Asymptomatic spinal lesions in patients with AQP4-IgG-positive NMOSD: A real-world cohort study
Abstract
Objective
This study aims to explore the frequency and influencing factors of asymptomatic spinal lesions (ASLs) and their impact on subsequent relapses in patients with AQP4-IgG-positive NMOSD (AQP4-NMOSD) in a real-world setting.
Methods
We retrospectively reviewed clinical information and spinal MRI data from AQP4-NMOSD patients who had at least one spinal cord MRI during their follow-ups. Kaplan–Meier curves and Cox proportional hazards models were employed to ascertain potential predictors of remission ASLs and to investigate factors associated with subsequent relapses.
Results
In this study, we included 129 patients with AQP4-NMOSD and reviewed 173 spinal MRIs during attacks and 89 spinal MRIs during remission. Among these, 6 ASLs (3.5%) were identified during acute attacks, while 8 ASLs (9%) were found during remission. Remission ASLs were linked to the use of immunosuppressive agents, particularly conventional ones, whereas no patients using rituximab developed ASLs (p = 0.005). Kaplan–Meier curve analysis indicated that patients with ASLs had a significantly higher relapse risk (HR = 4.658, 95% CI: 1.519–14.285, p = 0.007) compared to those without. Additionally, the use of mycophenolate mofetil (HR = 0.027, 95% CI: 0.003–0.260, p = 0.002) and rituximab (HR = 0.035, 95% CI: 0.006–0.203, p < 0.001) significantly reduced the relapse risk. However, after accounting for other factors, the presence of ASLs did not exhibit a significant impact on subsequent relapses (HR = 2.297, 95% CI: 0.652–8.085, p = 0.195).
Interpretation
ASLs may be observed in patients with AQP4-NMOSD. The presence of ASLs may signify an underlying inflammatory activity due to insufficient immunotherapy. The administration of immunosuppressive agents plays a key role in the presence of remission ASLs and the likelihood of subsequent relapses.
Introduction
Neuromyelitis optica spectrum disorder (NMOSD) is a type of autoimmune inflammatory central nervous system (CNS) disease characterized by optic neuritis and/or acute myelitis as well as a group of brain syndromes.1 NMOSD, different from multiple sclerosis (MS), is primarily associated with aquaporin-4 immunoglobulin G (AQP4-IgG).2 In MS, the presence of new asymptomatic lesions on MRI aids in diagnosing the disease in patients with clinically isolated syndromes and can be used for therapeutic monitoring and prognostic assessment.3 In contrast, disability accumulation in NMOSD seems closely linked to attacks, with asymptomatic lesions indicating ongoing disease activity found in only a small fraction of NMOSD patients.4-10 These asymptomatic lesions can emerge during attacks or remission, suggesting MRI's heightened sensitivity compared to clinical symptoms. Performing a one-off MRI scan following an acute episode seems reasonable, allowing a reestablishment of the imaging baseline and enabling comparisons with future relapses.11, 12 The Latin American NMOSD Consensus recently recommended annual brain MRIs for NMOSD patients after starting immunosuppressive therapy to complement clinical follow-ups.13
Acute myelitis, a primary core symptom of NMOSD, frequently presents as longitudinally extensive transverse myelitis (LETM) in about 70% of cases and as short transverse myelitis (STM) in roughly 20% of cases.1, 13, 14 Studies on asymptomatic optic nerve and brain lesions are predominant in NMOSD, whereas fewer studies have been conducted especially on asymptomatic spinal lesions (ASLs) concerning the incidence, implications for treatment failure, relationship with subsequent relapses, and management strategy. Currently, there is no global consensus regarding the use of spinal MRI in NMOSD follow-ups (unless a clinical relapse occurs). Furthermore, a previous study showed that in AQP4-IgG-positive NMOSD (AQP4-NMOSD), reductions in the mean upper cervical cord area could be detected even without a prior history of clinical relapses or spinal cord lesions on MRI, suggesting that there may be a subtle or subclinical inflammatory pathological process within the spinal cord.15 Given accumulating evidence of asymptomatic tissue damage in NMOSD, the role of MRI in routine patient monitoring warrants reconsideration. Therefore, this study aims to investigate the frequency and influencing factors of ASLs and their impact on subsequent relapses by retrospectively collecting clinical and spinal MRI data from Chinese AQP4-NMOSD patients in a real-world setting.
Methods
Study design and patients
This study was a retrospective cohort study of AQP4-NMOSD patients treated and followed up at the Second Affiliated Hospital of Anhui Medical University and the First Affiliated Hospital of Soochow University from 30 June 2014 to 30 June 2023. Following ethical requirements, all participating patients or their guardians signed a written informed consent for the collection of their clinical and imaging data. The local ethics committee approved the study. Patients meeting the IPND 2015 diagnostic criteria for NMOSD and undergoing regular follow-ups for a minimum of 6 months were included, during which at least one spinal cord MRI was performed. These patients were excluded if they were AQP4-IgG-negative, had not undergone spinal MRI, or had spinal MRI but did not meet criteria, or we were unable to obtain images. All data were sourced from medical records, supported by telephone follow-ups, periodic reexaminations, and readmissions.
Baseline clinical data collection
Baseline clinical data encompassed gender, age at disease onset and enrollment, core clinical symptoms at first onset, concomitant other autoimmune diseases, AQP4-IgG test results (fixed cell-based assay), number of attacks before enrollment, and medication administered for each episode (including acute-phase treatment, sequential immunosuppressive agents, coadministration of corticosteroids, or other medications), enrollment time, follow-up duration, and subsequent medication usage during follow-up.
Spinal cord MRI data collection and analysis
Spinal cord lesions in NMOSD patients were evaluated using 1.5T or 3T MRI in accordance with protocols established by the respective local neuroscience departments at the time. Spinal MRI assessments encompassed T2-weighted imaging, T1-weighted imaging, and short-time inversion recovery sequences. Administration of gadolinium contrast was contingent upon local protocols and clinical circumstances. Evaluation of all spinal MRI scans was conducted by two neurologists (SGC and YFZ) and one neuroradiologist (PC) with consensus.
Spinal MRI scans were categorized as attack MRIs if performed during the initial episode or relapse, or as remission MRIs if conducted 6 months or longer from the last episode, with the patient being completely free of new clinical symptoms. MRIs between 1 and 6 months from the last episode were excluded from the remission category to prevent the inclusion of enhanced lesions evolving post-acute attack periods. Acute-attack MRIs were paired with remission MRIs to assess lesion evolution on spinal MRIs. If a subsequent relapse occurred, the spinal MRI taken during the relapse was redefined as the baseline for future comparisons. In cases lacking acute-attack spinal MRI, remission MRI served as the baseline for comparison with a subsequent remission MRI.
ASLs were defined as newly detected silent lesions or enlargement or sustained enhancement of preexisting lesions observed on spinal cord MRIs, without concurrent new or deteriorating clinical signs and symptoms associated with spinal cord impairment. Detailed records of spinal MRI dates, spinal lesion characteristics, and immunotherapy status during spinal MRI scans were maintained for each patient.
Follow-up and relapse evaluation
The study centered on gathering follow-up data from patients with paired remission MRIs. Patients were monitored for potential subsequent relapses using the time of remission MRI scan as the reference point. NMOSD relapse is defined as new or worsened symptoms occurring at least 1 month after a prior attack, lasting over 24 hours and without other recognized causes such as fever or infection.16 Detailed records were maintained for relapse timing, clinical symptoms, imaging findings, and treatment administered during relapse. The study concluded on 30 June 2023. If patients switched to an alternative immunosuppressive agent during follow-up, the follow-up duration was calculated using the time of switching to new immunosuppressive agents as the follow-up endpoint.
Statistical analysis
Statistical analysis was performed using SPSS 22.0 (SPSS Inc., Chicago, IL) software. Baseline clinical data were expressed as mean ± standard deviation or median (interquartile range) for continuous variables and as percentages for categorical variables. Group differences in continuous variables were assessed using Student's t-test or Mann–Whitney U-test. For categorical variables, group differences were evaluated using the chi-squared test or Fisher's exact test. Kaplan–Meier curves were used to depict the time from remission MRI scan to subsequent relapse with group comparisons analyzed through the log-rank test and Cox proportional hazard regression model. Hazard ratios (HRs) and 95% confidence intervals (CIs) were subsequently computed. All tests used a two-sided p-value of 0.05 as a threshold for significance. Graphs were generated using GraphPad Prism software (version 8.0) or Microsoft PowerPoint.
Results
Baseline clinical features
Over the span of 9 years, a total of 282 NMOSD patients were identified. Among them, 129 AQP4-NMOSD patients with available spinal MRI imaging were ultimately enrolled (Fig. 1), including 19 males and 110 females, with a median onset age of 42 years (range: 8–78) and a median enrollment age of 44 years (range: 19–78). Out of these, 22 patients presented concurrent autoimmune diseases, including 6 with systemic lupus erythematosus (1 with Sjogren's syndrome, 2 with Hashimoto's thyroiditis, and 1 with anticardiolipin antibody syndrome), 12 with Sjogren's syndrome (1 with autoimmune hepatitis), 2 with myasthenia gravis, 1 with Grave's disease, and 1 with autoimmune hepatitis and primary biliary cholangitis.
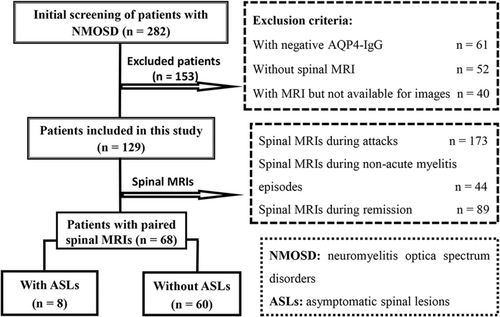
Acute myelitis (72 patients, 55.8%) and optic neuritis (38 patients, 29.5%) constituted the most prevalent initial core clinical symptoms, followed by area postrema syndrome (APS) (9 patients, 7%) and a combination of optic neuritis and acute myelitis (4 patients, 3.1%). Furthermore, a minor percentage of patients exhibited diverse core clinical symptoms such as APS combined with acute myelitis, cerebral syndrome, acute brainstem syndrome, acute mesencephalic syndrome, and acute myelitis combined with brainstem and cerebral syndrome (Table 1). The median number of attacks before enrollment was 1 (range: 0–11). Up until 30 June 2023, 3 patients were lost to follow-up, and 1 patient had passed away. The median follow-up period for the 129 AQP4-NMOSD patients was 42.8 (38.7) months.
| Age, median (range), years | |
| At onset | 42 (8–78) |
| At enrollment | 44 (19–78) |
| Gender, n (%) | |
| Female | 110 (85.3) |
| Male | 19 (14.7) |
| Concomitant autoimmune diseases, n (%) | 22 (17.1) |
| Number of attacks before enrollment, median (range) | 1 (0–11) |
| Follow-up duration, median (IQR), months | 42.8 (38.7) |
| Initial core clinical symptoms, n (%) | |
| Optic neuritis | 38 (29.5) |
| Acute myelitis | 72 (55.8) |
| APS | 9 (7) |
| Cerebral syndrome | 1 (0.8) |
| Acute mesencephalic syndrome | 1 (0.8) |
| Acute brainstem syndrome | 1 (0.8) |
| Diverse core clinical symptoms | 7 (5.4) |
| Period of onset at enrollment, n (%) | |
| Acute phase | 60 (46.5) |
| Relapse phase | 61 (47.3) |
| Remission phase | 8 (6.2) |
| Number of attack spinal MRIs during follow-up | 173 (121) |
| Number of remission spinal MRIs during follow-up | 89 (68) |
| Therapy at enrollment, n (%) | |
| None | 91 (70.5) |
| Steroids (including prednisone and methylprednisolone) | 27 (20.9) |
| AZA ± steroids | 6 (4.7) |
| MMF ± steroids | 3 (2.3) |
| RTX ± steroids | 2 (1.6) |
- APS, area postrema syndrome; AZA, azathioprine; IQR, interquartile range; MMF, mycophenolate mofetil; RTX, rituximab.
Spinal MRI findings
ASLs during acute attacks
Over a median follow-up of 43.7 (38.4) months, 121 patients underwent 173 spinal MRIs during acute attacks, with a median number of spinal MRIs of 1 (range: 1–4). Among them, 41 patients underwent 44 spinal MRIs during non-acute myelitis episodes: 28 times for optic neuritis, 7 for APS, 2 for cerebral syndrome, 1 for mesencephalic syndrome, 3 for brainstem syndrome, 2 for optic neuritis combined with cerebral syndrome, and 1 for ASP combined with mesencephalic syndrome. Within this group, 6 patients exhibited ASLs (2 with spinal cord enhancement, 1 without enhancement, and 3 without enhancement scans). Among these, 4 patients had new lesions identified on spinal MRI during episodes of optic neuritis, 1 during an episode of optic neuritis combined with cerebral syndrome, and 1 during an episode of APS, though no myelitis-related symptoms were present. The remaining 8 patients exclusively had remission spinal MRIs.
ASLs in remission stage
During the median follow-up of 50.9 (39.1) months, 68 patients had 89 spinal MRIs in remission, with a median number of spinal MRIs of 1 (range: 1–4). Among these, 3 remission spinal MRIs with durations less than 6 months were excluded and 5 patients had only remission MRIs, thus leaving 81 paired spinal MRIs. Although not included, these 8 remission spinal MRIs also did not reveal ASLs. The median interval between paired spinal MRIs was 17.5 months (range: 6.1–77.2). A total of 8 remission ASLs were observed in 8 patients (5 with enhancement of preexisting lesions, 1 with extension of a pre-existing C-segment lesion but no enhancement, 1 with enlargement of previous C2-3 lesion, and 1 with new T5–6 lesions). These ASLs were detected in 4 patients while on azathioprine (AZA) and prednisone, 2 while on mycophenolate mofetil (MMF) and prednisone, 1 while on cyclophosphamide (CTX) and prednisone, and 1 while on prednisone only.
Comparison of clinical and imaging data between patients with and without remission ASLs
Based on the presence of remission ASLs, 68 patients were classified into two groups: those with ASLs (8 patients) and those without ASLs (60 patients). A significant disparity in immunosuppressive agent usage during remission MRI assessment emerged (p = 0.005). Subsequent multiple comparisons highlighted a considerably higher proportion of AZA usage in patients with ASLs compared to those without (p < 0.05), whereas the proportion of rituximab (RTX) usage was significantly lower in patients with ASLs than in patients without ASLs (p < 0.05). Other indicators displayed no significant differences between the two groups (Table 2).
| Variables | With remission ASLs (n = 8) | Without remission ASLs (n = 60) | p value |
|---|---|---|---|
| Age at onset, median (IQR), years | 44.0 (14.0) | 42.0 (24.5) | 0.505 |
| Age at enrollment, median (IQR), years | 46.5 (14.5) | 44.0 (24.0) | 0.446 |
| Gender, n (%) | 0.624 | ||
| Female | 6 (75.0) | 53 (88.3) | |
| Male | 2 (25.0) | 7 (11.7) | |
| Concomitant autoimmune diseases, n (%) | 1 (12.5) | 14 (23.3) | 0.810 |
| Initial core clinical symptoms, n (%) | 0.541 | ||
| Optic neuritis | 1 (12.5) | 17 (28.3) | |
| Acute myelitis | 7 (87.5) | 35 (58.3) | |
| APS | 0 (0) | 6 (10.0) | |
| Acute mesencephalic syndrome | 0 (0) | 1 (1.7) | |
| Acute brainstem syndrome | 0 (0) | 1 (1.7) | |
| Number of attacks before enrollment, median (IQR) | 1.0 (3.0) | 1.0 (1.5) | 0.517 |
| Period of onset at enrollment, n (%) | 0.847 | ||
| First attack phase | 3 (37.5) | 28 (46.7) | |
| Relapse phase | 5 (62.5) | 27 (45.0) | |
| Remission phase | 0 (0) | 5 (8.3) | |
| Spinal cord segment involved, n (%) | 0.096 | ||
| None | 0 (0) | 13 (21.7) | |
| STM | 2 (25.0) | 4 (6.7) | |
| LETM | 8 (75.0) | 43 (71.7) | |
| Therapy at remission MRI scan, n (%) | 0.005 | ||
| None ± steroids | 1 (12.5) | 5 (8.3) | |
| AZA ± steroids | 4 (50.0) | 9 (15.0) | |
| MMF ± steroids | 2 (25.0) | 13 (21.7) | |
| RTX ± steroids | 0 (0) | 32 (53.3) | |
| CTX ± steroids | 1 (12.5) | 1 (1.7) | |
| Follow-up duration, median (IQR), months | 58.8 (40.1) | 50.3 (40.2) | 0.148 |
- APS, area postrema syndrome; ASLs, asymptomatic spinal lesions; AZA, azathioprine; CTX, cyclophosphamide; IQR, interquartile range; LETM, longitudinally extensive transverse myelitis; MMF, mycophenolate mofetil; RTX, rituximab; STM, short transverse myelitis.
Cox proportional hazard analysis of determinants of subsequent relapses in AQP4-NMOSD patients
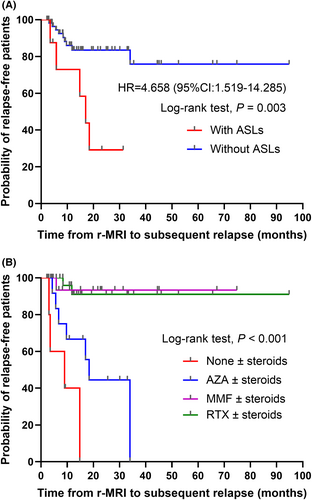
After a median follow-up of 13.3 (20.6) months among 68 AQP4-NMOSD patients, 14 experienced relapses. In patients with ASLs, the median time from remission scan to subsequent relapse was 15.9 months (range: 3.5–31.4 months). Kaplan–Meier curve analysis indicated a notable distinction in relapse risk between those with ASLs and those without (p = 0.003, log-rank test) (Fig. 2A). In Cox proportional hazard analysis, patients with ASLs displayed a significantly higher relapse risk compared to those without (HR = 4.658, 95% CI: 1.519–14.285, p = 0.007). Similarly, differing times to subsequent relapse were evident across various immunosuppressive agents (p < 0.001, log-rank test) (Fig. 2B). In contrast to individuals not using immunosuppressive agents, those using AZA exhibited a reduced but statistically nonsignificant relapse risk (HR = 0.320, 95% CI: 0.087–1.182, p = 0.087), whereas MMF (HR = 0.027, 95% CI: 0.003–0.260, p = 0.002) and RTX users (HR = 0.035, 95% CI: 0.006–0.203, p < 0.001) experienced a significantly lower relapse risk. Following adjustment for factors like the number of attacks before enrollment and immunosuppressive agents, the presence of ASLs exhibited no statistically significant impact on subsequent relapses (HR = 2.297, 95% CI: 0.652–8.085, p = 0.195). However, the number of attacks before enrollment exhibited a significant capacity to increase the risk of subsequent relapses (HR = 1.299, 95% CI: 1.019–1.657, p = 0.035) (Table 3).
| Variables | Unadjusted HR (95% CI) | p value | Adjusted HR (95% CI) | p value |
|---|---|---|---|---|
| Age at onset | 1.016 (0.981–1.052) | 0.366 | ||
| Female, n (%) | 0.784 (0.175–3.520) | 0.751 | ||
| Concomitant autoimmune diseases | 0.208 (0.027–1.599) | 0.131 | ||
| Initial core clinical symptoms, n (%) | ||||
| Optic neuritis | Ref | |||
| Acute myelitis | 5.666 (0.741–43.355) | 0.095 | ||
| APS | – | – | ||
| Acute mesencephalic syndrome | – | – | ||
| Acute brainstem syndrome | – | – | ||
| Number of attacks before enrollment | 1.158 (0.959–1.397) | 0.127 | 1.299 (1.019–1.657) | 0.035 |
| Period of onset at enrollment, n (%) | ||||
| Initial attack phase | Ref | |||
| Relapse phase | 0.941 (0.329–2.689) | 0.909 | ||
| Remission phase | – | – | ||
| Spinal cord segment involved | ||||
| None | – | – | ||
| STM | 0.885 (0.197–3.983) | 0.873 | ||
| LETM | Ref | |||
| Remission ASLs | 4.658 (1.519–14.285) | 0.007 | 2.297 (0.652–8.085) | 0.195 |
| Therapy at remission MRI scan | ||||
| None | Ref | Ref | ||
| AZA ± steroids | 0.320 (0.087–1.182) | 0.087 | 0.335 (0.082–1.366) | 0.127 |
| MMF ± steroids | 0.027 (0.003–0.260) | 0.002 | 0.027 (0.003–0.282) | 0.003 |
| RTX ± steroids | 0.035 (0.006–0.203) | <0.001 | 0.037 (0.005–0.275) | 0.001 |
- HR, hazard ratio; 95% CI, 95% confidence interval; APS, area postrema syndrome; ASLs, asymptomatic spinal lesions; AZA, azathioprine; LETM, longitudinally extensive transverse myelitis; MMF, mycophenolate mofetil; RTX, rituximab; STM, short transverse myelitis.
Management strategies and follow-up after ASL detection
Among 8 patients with ASLs, Case 1 presented an extension of a preexisting C2 lesion to C3–4 without enhancement, continued on prednisone, and relapsed after 3.5 months. In Cases 2 and 3, an enlarged preexisting lesion and a new thoracic segment lesion were observed, respectively, without enhanced scans. Acute phase strategies were not applied, but they persisted on AZA, later altering prednisone dosages for maintenance. Unfortunately, both experienced relapses over a year later. Enhanced ASLs were identified in Cases 4–8. Case 4 received only 40 mg of intravenous methylprednisolone (IVMP) and later switched to MMF, relapsing after 5.8 months. Cases 5 and 7 remained relapse-free with sequential MMF following methylprednisolone pulse therapy (MPPT). In Case 6, significant atrophy and enhancement of the initial spinal lesion transpired; she did not relapse following MMF up-dosing with prednisone. Case 8 was treated with CTX at the time of ASL detection. He continued to use CTX without receiving IVMP and solely used prednisone following the CTX course, relapsing 14.8 months after ASL detection (Fig. 3 and Table 4).
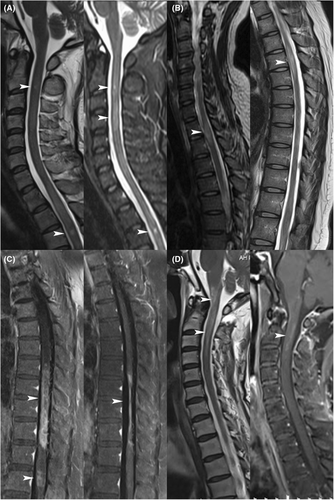
| Case No. | Therapy at remission MRI scan | Time since reference MRI, months | ASL location and features | Therapy after ASL detection | Outcomes |
|---|---|---|---|---|---|
| 1 | Prednisone 10 mg once daily | 8.8 | Extension of a preexisting C2 lesion to C3–4 (without enhancement) | Prednisone 10 mg once daily | T-segment myelitis relapse 3.5 months later |
| 2 | AZA 50 mg twice daily | 7.6 | Enlargement of previous C2–3 lesion (without enhanced MRI scan) | AZA 50 mg twice daily and prednisone 10 mg once daily | C-segment myelitis relapse 18.4 months later |
| 3 | AZA 50 mg twice daily and prednisone 10 mg once daily (irregularly) | 22.2 | New T5–6 lesion (without enhanced MRI scan) | AZA 50 mg twice daily and prednisone 30 mg once daily and tapered | C- and T-segment myelitis relapse 17 months later |
| 4 | AZA 50 mg twice daily and prednisone 5 mg once daily | 11 | C- and T-segmental spinal lesions with speckled enhancement | IVMP 40 mg; MMF 0.5 g twice daily and prednisone 5 mg once daily | Optic neuritis relapse 5.8 months |
| 5 | AZA 25 mg twice daily and prednisone 5 mg once daily | 19.2 | T1–6 lesion with a slight enhancement | IVMP 1000 mg; MMF 0.5 g twice daily and prednisone 60 mg once daily and tapered | No relapse |
| 6 | MMF 0.5 g twice daily and pred 5–10 mg once daily | 10.8 | T9–T12 spinal cord atrophy with lesion enhancement | MMF 0.75 g twice daily and prednisone 10 mg once daily | No relapse |
| 7 | MMF 0.75 g twice daily and prednisone 5 mg once daily | 53.3 | C- and T-segmental spinal lesions with C3–6 enhancement | IVMP 500 mg; MMF 0.75 g twice daily and prednisone 10 mg once daily | No relapse |
| 8 | CTX 0.6 g every month and prednisone 5 mg once daily | 6.1 | Medulla oblongata and upper C-segmental lesions with a slight enhancement | Prednisone 10 mg once daily following the CTX course | C-segment myelitis relapse 14.8 months later |
- ASL, asymptomatic spinal lesion; AZA, azathioprine; CTX, cyclophosphamide; IVMP, intravenous methylprednisolone; MMF, mycophenolate mofetil.
Discussion
In this retrospective cohort study conducted in a real-world setting, we found that ASLs may be observed among patients with AQP4-NMOSD, occurring in approximately 5.3% (14 out of 262) of cases. The presence of ASLs was found to be linked to the use of immunosuppressive agents, primarily observed in patients on conventional ones, whereas no instances of ASLs emerged among patients using RTX. Kaplan–Meier curve analysis indicated a significantly elevated risk of relapse in patients with ASLs compared to those without. However, subsequent analysis after adjusting for variables like the number of attacks before enrollment and immunosuppressive agents demonstrated that the presence of ASLs did not exhibit a statistically significant impact on subsequent relapses. This suggests that the primary determinant of ASLs and subsequent relapse is the choice of immunosuppressive agents.
ASLs refer to newly detected silent lesions or enlargement or sustained enhancement of pre-existing lesions on spinal MRI, even in the absence of new neurological deficits. In this context, we encompass enhanced lesions on remission spinal MRI 6 months or longer from the last episode as ASLs, as enhanced lesions frequently denote potential disease activity, even if clinical symptoms are lacking. The actual occurrence rate of ASLs in AQP4-NMOSD patients remains uncertain. Paolilo et al.4 monitored MRI findings of 16 pediatric AQP4-NMOSD patients, identifying ASLs in one case (6.3%), indicating a potentially higher incidence of ASLs. However, in a cohort of 220 AQP4-NMOSD cases, attack-associated silent lesions were mainly localized in the brain, with only three attack ASLs discovered among 331 spinal MRIs (0.9%); new ASLs during remission were identified in 1 of 116 patients (0.9%) and 2 of 200 spinal cord remission MRIs (1%).7 In the present study, we observed six ASLs in 6 patients (6 out of 173, 3.5%) out of 121 patients with 173 spinal MRIs during acute attacks, as well as eight ASLs in 8 patients (8 out of 89, 9%) among 68 patients with 89 spinal MRIs during remission. Nonetheless, the infrequency of regular MRI scans might lead to the oversight of certain ASLs. A preliminary analysis from the N-MOmentum trial (inebilizumab study) indicated that nearly 18% of NMOSD patients displayed ASLs on gadolinium contrast-enhanced MRI during inter-attack surveillance MRI.17 These findings, combined with prior research, suggest that the actual incidence of ASLs might be higher than initially perceived, albeit with reduced concern.
The occurrence of ASLs in NMOSD patients can be influenced by various factors, including the frequency of remission MRI scans, short-segmental spinal cord lesions, the type of immunosuppressants used, and non-standardized treatment approaches. As previously noted, systematic and rigorous surveillance tends to reveal asymptomatic lesions more frequently.17 Among asymptomatic lesions, ASL ranks as the second most prevalent type in NMOSD, trailing behind asymptomatic optic nerve lesions occurring during relapse-free periods and attacks, particularly in cases with short-segmental spinal cord lesions.17 Flanagan et al. documented three NMOSD patients displaying ASLs, all of whom exhibited enhanced cervical or thoracic cord lesions of a short segment.5 In our study, new or enlarged lesions were mostly short-segmented (Cases 1, 2, and 3), accounting for 37.5% of all ASLs, while the persistent enhancement of preexisting spinal lesions was not necessarily short-segmented, being mainly related to the length of the previous lesions. A study involving 165 individuals reported 27 MRIs (8%) from 24 patients (15%) displaying acute asymptomatic NMOSD-typical brain lesions; most asymptomatic brain lesions occurred before immunosuppressive therapy, with a few arising post-immunotherapies.9 The study also revealed the detection of five asymptomatic brain lesions from 5 patients during interferon treatment (for MS).9 These findings imply that appropriate and standardized immunosuppressive therapy may significantly diminish the likelihood of asymptomatic lesions. Our study identified a higher proportion of ASLs among AQP4-NMOSD patients who were either not employing immunosuppressants, using conventional agents like AZA or CTX, or irregularly taking such medications. Conversely, no ASLs were detected in patients using RTX, indicating that the utilization of more potent monoclonal antibody analogs notably mitigates the risk of ASLs. Additionally, irregular immunosuppressant usage emerged as a significant factor affecting ASLs. For instance, in Case 3, irregular AZA intake after 1 year of clinical stabilization led to the discovery of a thoracic lesion on repeat spinal MRI, despite the absence of new clinical symptoms. Similarly, Case 5, who only took AZA 25 mg twice daily after approximately 2.5 years of clinical stabilization, exhibited T1–6 spinal enhanced lesions during routine MRI reviews. This suggests that subtherapeutic dosages could contribute to ASL occurrence. Presently, no laboratory or radiological biomarkers exist to predict an inadequate response to immunosuppressive treatment.18 The potential of using ASLs as an imaging marker for evaluating subclinical disease activity and monitoring immunosuppressive treatment response merits further investigation.
Prior research has highlighted that AQP4-NMOSD patients may not remain “silent” in the presence of asymptomatic lesions during remission, which may have implications for future relapses.7 Recent findings from an NMOSD cohort study indicated that asymptomatic lesions during remission often anticipated imminent relapses, with the location of these lesions providing insight into subsequent relapse symptoms. Notably, patients with asymptomatic lesions experienced relapses in a median time of 5 months after the remission scans (with relapses occurring at 1, 2, 3, 5, 6, 6, and 7 months). In contrast, patients without asymptomatic lesions had a substantially longer median time of 85 months from remission scans to subsequent relapses. This suggests that the presence of asymptomatic lesions significantly elevates the risk of future relapses (HR = 21.23, 95% CI: 8.05–53.65).7 Consistently, our current study also demonstrated a markedly increased risk of relapse in patients with ASLs compared to those without (HR = 4.658, 95% CI: 1.519–14.285). This underscores the possibility that ASLs may signify an underlying inflammatory activity that could potentially evolve into a clinical relapse if not identified and addressed on time. However, the presence of ASLs did not exhibit a statistically significant impact on subsequent relapses in multivariate analyses. Another study also showed that the presence of new asymptomatic MRI lesions during the relapse-free period was not associated with a shorter time to develop subsequent relapses.10 We speculated that this might be related to treatments and immunosuppressant switches after ASL detection. Furthermore, previous investigations have indicated that a higher frequency of prior relapses is linked to an elevated risk of subsequent relapses.19-21 In alignment with this, our study also revealed that the number of attacks experienced before enrollment was significantly associated with a higher risk of subsequent relapses. In this context, considering ASLs as manifestations of underlying subclinical acute attacks could elucidate the notable increase in the risk of subsequent relapses.
Undoubtedly, the management of ASLs during acute attacks aligns with acute phase treatment strategies following primary symptomatic attacks. However, limited research exists on how to effectively manage ASLs during remission. In our investigation, we delved into the treatment of NMOSD patients after ASL discovery and its influence on subsequent relapses. Intriguingly, we observed that patients who received high-dose IVMP following ASL detection did not experience relapses. Conversely, those who did not undergo this therapy faced a heightened risk of subsequent relapses (as illustrated in Table 4). Additionally, the risk of relapse was notably diminished in patients on MMF in comparison to those on AZA. This risk reduction was further evident in patients who switched to MMF, a more potent immunosuppressant, as exemplified by Cases 5 and 7. Following ASL identification, Case 6 remained relapse-free after augmenting MMF to the high therapeutic dose. In contrast, Case 4, characterized by recurrent relapses, experienced a relapse shortly after switching to MMF at a lower dosage. In light of these findings, it appears reasonable to tailor ASL management in NMOSD patients based on acute phase therapy principles if enhanced MRI confirms that the ASL represents an active lesion (reflecting the blood–brain barrier destruction). Conversely, if an enhanced MRI indicates an inactive lesion (e.g., absence of enhancement), the ASL may not necessitate management according to acute phase therapy guidelines. Nonetheless, both scenarios might signal the inadequacy of the previous immunotherapy regimen in controlling the condition, warranting dose adjustments or medication switches.22 Flanagan et al.5 also documented three cases with ASLs. In the first two cases, short-segmental cervical enhanced lesions were observed during acute episodes of optic neuritis and APS, respectively. These lesions disappeared following MPPT treatment, as verified by subsequent MRIs conducted 2 months and 10 days later. In the third case, the patient was not treated with MPPT but started on AZA after the detection of short-segment thoracic enhanced lesions; unfortunately, the patient developed a symptomatic long-segmental cervical lesion after 2 months. These observations suggest that the post-ASL relapse occurrence might hinge on the subsequent management strategy. However, further longitudinal investigations involving larger sample sizes are imperative to validate these findings.
This study represents the first comprehensive analysis of ASL incidence, associated factors, and their influence on subsequent relapses, meanwhile offering preliminary insights into ASL management. Nevertheless, several limitations warrant consideration. First, being a retrospective cohort study, its findings should be approached with caution due to inherent biases and confounders. Second, the study predominantly examined spinal MRI data evolution across varying scanners and medical centers, each with distinct magnetic field strengths, parameters, and intervals, potentially introducing variability. Furthermore, due to irregular scans and inaccessible MRIs from external institutions, some ASL instances might have been overlooked. Third, our focus was solely on ASLs, without considering asymptomatic optic nerve or brain lesions, which could also impact subsequent relapses, an aspect we could not exclude. Last, the lack of a standardized ASL management strategy poses an additional consideration in subsequent relapses; however, due to the limited ASL cases, further subgroup analyses on its impact were unfeasible, allowing only preliminary observations from real-world clinical practice.
Conclusions
In summary, our findings underscore the relatively frequent occurrence of remission ASLs, primarily prevalent in AQP4-NMOSD patients using conventional immunosuppressive agents, thereby heightening the risk of subsequent relapses. Given the pronounced activity of NMOSD, assessing immunotherapy efficacy solely based on clinical data might inadequately capture disease activity and progression. The presence of ASLs may signify an underlying inflammatory activity due to insufficient immunotherapy. Hence, extensive multicenter longitudinal studies, incorporating regular follow-up spinal MRIs, are imperative to elucidate ASL incidence during follow-up, their implications for subsequent relapses and disability progression, and the formulation of ASL management strategies, including detecting potential subclinical disease activity and optimizing treatment shifts.
Author Contributions
CS and ZY: study design, patient selection, analysis and acquisition of data, and writing the manuscript; WX: study design, patient selection, and acquisition and interpretation of data; DJ: patient selection, analysis of imaging data, and revising the manuscript; XS: acquisition of data; CP: analysis of imaging data; LQ and XM: interpretation of data and revising the manuscript; TY and XQ: study design, interpretation of data, and revising the manuscript.
Acknowledgments
We thank Jingluan Tian and Xiaopei Ji of the Department of Neurology at First Affiliated Hospital of Soochow University for their invaluable assistance in conducting this study.
Funding Information
This study was supported by grants from the Natural Science Foundation of Jiangsu Province (BK20211075) and the Key Research and Development Plan of Jiangsu Province (BE2019666).
Conflict of Interest
The authors have no conflicts of interest to declare.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




