increase in MD have been observed from the early stages of neurodegeneration due to both AD1 and cSVD,2 highlighting the potential of DTI metrics as early and non-invasive imaging biomarkers. Previous studies suggest that the susceptibility to WM alterations could present disease-specific spatial patterns. In particular, while the amyloid-β (Aβ) pathological cascade of events has mostly been associated with reduction of temporal and parietal WM integrity,11 cSVD typically relates to alterations at the level of the genu of corpus callosum (CC),12 suggesting that these diseases could exert differential regional effects. However, although it has been shown that these pathologies often coexist,3 whether and to what extent they interact with each other in determining WM microstructural disruption remains unclear.
The development of new targeted therapies for AD and cSVD13, 14 highlights the need for identifying the determinants of WM alterations and disentangling their individual contributions.15 In recent years, intervention studies have been progressively focusing on the preclinical and prodromal stages of dementia, using mixed strategies to target the risk factors associated with AD13 and cSVD.16 As new primary and secondary prevention trials are starting, we need robust and possibly noninvasive biomarkers to properly define the enrollment criteria and serve as outcome measures of treatment efficacy in absence of cognitive decline.13
This study aims to explore the differential impact and possible interactions of AD pathology and cSVD, along with their associated genetic (APOE-ε4) and acquired (cardiovascular) risk factors, on WM microstructural alterations as assessed by DTI in a large sample of non-demented older adults from the European Prevention of Alzheimer Dementia (EPAD) cohort.
Methods
Study participants
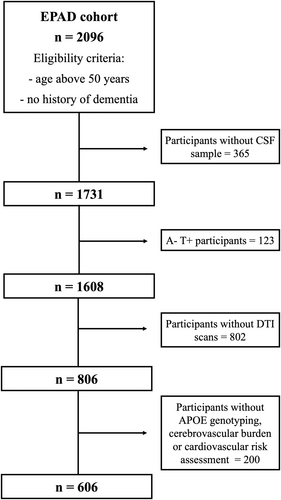
Data were drawn from the vIMI release of the EPAD cohort (www.ep-ad.org). EPAD eligibility criteria included age above 50 years and no diagnosis of dementia (Clinical Dementia Rating [CDR] scale ≤ 0.5).17 This prospective study included 606 individuals with available cerebrospinal fluid (CSF), APOE, cerebrovascular and cardiovascular data, and MRI scans including at least T1-weighted, T2*-weighted, T2-FLAIR, and DTI sequences (voxel size = 2.0 × 2.0 × 2.0 mm3, diffusion-encoding directions = 30) (Fig. 1). Longitudinal DTI data were available for a subset of individuals (n = 223).
Standard protocol approvals, registrations, and patient consents
The study was approved by the ethical committees of all participating centers. All study participants provided written informed consent according to the Declaration of Helsinki.
CSF analysis and AT classification
CSF biomarkers were quantified using a harmonized pre-analytical protocol.18 Analyses were performed with the fully automated Roche Elecsys System at the Clinical Neurochemistry Laboratory, Mölndal, Sweden.17 Concentrations of amyloid-beta (Aβ1-42) and phosphorylated-tau (p-Tau181) were determined using the manufacturer's guidelines. Following previous work on the same cohort,18 CSF Aβ1-42 levels < 1000 pg/mL and CSF p-Tau181 levels > 27 pg/mL were used to define amyloid positivity (A+) and tau positivity (T+), respectively. Four AT groups were derived (i.e., A-T-, A+T-, A+T+ and A-T+). A-T+ participants were excluded as they belong to the suspected non-AD pathology group.
APOE genotype
APOE genotype was determined from Taqman Genotyping of blood samples and analyzed in a single laboratory at the University of Edinburgh using QuantStudio 12KL Flex. APOE-ε4 carriers were defined as having at least one ε4 allele. Total DNA was obtained through proteinase-K digestion and alcohol precipitation of the blood cellular fraction. Samples were genotyped, using the APOE-F 5′-TTGAAGGCCTA CAAATCGGAACTG-3′ and APOE-R 5′-CCGGCTGCCCAT CTCCTCCATCCG-3′ primers, for two single nucleotide polymorphisms, rs429358 and rs7412. Thus, the possible APOE alleles were determined as follows: ε1, rs429358 (C) + rs7412 (T); ε2, rs429358 (T) + rs7412 (T); ε3, rs429358 (T) + rs7412 (C); and ε4, rs429358 (C) + rs7412 (C).
Cerebrovascular factors
Visual MRI reads were centrally performed by three observers (two neuroradiologists with more than 15 years of experience; one in training) according to the STRIVE (Standards for Reporting Vascular Changes on Neuroimaging) criteria.19 Visual scores included a 0–4 scale for enlarged perivascular spaces (ePVS) in basal ganglia and centrum semiovale; Fazekas scale (0–3) for periventricular and deep WMHs; presence/absence of lobar CMBs, presence of more than 2 deep CMBs; and lacunes (0, 1, 2, >2). We used confirmatory factor analysis to build a cSVD-burden latent factor from the radiological indices. Subsequently, gaussian mixture modeling was applied to identify a cutoff to dichotomize the cohort in cSVD groups as cSVD+ and cSVD- (Fig. S2). More details are provided in supplementary material.
Cardiovascular risk factors (Framingham)
Individual vascular risk was computed using the Framingham Risk Score (FRS), encompassing information on age, sex, systolic blood pressure, antihypertensive medication, diabetes, smoking, total and HDL cholesterol,20 as previously described.21 FRS was computed with and without age correction, and uncorrected scores were used in later analysis.
MRI acquisition and processing
MRI acquisition and preprocessing details have been detailed elsewhere.22 Briefly, diffusion-weighted images underwent geometric distortion correction using the opposed phase-encoding polarities, head motion and eddy-current correction, brain extraction, and tensor-fitting to produce FA and MD maps.
Tract-based spatial statistics
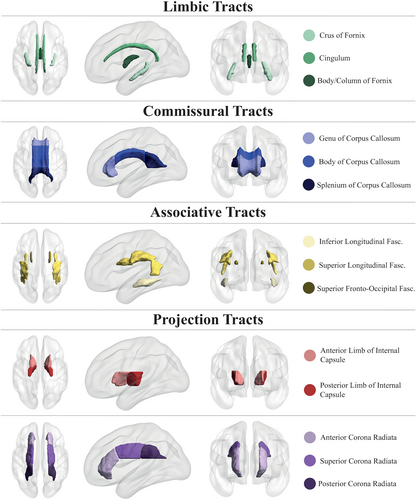
DTI maps were processed with the tract-based spatial statistics approach.23 Brain extracted FA maps were aligned into a common space with nonlinear registration, obviating the need for an inter-session subject-specific template, as longitudinal data were not available for all participants and therefore we did not want to introduce a bias by performing an additional registration step in a subset. We created a FA skeleton mask that comprised the WM regions common to the group. Using the JHU ICBM-DTI-81 atlas,24 FA and MD were extracted from 14 selected skeletonized tracts (Fig. 2), subdivided in commissural, associative, limbic (to distinguish them from other associative and commissural tracts, based on their involvement in AD pathophysiology)25 and projections tracts. DTI scalars were site-harmonized using the ComBat Toolbox26 and standardized. More details are given in supplementary material. We decided to not mask out WMHs from skeletonized tracts because we were interested in the effect of our putative determinants on the total WM, though their impact was negligible as the percentage of WMH voxels included in the skeletonized tracts was very low on average (1.5 ± 2.9%).
Statistical analysis
Demographics were compared using chi-square and t-tests, as appropriate.
Variables were tested for collinearity using the performance R package (v. 0.10.4).
For each tract and for both scalars, we performed a multimodel inference through MuMin R package (v. 1.47.5) to identify the best linear mixed-effects models. Briefly, starting from a reference model containing all variables of interest, we computed all possible models based on every combination of our predictors and selected the ones having the highest second-order Akaike information criterion scores.
The reference linear mixed-effects model (computed using the lme4 R package, v. 1.1–33) entered in the multimodel inference included a random intercept for the participant, and age and sex as covariates. The predictors included in the reference linear mixed-effects model were APOE-ε4, AT status, cSVD groups, and FRS (main effect of determinants); the time interval between MRI acquisitions (main effect of time); the pairwise interactions between APOE-ε4, AT status, cSVD groups, and FRS (interaction effect of determinants); the pairwise interactions between the time interval and APOE-ε4, AT status, cSVD groups, and FRS (main effect of determinants on longitudinal changes); the three-way interactions between the time interval and all possible pairs among APOE-ε4, AT status, cSVD groups, and FRS (interaction effect of determinants on longitudinal changes). Full formulas are given in supplementary material.
Age and sex were always included in the final models. p-values were adjusted for multiple testing using false discovery rate control (the Benjamini–Hochberg procedure) across the selected models and considered significant when pFDR < 0.05.
All statistical analyses were performed using R (v. 4.2.1).
Results
Demographic and clinical data of the study participants (n = 606) are shown in Table 1. Mean baseline age was 64.9 ± 7.2 years, 376 participants (62.0%) were female, and 142 (23.4%) had CDR = 0.5. Mean follow-up time was 1.3 ± 0.5 years.
| Overall | A-T- | A+T- | A+T+ | p-value | |
|---|---|---|---|---|---|
| N [baseline (longitudinal)] | 606 (223) | 410 (143) | 137 (49) | 59 (31) | n.a. |
| Age [mean (SD)] | 64.93 (7.16) | 63.81 (6.99) | 65.69 (6.69) | 70.97 (6.12) | <0.001 |
| Sex [m (%)] | 230 (38.0) | 146 (35.6) | 55 (40.1) | 29 (49.2) | 0.11 |
| Total follow-up time, years [mean (SD)] | 1.33 (0.48) | 1.36 (0.49) | 1.28 (0.46) | 1.30 (0.48) | 0.61 |
| CDR global score = 0.5 [n (%)] | 142 (23.4) | 71 (17.3) | 36 (26.3) | 35 (59.3) | <0.001 |
| MMSE score [mean (SD)] | 28.61 (1.72) | 28.85 (1.46) | 28.42 (2.01) | 27.44 (2.10) | <0.001 |
| APOE-ε4 carriers [n (%)] | 221 (36.5) | 110 (26.8) | 69 (50.4) | 42 (71.2) | <0.001 |
| Framingham risk score [mean (SD)] | 14.35 (4.19) | 13.84 (4.19) | 14.73 (3.73) | 17.02 (4.14) | <0.001 |
| cSVD+ [n (%)] | 258 (42.6) | 156 (38.0) | 61 (44.5) | 41 (69.5) | <0.001 |
| Number of visits | 0.07 | ||||
| 1 visit [n (%)] | 383 (63.2) | 267 (65.1) | 88 (64.2) | 28 (47.4) | |
| 2 visits [n (%)] | 170 (28.1) | 105 (25.6) | 40 (29.2) | 25 (42.4) | |
| 3 visits [n (%)] | 53 (8.7) | 38 (9.3) | 9 (6.6) | 6 (10.2) | |
- CDR, clinical dementia rating; cSVD, cerebral small vessel disease; MMSE, mini mental-state examination; n.a., not applicable; SD, standard deviation.
Participants had moderate cardiovascular risk according to FRS (mean FRS = 14.4 ± 2.3). The cSVD severity was generally low (median total Fazekas-score = 1, interquartile range [IQR] = 1.8). In total, 19 (3.1%) and 75 (12.4%) participants had at least two deep or one lobar CMBs, respectively. Thirty-four (5.6%) participants had at least one lacune. Median of basal ganglia and centrum semiovale ePVS was 1 for both (IQR = 0 and 1, respectively).
Effect of age and sex on WM integrity
Older age was associated with lower FA (β ranging from −0.048 to −0.015, all p ≤ 0.02) and higher MD values (β ranging from 0.020 to 0.055, all p ≤ 0.001) in most of the analyzed tracts. Men showed higher MD (β ranging from 0.166 to 0.367, all p ≤ 0.03), coupled with lower FA in the column body of the fornix (β = −0.343, p < 0.001) and higher FA in the cingulum (β = 0.266, p = 0.01).
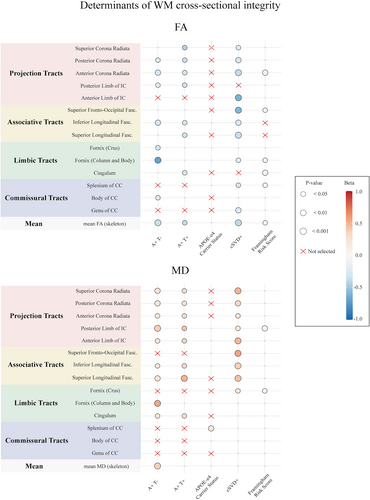
Determinants of WM integrity
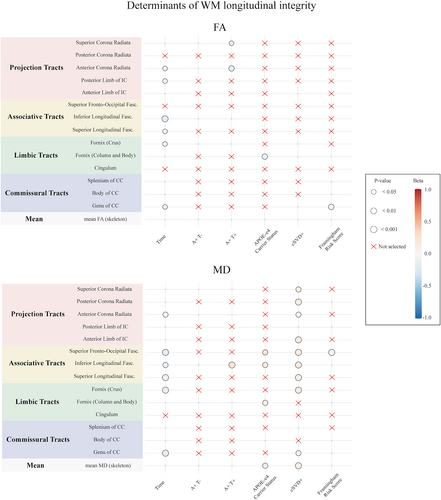
Multimodel inference and linear mixed-effects models full results are shown in Tables 2, 3 and Figs. 3, 4. Predictors selected by the multimodel inference for each linear mixed-effects model are listed in Tables S1 and S2. Plots exemplifying significant longitudinal effects are reported in Fig. S2. The analysis was repeated after excluding the participants having a CDR = 0.5, and the main results were unchanged (data not shown). We decided to include those subjects in the main analysis for two main reasons. Firstly, as clinical trials are starting to move toward the preclinical stages, we wanted to simulate a real-life scenario by including all those subjects that are still non-demented and that can benefit the most from early intervention therapies. Secondly, even if by excluding those subjects we were able to confirm our main results, this comes at the cost of losing statistical power to detect meaningful biological effects, as most of the A+T+ participants belong to the CDR = 0.5 group (as shown in Table 1).
| Genu of CC | Body of CC | Splenium of CC | Cingulum | Column and body of the fornix | Crus of the fornix | Superior long. fasc. | Inferior long. fasc. | Superior fronto-occipital fasc. | Anterior limb of internal capsule | Posterior limb of internal capsule | Anterior corona radiata | Posterior corona radiata | Superior corona radiata | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | −0.015; 0.02 | −0.022; 0.002 | −0.011; 0.14 | −0.029; <0.001 | −0.047; <0.001 | −0.048; <0.001 | −0.003; 0.64 | −0.017; 0.02 | −0.008; 0.31 | −0.005; 0.51 | 0.005; 0.46 | −0.029; <0.001 | 0.003; 0.65 | −0.006; 0.42 |
| Sex (m) | 0.085; 0.38 | −0.084; 0.38 | −0.053; 0.52 | 0.266; 0.01 | −0.343; <0.001 | −0.059; 0.47 | −0.102; 0.38 | −0.102; 0.38 | 0.089; 0.38 | 0.161; 0.22 | −0.100; 0.38 | −0.060; 0.47 | −0.096; 0.38 | −0.107; 0.38 |
| A+T- | / | −0.221; 0.03 | / | −0.165; 0.09 | −0.701; <0.001 | −0.290; 0.03 | −0.106; 0.27 | −0.292; 0.007 | −0.167; 0.08 | / | −0.225; 0.03 | −0.284; 0.005 | −0.240; 0.03 | −0.192; 0.06 |
| A+T+ | / | −0.117; 0.42 | / | −0.300; 0.047 | 0.068; 0.75 | 0.210; 0.40 | −0.332; 0.04 | −0.309; 0.047 | −0.242; 0.10 | / | −0.344; 0.04 | −0.302; 0.04 | −0.364; 0.03 | −0.434; 0.02 |
| APOE-ε4 | / | / | −0.214; 0.21 | / | −0.024; 0.86 | −0.027; 0.86 | / | 0.143; 0.21 | / | / | / | / | / | / |
| cSVD | −0.233; 0.009 | −0.132; 0.36 | −0.267; 0.02 | / | −0.162; 0.046 | 0.107; 0.40 | −0.365; <0.001 | −0.262; 0.004 | −0.596; <0.0001 | −0.607; <0.001 | / | −0.381; <0.0001 | −0.304; 0.001 | −0.214; 0.02 |
| FRS | −0.007; 0.70 | −0.006; 0.76 | 0.034; 0.045 | −0.049; 0.005 | −0.048; 0.01 | −0.012; 0.60 | / | / | −0.048; 0.03 | −0.031; 0.05 | −0.029; 0.07 | −0.054; 0.001 | / | −0.026; 0.10 |
| APOE-ε*A+T- | / | / | / | / | 0.271; 0.12 | 0.387; 0.06 | / | / | / | / | / | / | / | / |
| APOE-ε4*A+T+ | / | / | / | / | −0.352; 0.18 | −0.451; 0.18 | / | / | / | / | / | / | / | / |
| APOE-ε4*FRS | / | / | / | / | 0.037; 0.15 | / | / | / | / | / | / | / | / | / |
| APOE-ε4*cSVD | / | / | 0.254; 0.11 | / | / | / | / | / | / | / | / | / | / | / |
| FRS*cSVD | / | −0.04; 0.14 | / | / | / | −0.048; 0.14 | / | / | 0.044; 0.14 | / | / | / | / | / |
| Time | −0.118; 0.02 | −0.032; 0.42 | −0.024; 0.79 | / | −0.029; 0.49 | −0.096; 0.04 | −0.053; 0.01 | −0.193; <0.001 | / | 0.011; 0.80 | 0.066; 0.03 | 0.071; 0.01 | / | −0.001; 0.96 |
| A+T-*time | / | / | / | / | / | 0.128; 0.15 | / | 0.025; 0.75 | / | / | / | −0.086; 0.19 | / | −0.053; 0.29 |
| A+T+*time | / | / | / | / | / | −0.104; 0.22 | / | −0.181; 0.07 | / | / | / | −0.175; 0.009 | / | −0.138; 0.01 |
| APOE-ε4*time | / | / | / | / | −0.095; 0.006 | / | / | / | / | / | / | / | / | / |
| FRS*time | −0.026; 0.007 | −0.01; 0.08 | −0.023; 0.07 | / | 0.01; 0.07 | / | / | / | / | / | / | / | / | / |
| cSVD*time | −0.081; 0.11 | / | / | / | −0.047; 0.11 | −0.096; 0.11 | / | / | / | −0.090; 0.11 | / | / | / | / |
- Table showing coefficients and pFDR-values of the LMEs selected by the multimodal inference to test the effect of individual factors on FA values. Predictors that were not included in at least one LME are not reported. Results are expressed as “β coefficient; pFDR-value”. Significant results are highlighted in bold.
- cSVD, cerebral small vessel disease; fasc., fasciculus; FDR, false discovery rate; FRS, Framingham risk score; LMEs, linear mixed effect models; long., longitudinal; WM, white matter; “/”, predictor not included in the selected model.
| Genu of corpus callosum | Body of corpus callosum | Splenium of corpus callosum | Cingulum | Column and body of the fornix | Crus of the fornix | Superior long. fasc. | Inferior long. fasc. | Superior fronto-occipital fasc. | Anterior limb of internal capsule | Posterior limb of internal capsule | Anterior corona radiata | Posterior corona radiata | Superior corona radiata | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 0.045; <0.001 | 0.051; <0.001 | 0.037; <0.001 | 0.034; <0.001 | 0.055; <0.001 | 0.027; <0.001 | 0.029; <0.001 | 0.022; <0.001 | 0.039; <0.001 | 0.042; <0.0001 | 0.020; 0.001 | 0.043; <0.001 | 0.038; <0.001 | 0.036; <0.001 |
| Sex (m) | 0.034; 0.69 | 0.226; 0.004 | 0.174; 0.03 | −0.068; 0.46 | 0.226; 0.004 | 0.131; 0.14 | 0.222; 0.005 | 0.367; <0.001 | 0.166; 0.03 | 0.053; 0.53 | 0.164; 0.06 | 0.025; 0.72 | 0.291; <0.001 | 0.276; <0.001 |
| A+T- | / | / | / | 0.230; 0.01 | 0.546; <0.001 | / | 0.253; 0.004 | 0.208; 0.02 | / | 0.205; 0.02 | 0.391; <0.001 | 0.190; 0.02 | 0.247; 0.004 | 0.279; 0.001 |
| A+T+ | / | / | / | 0.314; 0.02 | −0.214; 0.34 | / | 0.510; <0.001 | 0.339; 0.02 | / | 0.353; 0.02 | 0.427; 0.01 | 0.302; 0.02 | 0.255; 0.04 | 0.307; 0.02 |
| APOE-ε4 | / | 0.083; 0.54 | 0.238; 0.008 | / | −0.032; 0.96 | / | / | 0.067; 0.96 | 0.125; 0.54 | 0.024; 0.96 | −0.144; 0.32 | / | / | / |
| cSVD | −0.045; 0.77 | 0.005; 0.97 | −0.054; 0.77 | 0.117; 0.44 | 0.160; 0.05 | 0.210; 0.03 | 0.443; <0.001 | 0.380; <0.001 | 0.562; <0.001 | 0.480; <0.001 | 0.191; 0.046 | 0.254; 0.046 | 0.357; 0.006 | 0.496; <0.001 |
| FRS | 0.001; 0.95 | 0.005; 0.83 | −0.029; 0.17 | 0.009; 0.76 | 0.036; 0.07 | 0.045; 0.01 | 0.031; 0.07 | 0.034; 0.11 | 0.026; 0.17 | 0.020; 0.40 | 0.050; 0.008 | 0.019; 0.34 | 0.005; 0.83 | 0.028; 0.07 |
| APOE-ε4*A+T- | / | / | / | / | −0.212; 0.42 | / | / | / | / | / | / | / | / | / |
| APOE-ε4*A+T+ | / | / | / | / | 0.441; 0.08 | / | / | / | / | / | / | / | / | / |
| APOE-ε4*FRS | / | / | / | / | −0.027; 0.44 | / | / | −0.025; 0.44 | −0.013; 0.59 | −0.037; 0.37 | / | / | / | / |
| APOE-ε4*cSVD | / | / | / | / | / | / | / | −0.106; 0.49 | / | / | / | / | / | / |
| FRS*cSVD | 0.058; 0.04 | 0.067; 0.03 | 0.053; 0.06 | 0.071; 0.03 | / | / | / | / | / | 0.037; 0.13 | / | 0.042; 0.10 | 0.039; 0.12 | / |
| Time | −0.148; 0.001 | 0.045; 0.27 | 0.081; 0.13 | / | 0.024; 0.32 | −0.135; 0.004 | −0.088; 0.001 | −0.120; 0.02 | −0.167; 0.002 | −0.021; 0.50 | −0.069; 0.27 | −0.076; 0.01 | −0.034; 0.32 | −0.037; 0.24 |
| A+T-*time | / | / | / | / | −0.050; 0.11 | / | / | 0.127; 0.06 | / | / | / | 0.082; 0.10 | / | 0.097; 0.06 |
| A+T+*time | / | / | / | / | 0.068; 0.15 | / | / | 0.248; 0.002 | / | / | / | 0.075; 0.18 | / | 0.078; 0.18 |
| APOE-ε4*time | / | 0.066; 0.14 | / | / | 0.107; 0.02 | / | / | 0.176; 0.03 | 0.239; 0.01 | / | / | / | / | / |
| FRS*time | 0.014; 0.07 | 0.016; 0.07 | / | / | 0.001; 0.83 | / | / | 0.018; 0.07 | 0.029; 0.007 | / | −0.018; 0.11 | / | 0.015; 0.07 | / |
| cSVD*time | 0.098; 0.03 | / | 0.098; 0.13 | / | / | 0.178; 0.005 | 0.098; 0.004 | 0.193; 0.001 | 0.168; 0.001 | 0.152; 0.002 | 0.095; 0.13 | 0.081; 0.03 | 0.157; 0.003 | 0.096; 0.01 |
| APOE-ε4*FRS*time | / | / | / | / | −0.021; 0.03 | / | / | −0.040; 0.03 | −0.042; 0.03 | / | / | / | / | / |
| APOE-ε4*cSVD*time | / | / | / | / | / | / | / | −0.218; 0.02 | / | / | / | / | / | / |
| FRS*cSVD*time | / | / | / | / | / | / | / | / | / | / | / | / | −0.016; 0.13 | / |
- Table showing coefficients and pFDR-values of the LMEs selected by the multimodal inference to test the effect of individual factors on MD values. Predictors that were not included in at least one LME are not reported. Results are expressed as “β coefficient; pFDR-value”. Significant results are highlighted in bold.
- cSVD, cerebral small vessel disease; fasc., fasciculus; FDR, false discovery rate; FRS, Framingham risk score; LMEs, linear mixed effect models; long., longitudinal; WM, white matter; “/”, predictor not included in the selected model.
Limbic tracts
White matter integrity in limbic tracts, including cingulum, column body, and crus of the fornix, was generally associated with both AD and vascular related factors, with stronger effects dependent on the AT status.
Compared to A-T-, participants in the A+T- group had lower FA in the column body (β = −0.701, p < 0.001) and crus of the fornix (β = −0.290, p = 0.03), and higher MD in the cingulum (β = 0.230, p = 0.01) and column body of the fornix (β = 0.546, p < 0.001), while A+T+ had higher FA (β = −0.300, p = 0.047) and lower MD (β = 0.314, p = 0.02) in the cingulum. APOE-ε4 was associated with longitudinal decreases of FA (β = −0.095, p = 0.006) and increases of MD in the (β = 0.107, p = 0.02) in the column body of the fornix.
Moreover, cSVD+ was associated with lower baseline FA in the column body (β = −0.162, p = 0.046) and higher baseline (β = 0.210, p = 0.03) and longitudinal (β = 0.178, p = 0.005) MD in the crus of the fornix. FRS showed a cross-sectional effect on FA in the cingulum (β = −0.049, p = 0.005) in the column body of the fornix (β = −0.048, p = 0.01) and on MD in the crus of fornix (β = 0.045, p = 0.01). APOE-ε4 and FRS showed a significant three-way interaction with time on MD changes in the column body of the fornix (β = −0.021, p = 0.03).
Commissural tracts
White matter integrity in commissural tracts, including genu, body, and splenium of the CC, was mostly impacted by cerebrovascular and cardiovascular factors, most prominently in the genu of CC.
In particular, cSVD+ participants had lower baseline FA in the regions of the genu (β = −0.233, p = 0.009) and the splenium (β = −0.267, p = 0.02), and greater increases of MD over time in the genu (β = 0.098, p = 0.03). Higher FRS was associated with lower baseline FA in the splenium (β = 0.034, p = 0.045), and greater declines of FA over time in the genu of CC (β = −0.026, p = 0.007). The effects of AD-related factors were circumscribed to body and splenium of CC, with A+T- participants showing lower body FA (β = −0.221, p = 0.03) and APOE-ε4 being associated with higher splenium MD (β = 0.238, p = 0.008).
Associative tracts
White matter integrity in associative tracts, including superior longitudinal, inferior longitudinal, and superior fronto-occipital fasciculi, was similarly and independently impacted by both vascular and AD-related factors. However, the integrity of the superior fronto-occipital fasciculus was independent of AD pathology.
Compared to A-T-, A+T- participants at baseline had lower FA in the inferior longitudinal (β = −0.292, p = 0.007), and higher MD in the inferior (β = 0.208, p = 0.02) and superior (β = −0.042, p = 0.03) longitudinal fasciculi, while A+T+ participants had lower FA and higher MD in the superior (FA: β = −0.332, p = 0.04; MD: β = 0.510, p < 0.001) and inferior (FA: β = −0.309, p = 0.047; MD: β = 0.339, p = 0.02) longitudinal fasciculi, and greater increases of MD over time in the inferior longitudinal fasciculus (β = 0.248, p = 0.002). APOE-ε4 was only related to greater increases of MD over time in inferior longitudinal (β = 0.176, p = 0.03) and superior fronto-occipital fasciculi (β = 0.239, p = 0.01).
CSVD+ participants had lower FA, higher MD and greater longitudinal increases of MD in all investigated associative tracts (all coefficients in Tables 2 and 3). Higher FRS scores were distinctively related to lower baseline FA (β = −0.048, p = 0.03) and greater decreases of MD over time in the superior fronto-occipital fasciculus (β = 0.029, p = 0.007).
Even if some significant three-way effects on MD changes were retained for inferior longitudinal (APOE-ε4*FRS*time with β = −0.040 and p = 0.03, and APOE-ε4*cSVD+*time with β = −0.218 and p = 0.02) and superior fronto-occipital fasciculi (FRS*APOE-ε4*time with β = −0.042 and p = 0.03), none of them included interactions between AD pathology and cerebrovascular factors.
Projection tracts
Projection tracts included anterior and posterior limbs of internal capsule, and anterior, posterior, and superior corona radiata. While the corona radiata was comparably impacted by AD and cSVD, integrity of the anterior and posterior limbs of the internal capsule was mostly linked to cSVD and AD-related factors, respectively.
At baseline, both A+T- and A+T+ participants showed significant lower FA and higher MD in most investigated projection tracts, except for the FA in the anterior limb of the internal capsule (all coefficients in Tables 2 and 3). A+T+ participants also had greater FA decline over time in the anterior (β = −0.175, p = 0.01) and superior (β = −0.138, p = 0.01) corona radiata. APOE-ε4 was not related to either baseline or longitudinal WM integrity in projection tracts.
At baseline, CSVD+ participants showed lower FA and higher MD in all investigated projections tract, except for FA in the posterior limb of the internal capsule (all coefficients in Tables 2 and 3). Similarly, greater increases of MD over time were observed in cSVD+ participants in all projection tracts but the posterior limb of the internal capsule (all coefficients in Table 3). Higher FRS scores were associated with lower FA in the anterior corona radiata (β = −0.054, p = 0.001) and higher MD in the posterior limb of the internal capsule (β = 0.050, p = 0.008).
Discussion
We investigated the effect of AD biomarkers, cSVD burden, and cardiovascular risk factors on WM microstructural integrity in non-demented older adults in the multi-center EPAD cohort. AD pathology (Aβ1-42 and p-Tau181) and APOE-ε4 had prominent effects on limbic tracts, while cSVD and FRS mostly impacted commissural, associative, and projection tracts. We showed that AD biomarkers and cSVD are independently associated with WM disruption in the explored tracts.
WM microstructural integrity of limbic tracts such as fornix (column body) and cingulum was found to be mostly impacted by AD biomarkers and APOE-ε4. This is in line with previous studies highlighting the involvement of these tracts, starting from AD preclinical stages,27, 28 as key structures of the Papez circuit, responsible for episodic memory functioning.25 Indeed, diffusivity alterations of these tracts have been shown to predict tau accumulation in amyloid-positive individuals.29 The strongest association was between A+T- status and fornix (column body) integrity, suggesting an early role of this structure. Similarly, decreases in fornix FA were previously shown to predict conversion from preserved cognition toward mild cognitive impairment and dementia,27 further supporting its role in disease progression. The lack of association between A+T+ status and fornix microstructure could be due to the relatively low number of A+T+ participants. Nevertheless, evidence suggests a biphasic relationship between AD pathology and DTI scalars, as the inflammatory mechanisms linked to amyloid and tau accumulation (i.e., glial activation and cellular swelling) may cause changes opposing the typical neurodegeneration pattern.1, 30
We observed widespread changes in white matter integrity in commissural, associative, and projection fibers, and demonstrated the presence of disease-specific gradients of WM disruption, anterior regions being more susceptible to vascular compromise and posterior regions to AD-related factors. Specifically, WM integrity in the genu (anterior part) of CC was distinctively associated with cSVD+ and FRS, while both AD pathology and cSVD had a significant effect on body and splenium (middle and posterior parts). Moreover, while the posterior limb of the internal capsule was mainly impacted by AD-related factors, alterations of the anterior limb were more often observed in individuals with high cerebrovascular burden. This posterior–anterior gradient of WM deterioration is in line with the evidence of frontal WM being more sensitive to vascular health, and generally more susceptible to cerebrovascular insults.31 Previous work has shown that global vascular health has a significant impact on white matter integrity, more strongly in the genu of the corpus callosum,32 and several studies have shown the detrimental effect of single cardiovascular risk factors on commissural WM tracts, such as hypertension33 and diabetes,34 suggesting that this region is a proxy for cerebrovascular health.32 Moreover, anterior patterns of WMHs have been shown to distinctively relate to cardiovascular etiologies.35
Conversely, WM lesions in parietal and occipital regions have been observed in association with amyloid pathology, independently of cardiovascular risk factors.11 In line with our results, previous studies have shown amyloid pathology in relationship with alterations in a group of regions including medial, temporal, and posterior white matter.36 The specific mechanisms through which amyloid deposition could impact white matter integrity are still unclear. On one hand, previous evidence has shown spatial correspondence between cortical amyloid deposition and white matter demyelination, possibly due to local alteration of neuronal and axonal function, thereby regulating oligodendrocyte functions, or resulting from Aβ-induced oxidative stress.37 Conversely, Aβ deposition may enhance capillary cortical amyloid angiopathy (CAA) and arteriosclerosis,38 resulting in hypoperfusion,39 which may eventually lead to white matter damage through secondary vascular events.37
Given that AD pathology and cSVD often coexist from early stages of neurodegeneration,40 questions have arisen regarding their impact on brain structure,12, 41, 42 raising the need to determine whether they act separately or jointly in causing WM disruption.15 Specifically, a recent ex vivo study has shown that the accumulation of WMHs is related to cSVD independently of AD pathology in non-demented older adults.41 A fixel-based study has demonstrated that cSVD mainly impacts fiber density, while AD pathology has comparable effects on fiber density and cross-section,42 leaving the question on their interaction unanswered. Here, we showed that they are both related to various degrees of disruption depending on the affected tracts, but seem to act largely independently. Only APOE-ε4 (independently of Aβ1-42 and p-Tau181, both accounted for in the linear mixed-effects models)43 showed interactions with FRS and cSVD+ in limbic and associative tracts. APOE-ε4 is involved in many amyloid-independent pathways affecting synaptic plasticity, metabolism, and vascular homeostasis,44 and therefore possibly synergizing with other cSVD-specific inherited and acquired risk factors.45 In this light, our results suggest that although sharing common etiopathological mechanisms, AD pathology and cSVD exert independent effects on white matter microstructure in preclinical stages. They also provide context for the selection of DTI metrics as outcome measures in primary and secondary prevention clinical trials, possibly representing a noninvasive alternative to PET imaging and a more sensitive MRI biomarker compared to hippocampal atrophy.46 In particular, our findings indicate that limbic tracts would be most relevant for therapeutic strategies targeting the AT pathway, whereas commissural, associative, or projection tracts might be better choices for interventions targeting cerebrovascular mechanisms.
In line with previous evidence,47 lower integrity measures were associated with higher age and male sex, with men showing higher MD in most of the tracts. In the posterior limb of the internal capsule, FA increased over time, possibly resulting from the relative sparing of the sensorimotor pathway in a crossing fibers region.48 Similarly, in the anterior limb of internal capsule FA increased, due to the faster degradation of genu of CC fibers.49 Some tracts showed a longitudinal MD decrease, supposedly due to the neuroinflammatory changes characterizing age-related neurodegeneration that may precede or overlap with microstructural disruption, causing temporary MD increases.50 We were able to identify a greater number of significant longitudinal effects on MD compared to FA, supporting the evidence of MD being more sensitive than FA.51
Our study relies on a relatively large sample size and on the advantage of longitudinal data, but has also some limitations. Firstly, we used an atlas-based approach. As DTI tractography yields subject-specific masks and connectomes, further studies are warranted to confirm and expand our results. Using a simpler and robust approach, we showed that TBSS is sensitive to AD and cSVD associated factors. Considering its less demanding computational time and power compared to connectome analysis, TBSS application to large datasets and in the context of clinical trials is favored. Secondly, since our acquisition protocol comprised 30 diffusion directions only, we were limited to classical tensor-based analyses, with the limitation of being unable to resolve crossing fibers. Therefore, further studies are needed to confirm our findings, and possibly expand on them by exploring other DTI scalars, which we did not include to reduce the number of tests and increase our power to identify biological effects. Another limitation is the multicentric nature of the study, representing a confounding factor that could not be entirely removed using state-of-art harmonization methods. Nonetheless, this also implies the effects that we found must be robust as we were still able to observe them. Importantly, while our results provide information on the biological mechanisms underlying myelin disruption in healthy aging and disease, further studies are needed to translate our group findings to single subjects, which still represents one of the most demanding and still unsolved challenges of the entire field. While we decided to dichotomize Aβ1-42 and p-Tau181 to adhere to the biological definition of AD and to the ATN framework, and subsequently we used gaussian mixture modeling to identify a cut-off to dichotomize cSVD and make it comparable to the ATN classification, further studies could explore the relationship between WM integrity and continuous measure of amyloid and tau accumulation or cSVD burden. Lastly, further research is needed to model the nonlinear relationship between amyloid and WM integrity.1, 30
In conclusion, we showed that AD pathology and cSVD are independently associated with myelin microstructural integrity within several tracts, with pathology-specific spatial patterns of WM vulnerability. This evidence supports the role of DTI for disease monitoring and subjects selection for early intervention targeting the factors associated with increased risk of developing dementia.
Acknowledgements
We thank the study participants. EPAD is supported by the EU/EFPIA Innovative Medicines Initiative (IMI) grant agreement 115736. The project leading to this paper has received funding from the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement No 115952. This Joint Undertaking receives the support from the European Union's Horizon 2020 research and innovation program and EFPIA. This communication reflects the views of the authors and neither IMI nor the European Union and EFPIA are liable for any use that may be made of the information contained herein.
Author Contributions
M.T, L.L., and F.B. contributed to the conception and design of the study and to drafting the manuscript; M.T, L.L., L.E.C., D.V.G., and F.B. contributed to the acquisition and analysis of the data and interpretation of the results; M.T, L.L., L.E.C., D.V.G., S.I., G.P., L.P., A.M., R.W., S.H., K.B., G.F., C.H.S., G.C., M.E., P.P., A.W., P.M.L., A.J.S., C.W.R., J.M.W., J.D.G., A.B., H.J.M.M.M., A.M.W., and F.B. contributed to reviewing and editing the manuscript. All authors have approved the final version of the manuscript.
Conflict of Interest Statement
L.E.C. is supported by AMYPAD (IMI 115952) and has received research support from GE HealthCare Ltd. (paid to institution); H.J.M.M.M. is supported by the Dutch Heart Foundation (2020 T049), by the Eurostars-2 joint program with co-funding from the European Union Horizon 2020 research and innovation program (ASPIRE E!113701), provided by the Netherlands Enterprise Agency (RvO), by the EU Joint Program for Neurodegenerative Disease Research provided by the Netherlands Organization for health Research and Development and Alzheimer Nederland (DEBBIE JPND2020-568-106), and by AMYPAD (IMI 115952); J.M.W. is supported by the UK Dementia Research Institute (funded by the MRC, Alzheimer's Society and Alzheimer's Research UK), the British Heart Foundation, The Fondation Leducq Network on Perivascular Spaces and the Row Fogo Centre for Research into Small Vessel Diseases; F.B. is supported by EPSRC, EU-JU (IMI), NIHR-BRC, GEHC, ADDI (paid to institution) and by AMYPAD (IMI 115952), is a consultant for Combinostics, IXICO, and Roche, participates in Advisory boards of USC-ATRC, Biogen, Prothena, and Merck, and is a co-founder of Queen Square Analytics; R.W. is an employee of IXICO; C.R. has done paid consultancy work in the last 3 years for Eli Lilly, Biogen, Actinogen, Brain Health Scotland, Roche, Roche Diagnostics, Novo Nordisk, Eisai, Signant, Merck, Alchemab, Sygnature, and Abbvie. His group has received Research Income to his Research Unit from Biogen, AC Immune and Roche. He has out licensed IP developed at University of Edinburgh to Linus Health and is CEO and Founder of Scottish Brain Sciences; S.H. is consultant for WYSS Center, Geneva, Switzerland, and consultant for SPINEART, Geneva, Switzerland; G.C. has received research support from the European Union's Horizon 2020 research and innovation program (grant agreement number 667696), Fondation d'entreprise MMA des Entrepreneurs du Futur, Fondation Alzheimer, Agence Nationale de la Recherche, Région Normandie, Association France Alzheimer et maladies apparentées, Fondation Vaincre Alzheimer, Fondation Recherche Alzheimer and Fondation pour la Recherche Médicale (all to Inserm), and personal fees from Inserm and Fondation Alzheimer; A.J.S. is an employee and minor shareholder of Takeda Pharmaceutical Company Ltd. The other authors report no competing interests.
Open Research
Data Availability Statement
Raw and processed data can be accessed from the EPAD website (www.ep-ad.org) upon request.




