Associations of inflammatory cytokines and cortisol with nonmotor features of Huntington's disease
Abstract
Objective
Huntington's disease (HD) is an inherited neurodegenerative disease involving progressive motor abnormalities, cognitive decline, and psychiatric disturbances. Depression and cognitive difficulties are among the most impactful symptoms of HD, yet the pathogenesis of these symptoms is not fully understood. HD involves low-level chronic inflammation and dysregulation of the hypothalamic–pituitary–adrenal (HPA) axis, which are linked to depression and cognitive impairment in non-HD populations. However, previous research on the relationships of these pathologies with depression and cognition in HD is limited and inconsistent.
Methods
Fifty-three adults with the HD gene expansion (30 premanifest and 23 manifest) completed measures of depression and cognitive functioning. Forty-eight out of 53 participants provided hair samples for quantification of cortisol, and 34 participants provided blood samples for quantification of peripheral inflammatory cytokines. We examined the associations of four cytokines (interleukin [IL]-6, IL-10, IL-1β, and tumor necrosis factor [TNF]-α) and cortisol levels with depression and cognitive scores.
Results
In unadjusted models, higher levels of plasma IL-6, IL-10, and TNF-α correlated with higher depression scores, and higher levels of IL-10 and TNF-α correlated with poorer cognitive performance. After controlling for age, sex, and body mass index, only the correlations of IL-10 with depression and cognitive performance remained significant. No correlations were evident with hair cortisol.
Interpretations
Peripheral inflammation is associated with depression symptoms and cognitive impairment in HD. Our findings suggest that interactions between the immune and nervous systems are important in HD, and highlight the potential of chronic inflammation as a therapeutic target in early stages of HD.
Introduction
Huntington's disease (HD) is an inherited neurodegenerative disease caused by an abnormal expansion in the number of cytosine–adenine–guanine (CAG) repeats in the huntingtin gene, which causes a mutated form of the huntingtin protein to be encoded and expressed throughout the body.1 Motor abnormalities are traditionally considered to be the hallmark feature of HD, and clinical diagnosis of HD requires the unequivocal presence of motor signs.2 Yet, a growing body of research indicates that cognitive and psychiatric disturbances have a more significant impact on the lives of people with HD than motor symptoms.3-5 Moreover, psychiatric disturbances and subtle changes in cognitive functioning can emerge 10 to 15 years before clinical diagnosis of HD6-8 and may consequently begin to affect quality of life early in the disease course. Therefore, identifying and addressing the factors that contribute to cognitive and psychiatric difficulties in HD is essential for optimizing the quality of life of people with HD.
Depression is one of the most common psychiatric syndromes to affect HD CAG expansion carriers.9, 10 Depression can emerge in any stage of HD but appears most commonly just before HD diagnosis and in the early stages of HD.11, 12 Moreover, depression does not appear to increase in prevalence or severity as HD progresses, instead following a nonlinear course across the disease.11-14 The etiology of depression in HD appears to involve both neurobiological and psychosocial factors15 but is not well understood. Unlike depression, cognitive functioning is known to decline as HD progresses.7, 16 Cognitive deficits in HD are thought to result from progressive brain atrophy caused by the expression of mutant huntingtin throughout the body.17 However, other factors are also likely to affect cognitive functioning in HD, given that the onset and progression of cognitive decline in HD are highly variable, and because cognitive deficits do not appear to map onto brain changes in premanifest HD.17-19
In non-HD populations, depression and cognitive impairment are strongly linked to dysregulation of the hypothalamic–pituitary–adrenal (HPA) axis, and inflammation in the central nervous system (CNS) and periphery.20-27 Notably, both the HPA axis and the immune system are known to be dysregulated in HD, as indicated by altered salivary and plasma cortisol levels,28-31 higher levels of inflammatory cytokines within brain tissue, cerebrospinal fluid, and blood plasma,32-36 and increased microglial activation within the striatum and cortex.37-40 However, current evidence for the relationships of HPA axis functioning with depression and cognition in HD is inconsistent. Several studies have reported that cortisol levels are associated with depression symptoms and cognitive functioning in HD,29, 30, 41 whereas other studies have reported no significant associations among these measures.42, 43 Meanwhile, the relationship of inflammation with depression and cognition in HD has only been investigated in one study,44 in which peripheral inflammatory cytokines were significantly associated with cognitive performance, but not depression symptoms. Given the limited research in this area, and discrepancies among existing studies, the relationships of HPA axis functioning and inflammation with depression and cognition in HD remain poorly understood.
In this study, we assessed HPA axis functioning by quantifying cortisol levels in hair samples. Hair samples enable quantification of cumulative cortisol release across several months, which render them suitable for measuring chronic HPA axis functioning relative to traditional cortisol measures (i.e., blood, urine, and saliva) that are influenced by diurnal rhythms and other sources of contextual variability.45 We also measured systemic inflammation by quantifying four inflammatory cytokines (interleukin [IL]-6, IL-10, IL-1β, and tumor necrosis factor [TNF]-α) in blood plasma, using highly sensitive immunoassays. These cytokines are known to be elevated in HD relative to healthy control groups32, 34 and have previously been linked to depression and cognitive functioning in non-HD populations.21, 46 The primary aims of this study were to examine the associations of chronic HPA axis activity (hair cortisol) and systemic inflammation (blood-based inflammatory cytokines) with depression symptoms and cognitive performance in premanifest and early manifest HD. A secondary aim was to examine whether these associations differed according to disease stage (premanifest versus manifest HD groups). We also explored the associations of chronic HPA axis activity and systemic inflammation with demographic and clinical outcomes in HD.
Methods
Participants
Fifty-three people with the HD CAG expansion (CAG repeat length ≥ 36) were recruited through a local HD research volunteer database, HD specialist clinics and advocacy groups in Australia, and social media. We recruited people in the premanifest or early manifest stages of HD. The premanifest group (n = 30) comprised CAG-expanded participants who did not meet criteria for clinical (motor) diagnosis at the time of participation, based on their most recent motor examination using the Unified Huntington's Disease Rating Scale (UHDRS).47 The early manifest group (n = 23) included clinically diagnosed participants who were in Stage I or II of HD (Total Functional Capacity [TFC] Score ≥7).47 The TFC scale assesses the ability to independently carry out activities such as domestic chores, management of finances, personal grooming, and hygiene. Total scores can range from 0 to 13, with a lower score indicating poorer functional capacity and greater disease severity. We calculated a Disease Burden Score (DBS; [CAG – 35.5] × Age) for each participant to estimate lifelong exposure to mutant huntingtin.48
Exclusion criteria for this study included psychiatric conditions other than depression and anxiety, neurological disorders other than HD, previous significant traumatic brain injury and/or concussion sustained in the past 12 months, consumption of excessive alcohol (>10 standard drinks per week) or illicit drugs, and current participation in a clinical drug trial. Additional exclusion criteria for the blood collection phase were autoimmune or inflammatory disorders, and current use of anti-inflammatory or immunomodulatory medications. For the hair sample collection phase, individuals with hair length <3 cm were excluded. The Monash University Human Research Ethics Committee (MUHREC) approved this study (MUHREC IDs: 23043 and 18894). All participants provided written informed consent in accordance with the Declaration of Helsinki.
From the 53 participants recruited, 48 provided hair samples, and 34 provided blood samples. Several participants were unable to provide a blood sample because of restrictions, either due to the COVID-19 pandemic, or because no collection center was available within a reasonable distance from their residence. Blood sample providers and nonproviders did not significantly differ with respect to any demographic or clinical variables, except for previous history of major depressive disorder (Table S1). With respect to hair samples, of the 53 total participants, four had insufficient hair for sample collection or quantification, and one participant opted out. Table 1 reports descriptive statistics for the hair and blood sample subgroups, stratified by disease stage (premanifest and manifest groups).
| Hair samples (n = 48) | Blood samples (n = 34) | |||
|---|---|---|---|---|
| Premanifest (n = 26) | Manifest (n = 22) | Premanifest (n = 20) | Manifest (n = 14) | |
| Age | ||||
| M (SD) | 45.1 (10.8)* | 55.3 (8.1) | 45.3 (11.5)* | 55.6 (7.8) |
| Range | 29–65 | 44–66 | 29–65 | 44–66 |
| Sex (female) | ||||
| N (%) | 21 (80.8%) | 13 (59.1%) | 15 (75.0%) | 9 (64.3%) |
| BMI | ||||
| M (SD) | 26.4 (5.7) | 28.8 (6.4) | 27.3 (6.1) | 27.6 (6.7) |
| Range | 20.2–42.5 | 20.2–45.2 | 20.9–42.5 | 20.2–45.2 |
| Smoker | ||||
| N (%) | 1 (3.8%) | 2 (9.1%) | 1 (5.0%) | 1 (7.1%) |
| Previous MDD diagnosis | ||||
| N (%) | 11 (42.3%) | 15 (68.2%) | 4 (70.0%) | 11 (78.6%) |
| Antidepressant use | ||||
| N (%) | 10 (38.5%)* | 17 (77.3%) | 11 (55.0%) | 12 (85.7%) |
| CAG repeat length | ||||
| M (SD) | 41.7 (1.9) | 42.3 (1.9) | 41.7 (2.1) | 41.8 (2.1) |
| Range | 38–45 | 37–45 | 38–45 | 37–45 |
| DBS | ||||
| M (SD) | 277.1 (86.4)* | 371.8 (103.2) | 281.2 (96.4) | 355.2 (125.7) |
| Range | 101.5–450.0 | 94.5–535.5 | 101.5–450.0 | 94.5–535.5 |
| TFC | ||||
| M (SD) | 12.6 (0.9)* | 9.1 (1.9) | 12.6 (0.9)* | 8.5 (1.7) |
| Range | 9–13 | 6–13 | 9–13 | 6–11 |
| COGTEL | ||||
| M (SD) | 33.2 (10.4)* | 19.4 (7.9) | 32.1 (10.7)* | 18.5 (7.8) |
| Range | 13.6–51.4 | 8.1–32.4 | 14.6–48.3 | 8.7–32.4 |
| NQ-depression (T-score) | ||||
| M (SD) | 49.2 (6.1) | 50.5 (8.8) | 50.5 (6.2) | 50.5 (10.6) |
| Range | 36.9–64.6 | 36.9–69.6 | 36.9–63.0 | 36.9–69.6 |
- In the pooled sample, CAG (and therefore DBS) data are missing for four participants (three manifest and one premanifest). COGTEL data are missing for one manifest participant.
- BMI, body mass index; CAG, cytosine–adenosine–guanine; COGTEL, Cognitive Telephone Screening Instrument; DBS, Disease Burden Score; MDD, major depressive disorder; NQ, Neuro-QoL.; TFC, Total Functional Capacity.
- * p < 0.05 for difference between premanifest and manifest groups (assessed using independent-samples t-tests and Mann–Whitney U tests for continuous variables, and chi-square tests for categorical variables).
Measures
Quality of Life in Neurological Disorders (Neuro-QoL) – Depression Questionnaire
Depression severity was self-rated by the participants using the short form of the Neuro-QoL Depression questionnaire,49 which assesses depressive symptoms experienced in the past week. This measure is rated on a Likert scale ranging from 1 to 5, with higher scores indicating more severe depressive symptoms. Raw scores were converted into standardized T-scores with a mean of 50 and a standard deviation of 10.
Cognitive Telephone Screening Instrument (COGTEL)
We used the COGTEL50 to measure cognitive functioning. The COGTEL is a telephone-based cognitive screening test which consists of six subtests assessing prospective memory, immediate and delayed verbal memory, working memory, verbal fluency, and inductive reasoning. Scores from each of the six subtests were combined into a weighted total score.
Hair cortisol
We used hair cortisol to measure chronic cortisol production and HPA axis functioning. Hair samples were self-collected by participants from the posterior vertex of the head, which yields the most reliable measures of cortisol.51 Hair samples were stored in a dark, dry area at room temperature until they were ready to be analyzed. Hair cortisol was extracted and quantified by Stratech Scientific APAC Pty Ltd (Sydney, Australia). Cortisol levels were quantified in the three centimeters of hair closest to the scalp, with 1 cm of hair reflecting approximately 1 month of cortisol production.45 Hair samples were first cleaned and allowed to dry for 5 days before being bathed and sonicated in an extraction solvent for 24 hours. Samples were then removed from the solvent, dried, and reconstituted in phosphate-buffered saline for analysis. Cortisol was analyzed in duplicate using a commercially available ELISA assay (Salimetrics, USA) according to the manufacturer's instructions. Intra-assay variability for cortisol analysis was 4.1% and inter-assay variability was 4.6%.
Inflammatory cytokines
We quantified the inflammatory cytokines IL-6, IL-10, IL-1β, and ΤΝF-α in blood plasma. IL-6 is a cytokine with both anti- and (predominantly) pro-inflammatory properties, and stimulates the production of acute phase proteins, hematopoiesis, and immune reactions as part of the inflammatory response.52 IL-1β and TNF-α are pro-inflammatory cytokines that are secreted as part of the innate immune response and influence neuroendocrine and neurotransmitter activity, as well as inducing “sickness behaviors” such as fever, reduced food consumption, and social withdrawal.53, 54 IL-10 is an anti-inflammatory cytokine that is involved in balancing the immune response via several immunoregulatory effects, such as inhibiting the production of pro-inflammatory cytokines, and enhancing B-cell proliferation and antibody production.55
Blood was collected from each participant using EDTA tubes at a Melbourne Pathology Services (MPS) collection center, and blood samples were delivered to a central lab for processing within 4 h of collection. Aliquots of 0.5 mL of plasma were extracted and stored at −70°C until analysis. Cytokines were quantified using four highly sensitive, single-plex Advantage assays on the SIMOA HD-X analyser (Quanterix, Billierica, MA, USA). All samples were tested in duplicate. Except for two IL-1β samples, all samples measured above their lower limit of quantification for each inflammatory marker (IL-6: 0.01 pg/mL; IL-10: 0.021 pg/mL; IL-1β: 0.016 pg/mL; TNF-α: 0.016 pg/mL). For the two IL-1β samples which fell below the lower limit of quantification, values were replaced with the lower limit of detection for IL-1β (0.0055 pg/mL). The average coefficient of variation for duplicate samples was 4.77% for IL-6, 4.32% for IL-10, 21.71% for IL-1β, and 4.06% for TNF-α.
Study procedure
All study components were completed remotely as part of a broader 4-week investigation of depression in HD. Participants first completed study screening and the TFC scale via telephone with the first author (HB). On Day 1 of the study, participants completed the study consent form and Neuro-QoL Depression questionnaire electronically via the Research Electronic Data Capture (REDCap) system.56 Participants completed the COGTEL with HB via telephone, approximately 1 week after baseline. Participants provided a blood sample at a Melbourne Pathology Services (MPS) collection center on any weekday during the 4-week period of testing. Because inflammatory molecules are sensitive to time of day,57 all blood test appointments were scheduled in the morning, as close as possible to the participant's usual waking time. Participants also self-collected a hair sample at the end of the 4-week period of testing, with the assistance of a companion.
Seven participants provided a blood sample following completion of the 4-week study, after restrictions related to COVID-19 had eased; these participants re-completed the COGTEL (alternate form) and Neuro-QoL Depression questionnaire on the same day as their blood draw, so that their cytokine data could be correlated with up-to-date clinical data. Blood and hair samples were collected from three additional participants during on-site research visits at Monash University. These three participants completed the Neuro-QoL Depression questionnaire on the same day as biological sample collection, and the COGTEL with HB via telephone within 2 weeks.
Data analysis
Statistical analyses were conducted using SPSS Version 27. Preliminary assumption testing revealed a significant positive skew for all inflammatory cytokines and hair cortisol, which were log-transformed for subsequent statistical analyses. To examine whether hair cortisol and cytokine concentrations differed between premanifest and manifest groups, we performed independent-samples t-tests or Mann–Whitney U tests for variables whose distributions continued to deviate from normality after log transformations (IL-1β, TNF-α, and hair cortisol). Cohen's d was calculated to obtain effect sizes for all between-groups comparisons. We used Spearman's rank order correlations to examine correlations among biological measures and quantify associations between each biological measure and depression symptoms, cognitive performance, demographic variables (i.e., age, sex, body mass index (BMI), smoking status, antidepressant use), disease burden, and functional capacity. We also performed nonparametric partial correlation tests to examine the associations of each biological measure with depression severity and cognitive performance after controlling for age, sex, and BMI. We then performed multiple regression analyses to assess the independent associations of each inflammatory cytokine with depression severity and cognitive performance, and to assess whether there were significant interactions between each biological measure and disease stage (premanifest and manifest) for depression and cognition. Simple slope analyses were performed for post hoc characterization of statistically significant interactions.
Results
Cytokine and cortisol concentrations relative to demographic and clinical variables
As depicted in Figure 1, the premanifest and manifest groups did not significantly differ for any biological measure: hair cortisol (p = 0.56, d = 0.17), IL-6 (p = 0.12, d = 0.56), IL-10 (p = 0.06, d = 0.70), IL-1β (p = 0.55, d = 0.22), or TNF-α (p = 0.27, d = 0.40), although medium effect sizes were observed for IL-6 and IL-10.
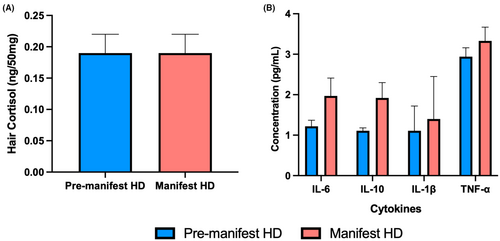
Higher concentrations of IL-6 significantly correlated with older age, higher BMI, and current use of antidepressants (Table 2). Moreover, higher concentrations of IL-10 significantly correlated with older age, antidepressant use, and poorer functional capacity, while higher concentrations of TNF-α correlated with older age and antidepressant use. Hair cortisol did not significantly correlate with any demographic or clinical variables.
| Hair cortisola | IL-6b | IL-10b | IL-1βb | TNF-αb | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| r s | p | r s | p | r s | p | r s | p | r s | p | |
| Age | 0.005 | 0.98 | 0.35 | 0.04 | 0.39 | 0.02 | 0.11 | 0.55 | 0.48 | 0.004 |
| Sex | 0.003 | 0.98 | −0.10 | 0.71 | 0.16 | 0.37 | −0.30 | 0.09 | 0.09 | 0.60 |
| BMI | 0.06 | 0.68 | 0.47 | 0.006 | 0.22 | 0.22 | 0.22 | 0.21 | 0.17 | 0.36 |
| Smoking status | −0.18 | 0.21 | 0.01 | 0.94 | 0.19 | 0.28 | 0.17 | 0.35 | −0.04 | 0.83 |
| Antidepressant use | 0.03 | 0.83 | 0.47 | 0.006 | 0.62 | <0.001 | 0.20 | 0.25 | 0.36 | 0.04 |
| DBS | −0.10 | 0.53 | 0.05 | 0.80 | 0.27 | 0.14 | 0.09 | 0.62 | 0.13 | 0.48 |
| TFC | −0.21 | 0.16 | −0.31 | 0.08 | −0.44 | 0.01 | −0.11 | 0.55 | −0.25 | 0.17 |
- Correlation tests were performed on log-transformed values of cortisol and cytokine data. rs = Spearman's rho. Significant correlations (p < 0.05) are in bold text.
- a n = 48.
- b n = 34.
With respect to correlations among the biological measures, higher levels of IL-6 significantly correlated with higher levels of IL-10 (rs = 0.45, p = 0.008) and TNF-α (rs = 0.35, p = 0.046). Additionally, higher levels of IL-10 significantly correlated with higher levels of TNF-α (rs = 0.43, p = 0.01). No significant intercorrelations were observed for IL-1β or hair cortisol, although we observed trends for correlations between TNF-α and IL-1β (rs = 0.34, p = 0.05), as well as TNF-α and hair cortisol (rs = −.35, p = 0.06).
Associations of depression and cognition with cytokine and cortisol concentrations
Higher concentrations of IL-6, IL-10, and TNF-α significantly correlated with higher (more severe) depression scores, and higher concentrations of IL-10 and TNF-α significantly correlated with poorer cognitive performance; medium to large effect sizes were observed for all significant correlations (see Table 3 and Fig. S1). Neither hair cortisol nor IL-1β had significant correlations with depression symptoms or cognitive performance. After including age, sex, and BMI as covariates, only the correlations of IL-10 with depression and cognitive performance remained statistically significant, although a trend was noted for the correlation between TNF-α and depression symptoms (Table 3).
| Hair cortisol | IL-6 | IL-10 | IL-1β | TNF-α | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| r s | p | r s | p | r s | p | r s | p | r s | p | |
| Unadjusted | ||||||||||
| NQ-depression | −0.01 | 0.96 | 0.34 | 0.049 | 0.54 | <0.001 | 0.17 | 0.35 | 0.37 | 0.03 |
| COGTEL | 0.07 | 0.65 | −0.11 | 0.54 | −0.52 | 0.002 | −0.24 | 0.18 | −0.37 | 0.04 |
| Controlling for age, sex & BMI | ||||||||||
| NQ-depression | −0.03 | 0.85 | 0.26 | 0.16 | 0.53 | 0.002 | 0.17 | 0.37 | 0.36 | 0.05 |
| COGTEL | 0.10 | 0.53 | 0.17 | 0.37 | −0.41 | 0.03 | −0.19 | 0.31 | −0.18 | 0.34 |
- Correlation tests were performed on log-transformed cortisol and cytokine data. Significant correlations (p < 0.05) are in bold text.
Multiple regression analyses indicated that IL-6, IL-10, TNF-α, and IL-1β together accounted for 30.4% of the variance in depression scores, (F(4, 33) = 3.16, p = 0.03), and 29.8% of the variance in cognitive performance (F(4, 32) = 2.97, p = 0.04). When included together in the same multiple regression model, none of the cytokines had statistically significant independent associations with depression: IL-6 (p = 0.35), TNF-α (p = 0.27), IL-1β (p = 0.48), although a trend was observed for IL-10 (p = 0.07). Cognitive performance had a significant independent association with IL-10 (β = −.48, p = 0.02), but not with IL-6 (p = 0.49), TNF-α (p = 0.53), or IL-1β (p = 0.58).
Interactions of cortisol and cytokine concentrations with disease stage
As illustrated in Figure 2A, a significant interaction between TNF-α and disease stage was evident for depression symptoms (β = 0.49, p = 0.02). Specifically, higher concentrations of TNF-α were significantly associated with higher (more severe) depression scores in the manifest group (r = 0.67, p = 0.009), but not in the premanifest group (r = 0.14, p = 0.55). Interactions with disease stage were not statistically significant for IL-6 (p = 0.55), IL-10 (p = 0.99), IL-1β (p = 0.33), or hair cortisol (p = 0.24).
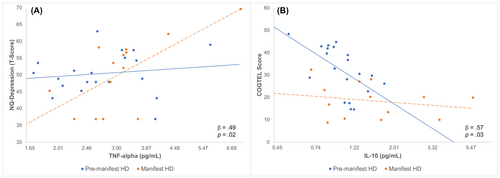
As illustrated in Figure 2B, a significant interaction between disease stage and IL-10 was observed for cognitive performance (β = 0.57, p = 0.03). Higher concentrations of IL-10 were associated with poorer cognitive performance in the premanifest group (r = −0.63, p = 0.003), whereas no significant associations were observed between IL-10 and cognitive performance in the manifest group (r = −0.22, p = 0.47; Fig. 2B). Interactions with disease stage were not significant for IL-6 (p = 0.16), IL-1β (p = 0.27), TNF-α (p = 0.57), or hair cortisol (p = 0.88).
Discussion
This study investigated the associations of systemic inflammation and chronic HPA axis activity with depression symptoms and cognitive functioning in HD. We report that more severe depression symptoms and poorer cognitive performance were associated with higher plasma levels of the inflammatory cytokine IL-10. Associations of depression and cognition with the inflammatory cytokines IL-6 and TNF-α were also evident; however, these associations were not statistically significant after adjusting for covariates. Conversely, we did not observe any significant associations between hair cortisol concentrations and depression or cognitive performance. Our findings provide evidence for a relationship between peripheral inflammation and cognitive and psychiatric disturbances in HD, which may have important implications for disease modeling and treatment of HD symptoms.
We found that depression symptoms and cognitive performance had the strongest associations with IL-10, an anti-inflammatory cytokine; these associations remained significant even after controlling for demographic factors and were independent of the effects of other inflammatory cytokines. IL-10 is elevated in many conditions involving CNS pathology, including HD, likely to regulate the production of pro-inflammatory cytokines and thus reduce inflammation.36, 55 Yet, the elevations previously observed in both pro- and anti-inflammatory cytokines in HD suggest that immune dysregulation persists.32, 34, 36 The uncontrolled and prolonged over-expression of inflammatory markers may contribute to depression and cognitive impairment in HD.
We additionally found that depression symptoms and cognitive disturbances in HD are associated with markers of general immune reactivity (IL-6) and pro-inflammatory processes (TNF-α). These results are in keeping with the large body of research indicating that depression and cognition are associated with elevations in IL-6 and TNF-α in the periphery, both in neurologically healthy populations,21, 58 and in neurodegenerative diseases like Alzheimer's and Parkinson's disease.20, 23, 24 Our results are also partially consistent with a previous study in HD, in which higher levels of IL-6 and IL-1ra were associated with poorer executive functioning,44 although no significant associations were observed between inflammatory cytokines and depression scores. Unlike IL-10, the associations of depression and cognitive performance with TNF-α and IL-6 did not remain significant after adjusting for age, sex, and BMI, and should therefore be interpreted with caution. However, the observation of medium effect sizes motivates further investigation and replication of these relationships in larger samples.
Intriguingly, pro-inflammatory activity, represented by TNF-α, was significantly associated with depressive symptoms in the manifest group but not the premanifest group, even though TNF-α levels and depression scores were comparable in both groups. This finding reinforces the complex etiology of depression in HD and suggests that inflammation may be more relevant to depression in manifest HD. We also found that anti-inflammatory activity, represented by IL-10, was significantly associated with worse cognitive performance in the premanifest group, but not the manifest group. Several studies have shown that inflammatory markers in the CNS and periphery are elevated before the onset of clinical symptoms of HD,32, 38, 39 suggesting that inflammation emerges early in disease course. Accordingly, low-grade inflammation may disrupt cognitive functioning in premanifest HD, when other pathophysiological changes associated with the disease are more subtle,7, 16 whereas other pathophysiological processes may have a stronger impact on cognition in the manifest stage.
Several possible mechanisms may underpin the relationship of peripheral inflammation with depression and cognition in HD. Animal studies suggest that peripheral inflammatory cytokines may elicit depression and cognitive changes by crossing the blood–brain barrier to influence brain structure and function, or by communicating with the brain via peripheral nerves and endothelial cells of the blood–brain barrier.21, 59 Alternatively, peripheral inflammatory cytokines may be generated by glial cells in the CNS in response to progressive neurodegeneration and other pathological changes in HD.60 Further research is needed to clarify whether peripheral cytokines directly influence depression and cognitive functioning in HD, or are simply a proxy for the actual mechanisms that are driving these changes.
Our findings suggest that inflammation is a promising target for the treatment of depression and cognitive symptoms in HD. Preclinical studies have demonstrated that reducing inflammation improves neuronal, neuropathological, motor, and behavioral abnormalities in mouse models of HD.61-66 Clinical trials using immunomodulators have also proven effective in reducing caudate atrophy and cognitive decline in HD patients.67, 68 Our findings further support the inclusion of cognitive functioning and depression as outcomes of interest in future trials targeting inflammation in HD. This is particularly salient for future trials in premanifest groups, where cognitive and psychiatric symptoms are evident and clinically relevant even in the absence of motor features.
Our study suggests that chronic HPA axis functioning may not be strongly associated with depression or cognitive functioning in HD, replicating one previous study that also assessed hair cortisol concentrations in relation to clinical and disease-related outcomes in premanifest HD.43 Moreover, studies in HD which have quantified cortisol levels in bodily fluids have obtained inconsistent findings regarding associations with depression and cognition.29, 30, 41-43 These inconsistent findings may relate to methodological differences between studies, as well as the reliance on small sample sizes in HD.30, 41, 43 Notwithstanding these methodological limitations, previous studies also indicate that many of the factors that contribute to depression and cognitive disturbances in HD are unique to the disease.17, 69, 70 Therefore, unlike neurologically healthy populations, HPA axis functioning may not be strongly implicated in the etiology of depression and cognitive disturbances in HD.
Several limitations of this study should be noted. Firstly, this study had a relatively small sample size, particularly for the cytokine data, which may have limited statistical power to detect important effects. We noted several interesting trends in our cytokine data, which did not reach statistical significance but warrant follow-up in larger samples. Further, because this study did not include participants with advanced HD, the relationships of inflammatory cytokines and cortisol with depression and cognition across all disease stages are unknown. This study also did not include a healthy comparison group, which makes it difficult to determine the extent to which the associations observed in this study are unique to HD. Moreover, because of the cross-sectional nature of this study, the directionality of the associations observed between cytokines, depression, and cognition cannot be established with certainty. Lastly, because this study was conducted during the COVID-19 pandemic, it is feasible that psychological stress associated with the pandemic may have affected study outcomes to some extent.
In conclusion, this is the first study to show significant associations between peripheral inflammation and depression symptoms in premanifest and manifest HD. This study also extends previous research showing that peripheral inflammation is linked to poorer cognitive functioning in HD. We did not find significant associations between chronic HPA axis functioning and depression symptoms or cognitive performance in HD, although these relationships warrant further investigation in larger cohorts. Our findings highlight chronic inflammation as a possible mechanism underpinning depression and cognitive difficulties in HD. The results of this study also motivate further research on the suitability of chronic inflammation as a treatment target for key HD symptoms, and the role of interactions between the immune system and nervous system in the development, exacerbation, and/or progression of HD symptoms.
Acknowledgements
We thank those who took the time to participate in this study. We also thank Calvary Healthcare Bethlehem and the Neuropsychiatry Centre at the Royal Melbourne Hospital for their assistance with recruitment. This work was supported by internal funding from the Central Clinical School, Monash University, and by a Research Training Program (RTP) Scholarship awarded to Hiba Bilal by the Australian Government. Open access publishing facilitated by Monash University, as part of the Wiley - Monash University agreement via the Council of Australian University Librarians.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Hiba Bilal. The first draft of the manuscript was written by Hiba Bilal, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Conflict of Interest
Julie Stout has served on Scientific Advisory Boards for Spark Therapeutics and Teva-Australia within the past 3 years. Although these ad boards were focused on HD, neither focused on depression, which is the topic of the current manuscript. Julie Stout also serves in an ongoing role as a director of the company Zindametrix, which provides services to pharmaceutical companies to facilitate the implementation of cognitive assessment in Huntington's disease clinical trials. Several of these trials have been ongoing throughout the time of the research reported in this paper; however, none of the trials are focused on depression in HD, and therefore are not considered a conflict of interest for the work reported in the manuscript. Julie Stout has provided paid consulting services to Sage Therapeutics related to HD but not directly related to the work presented in this paper. Finally, Julie Stout receives an annual honorarium for her services as the Chair of the Scientific Oversight Committee of the global Enroll-HD study. Again, this role is not in conflict with or related to the contents of the report.




