Association of plasma sPD-1 and sPD-L1 with disease status and future relapse in AQP4-IgG (+) NMOSD
Abstract
Objective
Neuromyelitis optica spectrum disorder (NMOSD) is an autoimmune-mediated disorder with aquaporin 4-immunoglobulin G (AQP4-IgG) in most settings. Soluble programmed death-1 (sPD-1) and soluble programmed death ligand 1 (sPD-L1) play key roles in immunomodulation. We aim to assess the association of sPD-1 and sPD-L1 with cytokines and their clinical significance in AQP4-IgG (+) NMOSD.
Method
We measured plasma sPD-1, sPD-L1, and 10 cytokines levels of 66 AQP4-IgG (+) NMOSD patients, including 40 patients in attack (attack-NMOSD) and 26 patients in remission (remission-NMOSD) phases, and 28 healthy controls through ultrasensitive Simoa and SP-X platform, respectively. We also performed >2 years (median) of follow-up after testing and analyzed the relationship between the detection index and current and future clinical parameters.
Result
Plasma sPD-1 level discriminated attack-NMOSD from remission-NMOSD (AUC = 0.692, p = 0.009). sPD-1 and sPD-L1 levels positively correlated with IL-6 (rsPD-1 = 0.313; rsPD-L1 = 0.508), IFN-γ (rsPD-1 = 0.331; rsPD-L1 = 0.456), and TNF-α (rsPD-1 = 0.451; rsPD-L1 = 0.531) expression, as well as clinical indicators, including the EDSS score (rsPD-1 = 0.331; rsPD-L1 = 0.402), number of attacks (rsPD-1 = 0.431) and segments of spinal cord involvement (rsPD-1 = 0.462; rsPD-L1 = 0.508). The risk of relapse within 2 years after sampling was associated with higher sPD-1/sPD-L1 ratio in attack-NMOSD (p = 0.022; Exp(B) = 1.589).
Interpretation
Plasma sPD-1 and sPD-L1 levels reflected current disease severity and activity, and predicted future relapses in AQP4-IgG (+) NMOSD, suggesting that they hold the potential to guide timely and targeted treatment.
Introduction
Neuromyelitis optica spectrum disorder (NMOSD) is an inflammatory demyelinating disorder in the central nervous system (CNS), associated with the presence of aquaporin 4-immunoglobulin G (AQP4-IgG) in most pathological settings.1 Recently, with the advancing understanding of the pathophysiology, the crosstalk of various immunological components, including T cells, B cells, autoantibodies, and cytokines, integrally constituting a complex immunoregulatory network, has been suggested to be involved in the development of NMOSD.2, 3 The indicators related to immune activation, such as IL-6 and complement, are considered as available therapeutic targets for NMOSD. Accordingly, satralizumab, a drug targeting IL-6, and eculizumab, a drug targeting complement, have been approved for the clinical treatment of NMOSD.4-6 However, except for AQP4-IgG, which has been included in the diagnostic criteria of NMOSD, current assays for cytokines, chemokines, complement, and autoantibodies do not provide a favorable indication of disease status, implying the necessity of investigating objective circulating biomarkers in this disorder.7, 8
Programmed death-1 (PD-1)/programmed death ligand 1(PD-L1) pathway has been identified to modulate the co-stimulatory process of immune cell activation, and its potential for prognostic prediction and as a therapeutic target has been extensively studied in a variety of tumor, infective diseases, and autoimmune disorders.9, 10 PD-1 polymorphism, resulting in alterations in its expression or function has been associated with susceptibility and severity to multiple autoimmune conditions, such as multiple sclerosis (MS), rheumatoid arthritis (RA), Type 1 diabetes mellitus (T1DM), and systemic lupus erythematosus (SLE).11-14 PD-1/PD-L1 blockade increased the incidence and severity of collagen II-induced arthritis, lupus-like glomerulonephritis, and experimental autoimmune encephalomyelitis (EAE), an effect associated with enhanced T-cell proliferation, increased production of Th1- and Th17-related cytokines and decreased generation of Treg-related cytokines.15-17
Both PD-1 and PD-L1 have their soluble forms (sPD-1 and sPD-L1) aside from membrane bound forms (mPD-1 and mPD-L1). It is generally accepted that sPD-1 and sPD-L1 are produced by proteolytic cleavage of membrane bound proteins or translation of alternatively spliced mRNAs.18 Nowadays, sPD-1/sPD-L1 have also been linked to autoimmune diseases.19 Abnormal expressions of sPD-1 and sPD-L1 were found in the serum or synovial fluid of patients with RA, SLE, and systemic sclerosis, and were associated with levels of diverse immunological elements (complement component 3 and 4, rheumatoid factor titer), as well as disease severity and activity.20-22 Alternatively, Xue et al. revealed that patients with first-attack NMOSD had obviously enhanced serum levels of sPD-1 and sPD-L1 compared to those with longitudinally extensive transverse myelitis and optic neuritis and healthy control, suggesting that sPD-1 and sPD-L1 may be as valuable biomarkers for early-stage NMOSD diagnosis.23 Notably, there are no significant challenges in differential diagnosis of AQP4-IgG (+) NMOSD with the utilization of the current diagnostic criteria, but there is still widespread controversy as to whether AQP4-IgG is a good indicator of the disease severity and activity.8 Accurate identification of disease attack and prediction of relapse are essential for timely treatment to mitigate the relapse-related neurological disability accumulation. Considering the close relationship between sPD-1 and sPD-L1 and immunoregulation, studies on the association of sPD-1 and sPD-L1 with cytokines and disease states, as well as whether they serve as a reliable indicator of relapse in AQP4-IgG (+) NMOSD have not been reported. Therefore, in the present study, we tested the plasma levels of sPD-1, sPD-L1 and 10 cytokines for patients with AQP4-IgG (+) NMOSD in the attack and remission phase, and followed up the future relapses after testing to evaluate the clinical relevance and predictive value of these indicators in a prospective cohort study.
Methods
Participants
A total of 66 AQP4-IgG-seropositive patients who fulfilled with the 2015 International Consensus Diagnostic Criteria for NMOSD were recruited between the year of 2020–2022 in the department of Neurology, the Second Hospital of Hebei Medical University7; 40 patients were in the attack phase (attack-NMOSD) and 26 patients were in the remission phase (remission-NMOSD). An attack was defined as the new, recurring, or worsening neurological symptoms taking place for at least 24 h in the absence of fever or infection.2 The attack phases were defined as periods within 1 month of attack and the remission phases were defined as periods at least 1 month after the attack.2 Attack-NMOSD patients had not received any attack-therapy (high-dose intravenous methylprednisolone, plasma exchange, etc.) or attack-prevention treatment (hormones, immunosuppressants, disease-modifying therapy, etc.) within 3 months prior to sampling. AQP4-IgG and myelin oligodendrocyte glycoprotein (MOG)-IgG testing was performed by using a cell-based assay (CBA), and all patients included were seronegative for MOG-IgG. A flowchart of patient inclusion and exclusion are detailed in Fig. S1. Meanwhile, 28 age- and sex-matched healthy controls (HC) were enrolled from the Medical Examination Center, the Second Hospital of Hebei Medical University with no history of malignant diseases, systemic diseases, metabolic syndromes, central nervous system disorders and immunological/infectious illnesses, and no history of administration of hormones, immunosuppressants, and other medications within 3 months prior to sampling.
Patient characteristics
Demographic, clinical, laboratory, and medication data were collected. Medical records of attacks (date and sites), attack-prevention treatments, the Expanded Disability Status Scale (EDSS) score at sampling, autoimmune comorbidities or representative autoimmune antibodies tested at sampling, as well as inflammatory-related blood and cerebrospinal fluid (CSF) findings were reviewed. Neurological involvement was categorized into optic nerve (optic neuritis), spinal cord (myelitis), and brain (brain syndromes, including area postrema syndromes, brainstem syndromes, diencephalic syndromes, and cerebral syndromes), evaluated by clinical manifestations, as well as visual evoked potentials (VEP), optical coherence tomography (OCT) or magnetic resonance images (MRI). Optic nerve with gadolinium contrast on fat-suppressed T1-weighted imaging in the attack phase were regarded as acute ON lesions. The longitudinal length of the ON lesion was semiquantitatively represented by the number of ON-involved sub-segments on optic MRI in the following six areas of the optic nerves: anterior orbital, posterior orbital, canalicular, intracranial, chiasmal, and optic tract, which scored between 0 and 6.24 The interpretations of the optic MRI images were conducted separately by neurologists (J.L. and Z.J.) with reference to the reports of the neuroradiologist (who was blinded to the clinical information when analyzing the MRI images). If the optic nerves are involved bilaterally, the lesion segment score used for correlation analysis with levels of sPD-1, sPD-L1, and cytokines was defined as the sum of the score of each optic nerve. Patients were further categorized into two groups; mild disability: EDSS < 3 and severe disability: EDSS ≥ 3, according to the median EDSS score of NMOSD cohort in this study. Notably, we subsequently conducted prospective follow-up for patients with NMOSD after sampling and collected clinical information, including whether they experienced a new relapse (confirmed by a specialized neurologist), date of relapse, as well as attack-prevention treatment conditions.
Sample collection and plasma sPD-1, sPD-L1, and cytokines detection
A total of 5 mL venous blood samples were collected from NMOSD patients and HC by using ethylenediaminetetraacetic acid (EDTA)-containing tubes. The plasma was separated from blood by centrifugation at 1000 g for 10 min at 4°C. The plasma obtained was then dispensed and stored at −80°C until assayed.
Plasma sPD-1 (soluble PD-1 Discovery Kit, Quanterix, MA, US, Cat No:102929) and sPD-L1 (soluble PD-L1 Discovery Kit, Quanterix, Cat No:102648) levels were detected on the ultra-sensitive Single-molecule Array (Simoa) HD-X platform (GBIO, Hangzhou, China). Ten plasma cytokines including interleukin (IL)-1β, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12p70, IL-22, interferon (IFN)-γ, tumor necrosis factor (TNF)-α (CorPlex Human Cytokine 10-Plex panel 1 Assay Kit, Quanterix, Cat No:85-0329) were tested on the SP-X platform (GBIO). All assays were conducted following manufacturer's instruction. Plasma samples were diluted at a 1:4 ratio. Calibrators and quality controls were measured in duplicate. Operators were blinded to participants' disease status and clinical information. Levels below the lower limit of detection (LOD) were set at the midpoint between zero and the LOD.
Statistical analysis
Steel-Dwass multiple comparison was used to compare among three groups. Mann–Whitney U test was used for the comparison between two groups, and Spearman correlation was used for the correlation analysis between indexes. Wilcoxon matched-pairs signed rank test was used to compare sPD-1, sPD-L1, and cytokine levels from attack-remission paired samples. All categorical data was tested with the Pearson chi-quared test. We used receiver operating characteristic (ROC) curves to evaluate discriminatory power of sPD-1, sPD-L1, and cytokines levels between different disease status. The Youden index (sensitivity + specificity – 1) was calculated to determine the cutoff value. Binary Logistic Regression was used to identify the predictors that influence the binary outcome. Kaplan–Meier curves was used to analyze “time-to-relapse” data. A threshold of p < 0.05 was considered as statistically significant. The statistical analysis was performed using IBM SPSS Statistics 26.0 Software (IBM Japan), JMP13.2.0 software (SAS Institute Japan), and GraphPad Prism7 software (MDF Co. Ltd., Tokyo, Japan).
Protocol approvals and patient consents
The study was approved by the Ethics Committee of the Second Hospital of Hebei Medical University (Approval No. 2021-R513), and written informed consent was gained from each subject or his/her legal guardian.
Results
Demographic and clinical characteristics of the participants
The demographic and clinical features, laboratory findings, and treatment data were summarized at Tables 1 and 2. There was no significant difference in sex distribution (p > 0.05), or age at sampling (p > 0.05) among attack-NMOSD, remission-NMOSD, and HC. Patients with attack-NMOSD had comparable EDSS score, number of attacks, and site of onset to those with remission-NMOSD, whereas attack-NMOSD group possessed significantly shorter time elapse from the latest attack to sampling than the remission-NMOSD group (p < 0.001). Besides, 15 (22 eyes) of 40 patients with attack-NMOSD suffered from optic neuritis, of which 7 patients (10 eyes) had complete optic MRI recordings. The median (range) of optic nerve lesion segments score was 2 (0–3). Regarding the laboratory results, in addition to attack-NMOSD group having lower levels of Complement (C)-3 than the remission-NMOSD group (p = 0.045), the concentrations of inflammation-related indicators, including the white blood cell/lymphocyte count, C4, C-reactive protein (CRP), and erythrocyte sedimentation rate (ESR), and autoimmune-related parameters, including the antinuclear antibody (ANA), SSA antibody, double stranded DNA (dsDNA) antibody, and anti-cardiolipin antibodies (ACLA) were not statistical difference between two groups (p > 0.05). In terms of medication at sampling, a total of 16 (61.5%) patients in remission received preventive treatment, and 14 (53.8%) patients underwent rituximab therapy.
| Groups | Total NMOSD (n = 66) | Attack-NMOSD (n = 40) | Remission-NMOSD (n = 26) | HC (n = 28) | P1-value | P2-value |
|---|---|---|---|---|---|---|
| Demographic data | ||||||
| Male: Female | 7:59 | 3:37 | 4:22 | 7:21 | 0.136b | 0.309b |
| Age at sampling, yearsa | 45.0 (32.3–55.0) | 44.5 (32.0–54.3) | 49.0 (35.5–55.0) | 44.5 (41.8–49.8) | 0.801d | 0.563c |
| Disease duration, monthsa | 27.7 (6.5–69.4) | 21.0 (1.0–60.4) | 34.7 (8.2–109.9) | n/a | n/a | 0.121c |
| Clinical parameters | ||||||
| Site of onset | ||||||
| ON, n (%) | 33 (50.0) | 19 (47.5) | 14 (53.8) | |||
| Brain, n (%) | 12 (18.2) | 7 (17.5) | 5 (19.2) | n/a | n/a | 0.549b |
| Spinal cord, n (%) | 24 (36.4) | 14 (35.0) | 10 (38.5) | |||
| Unknown, n (%) | 3 (4.5) | 3 (7.5) | 0 (0) | |||
| Site of sampling | ||||||
| ON, n (%) | 15 (37.5) | |||||
| Brain, n (%) | n/a | 6 (15.0) | n/a | n/a | n/a | n/a |
| Spinal cord, n (%) | 25 (62.5) | |||||
| EDSS scorea | 3.0 (2.0–4.4) | 4.0 (2.0–6.0) | 2.8 (2.0–4.0) | n/a | n/a | 0.071c |
| Number of attacksa | 2.0 (1.0–3.0) | 2.0 (1.0–3.0) | 2.0 (1.0–3.8) | n/a | n/a | 0.812c |
| Time elapse from the latest attack to sampling, monthsa | 0.9 (0.2–3.5) | 0.3 (0.2–0.6) | 6.4 (3.2–17.1) | n/a | n/a | <0.001c |
| Medication at sampling | ||||||
| Preventive treatment, n (%) | 16 (24.2) | 0 (0%) | 16 (61.5) | n/a | n/a | n/a |
| RTX, n (%) | 14 (21.2) | 0 (0%) | 14 (53.8) | n/a | n/a | n/a |
| PSL, n (%) | 8 (12.1) | 0 (0%) | 8 (30.8) | n/a | n/a | n/a |
| MMF, n (%) | 1 (1.5) | 0 (0%) | 1 (3.9) | n/a | n/a | n/a |
- EDSS, Expanded Disability Status Scale; HC, healthy control; MMF, mycophenolate mofetil; NMOSD, Neuromyelitis Optica Spectrum Disorders; n/a, not available; ON, optic nerve; PSL, prednisolone; P1, comparisons were made among patients with attack-NMOSD, remission-NMOSD and HC; P2, comparisons were made between patients with attack-NMOSD and remission-NMOSD; RTX, rituximab.
- a Median (Q1, Q3);
- b Pearson chi-squared test;
- c Mann–Whitney U test;
- d Steel-Dwass test.
| Groups | Attack-NMOSD | Remission-NMOSD | p-value |
|---|---|---|---|
| White blood cell counta (^109/L) (40 vs. 26) | 6.0 (4.7–7.4) | 6.1 (4.9–8.5) | 0.576c |
| Lymphocyte counta (^109/L) (40 vs. 26) | 1.5 (1.0–2.4) | 1.4 (1.0–1.8) | 0.677c |
| Lymphocyte percentage, % (40 vs. 26) | 29.1 (18.1–36.2) | 22.4 (16.9–30.9) | 0.125c |
| C3a, g/L (26 vs. 6) | 0.8 (0.8–0.9) | 1.1 (0.9–1.1) | 0.045c |
| C4a, g/L (26 vs. 6) | 0.21 (0.16–0.24) | 0.20 (0.17–0.22) | 0.981c |
| CRPa, mg/L (27 vs. 6) | 1.8 (1.6–3.3) | 2.7 (1.8–3.3) | 0.852c |
| hs-CRPa, mg/L (37 vs. 16) | 1.3 (0.6–2.3) | 1.9 (1.1–3.6) | 0.181c |
| ESRa, mm/h (15 vs. 6) | 8.0 (5.5–10.0) | 7.0 (2.8–14.3) | 0.814c |
| Cells in CSFa, 106/L (12 vs. 3) | 88.5 (50.8–422.0) | 18.0 (13.0–41.0) | 0.194c |
| Proteins in CSFa, mg/dL (12 vs. 3) | 0.19 (0.12–0.22) | 0.24 (0.15–0.25) | 0.469c |
| Autoimmunity diseases, n (%) (40 vs. 26) | 8 (20.0) | 1 (3.9) | 0.062b |
| ANA antibody, n (%) (33 vs. 18) | 12 (36.4) | 3 (16.7) | 0.140b |
| SSA antibody, n (%) (34 vs. 18) | 8 (23.5) | 6 (33.3) | 0.448b |
| dsDNA antibodya, IU/mL (14 vs. 4) | 4.9 (1.4–17.7) | 20.9 (7.8–21.7) | 0.184c |
| ACLAa, RU/mL (11 vs. 3) | 2.8 (1.6–6.7) | 1.6 (1.0–n/a) | 0.533c |
- ACLA, anti-cardiolipin antibodies; ANA, antinuclear antibody; dsDNA, double stranded DNA; CRP, C-reactive protein; CSF, cerebrospinal fluid; C3, Complement 3; C4, Complement 4; ESR, erythrocyte sedimentation rate; hs-CRP, high-sensitivity C-reactive protein; NMOSD, neuromyelitis optica spectrum disorders.
- a Median (Q1, Q3);
- b Pearson chi-squared test;
- c Mann–Whitney U test.
Levels of sPD-1, sPD-L1, and cytokines in AQP4-IgG (+) NMOSD
The comparisons of concentrations of sPD-1, sPD-L1, and cytokines between NMOSD patients and HC were shown in Fig. 1 and Table S1. The attack-NMOSD group had significantly elevated sPD-1 level and sPD-1/sPD-L1 ratio than the remission-NMOSD group (psPD-1 = 0.024, pratio = 0.048) and HC (psPD-1 < 0.001, pratio < 0.001). The attack-NMOSD group also possessed higher concentrations of IL-6 (p = 0.029), IL-10 (p = 0.006) and IFN-γ (p = 0.037), compared to the HC. Besides, increased expression of IL-6 was observed in remission-NMOSD group, compared to that in HC (p = 0.015). Attack-remission paired plasma samples were collected from 10 NMOSD patients to observe the dynamic change of these indicators in a single case. And we found a significant increase in sPD-1 level (p = 0.037) and sPD-1/sPD-L1 ratio (p = 0.049), and an obvious decrease in the level of IL-10 (p = 0.048) during the attack phase than the remission phase (Fig. 1, Table S2). Notably, (1) sPD-1 level and sPD-1/sPD-L1 ratios were elevated in two patients (Patient 2 and Patient 7) and three patients (Patient 2, Patient 5, and Patient 7), respectively during the remission phase than the attack phase; 2) IL-10 level was increased in three patients (Patient 5, Patient 7, and Patient 8) during the attack phase than the remission phase. The detail clinical characteristics of each patient with attack-remission paired samples (Patient 1 to Patient 10) were listed in Table S3.
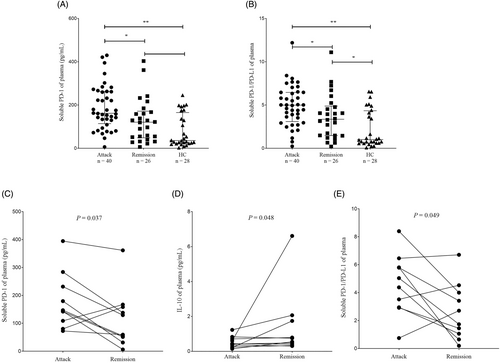
Since the cytokines detected were not significantly different in groups of attack-NMOSD and Remission-NMOSD, we further evaluated the discriminatory power of sPD-1 level and sPD-1/sPD-L1 ratio between the attack-NMOSD and remission-NMOSD with employment of ROC curve. The ROC analysis demonstrated that both sPD-1 level (AUC = 0.692, p = 0.009, cutoff = 129.50 pg/mL) and sPD-1/sPD-L1 ratio (AUC = 0.66, p = 0.028, cutoff = 4.09) could discriminate attack-NMOSD from remission-NMOSD, which yielded a sensitivity of 75.0% and 65.0% and a specificity of 61.5% and 69.2%, respectively (Fig. 2A).

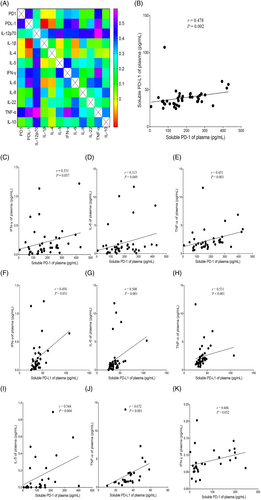
Correlation matrices of sPD-1, sPD-L1, and cytokines in AQP4-IgG (+) NMOSD
The findings of correlation analysis of sPD-1, sPD-L1, and cytokines were shown in Fig. 3. In attack-NMOSD group, level of sPD-1 positively correlated with that of sPD-L1 (p = 0.002, r = 0.478), whereas this was not observed in either the remission-NMOSD group or the HC group. In attack-NMOSD group, levels of sPD-1 and sPD-L1 positively correlated with those of IL-6 (psPD-1 = 0.049, rsPD-1 = 0.313; psPD-L1 = 0.001, rsPD-L1 = 0.508), IFN-γ (psPD-1 = 0.037, rsPD-1 = 0.331; psPD-L1 = 0.031, rsPD-L1 = 0.456), and TNF-α (psPD-1 = 0.003, rsPD-1 = 0.451; psPD-L1 < 0.001, rsPD-L1 = 0.531). In remission-NMOSD group, levels of sPD-1 and sPD-L1 positively correlated with those of IL-5 (p = 0.004, r = 0.544) and TNF-α (p < 0.001, r = 0.672), respectively. In HC, level of sPD-1 positively correlated with that of IFN-γ (p = 0.032, r = 0.406).
Clinical relevance of sPD-1 and sPD-L1 in AQP4-IgG (+) NMOSD
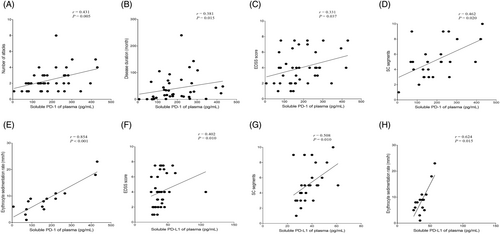
In attack-NMOSD group, level of sPD-1 positively correlated with the EDSS score (p = 0.037, r = 0.331), number of attacks (p = 0.005, r = 0.431), disease duration (p = 0.015, r = 0.381), segments of spinal cord involvement judged by the MRI analysis (p = 0.020, r = 0.462), and ESR (p < 0.001, r = 0.854); level of sPD-L1 positively correlated with the EDSS score (p = 0.010, r = 0.402), segments of spinal cord involvement (p = 0.010, r = 0.508), and ESR (p = 0.015, r = 0.624) (Fig. 4). Alternatively, TNF-α level positively correlated with the EDSS score (p = 0.022, r = 0.361), number of attacks (p = 0.006, r = 0.427), disease duration (p = 0.001, r = 0.490) and segments of spinal cord involvement (p = 0.018, r = 0.470); level of IL-6 positively correlated with that of CRP (p = 0.010, r = 0.485) and hsCRP (p = 0.004, r = 0.467); level of IL-4 positively correlated that of dsDNA antibody (p = 0.038, r = 0.558). The median (IQR) of time elapse from the previous attack to the present attack in attack-NMOSD group is 16.7 (10.5–45.5) months, and IL-10 level negatively correlated with this period (p = 0.043, r = 0.400) (Fig. S2). There was no correlation between sPD-1, sPD-L1, and cytokines levels and optic neuritis lesion length evaluated by MRI in attack-NMOSD [n = 7; optic neuritis lesion length vs. sPD-1(p = 0.350), sPD-L1(p = 0.899), IL-1β (p = 0.156), IL-4 (p = 0.898), IL-5 (p = 0.799), IL-6 (p = 0.606), IL-8 (p = 0.799), IL-10 (p = 0.109), IL-12p70 (p = 0.602), IL-22 (p = 0.213), IFN-γ (p = 0.350), TNF-α (p = 0.350)]. In addition, we assessed the correlation between the inflammatory markers (WBC/lymphocyte count, CRP, hsCRP, ESR) levels and disease status parameters in attack-NMOSD, which showed that hsCRP level negatively correlated with the time elapse from sampling to the latest attack (p = 0.014, r = 0.683); ESR level positively correlated with the number of attacks (p = 0.001, r = 0.755). In group of remission-NMOSD, TNF-α level negatively correlated with the time elapse from the latest attack to sampling (p = 0.018, r = 0.462).
Patients with attack-NMOSD were divided into two groups (severe disability vs. mild disability) according to EDSS score, levels of sPD-1 (p = 0.025, AUC = 0.721, sensitivity = 66.7%, specificity = 76.7%, cutoff = 159.78 pg/mL), sPD-L1 (p = 0.011, AUC = 0.752, sensitivity = 77.8%, specificity = 69.2%, cutoff = 34.22 pg/mL) and TNF-α (p = 0.015, AUC = 0.741, sensitivity = 85.2%, specificity = 69.2%, cutoff = 1.49 pg/mL), as well as the combinations of the above three indices (p = 0.002, AUC = 0.809, sensitivity = 77.8%, specificity = 84.6%) were able to distinguish between severe disability and mild disability, indicating the combination of sPD-1, sPD-L1, and TNF-α was superior to TNF-α alone in discriminating between severe and mild disability (Fig. 2B). Moreover, an increased sPD-1 level was identified to be a predictor for severe disability in attack-NMOSD (p = 0.044; Exp(B) = 1.009, 95% confidence interval (95% CI): 1.000–1.018), while levels of cytokines or inflammatory markers (WBC/lymphocyte count, CRP, hsCRP, ESR) did not have this predictive value (p > 0.05). Besides, attack-NMOSD patients with first attack had lower concentration of TNF-α (p = 0.038) and a trend toward lower level of sPD-1 (p = 0.082) than those with multiple attacks.
Predictive value of sPD-1 and sPD-L1 for future relapses of AQP4-IgG (+) NMOSD patients
We further performed follow-up for NMOSD patients after testing and the detailed information were listed in Table 3. There were 38 (95%) patients in attack-NMOSD group and 26 (100%) patients in remission-NMOSD group received this clinical follow-up with the median follow-up duration of 28.1 months (attack-NMOSD) and 26.7 months (remission-NMOSD), respectively. Thirteen patients (34.2%) in attack-NMOSD and five (19.2%) patients in remission-NMOSD suffered from a relapse during follow-up period, and patients in remission-NMOSD group had shorter time elapse from sampling to relapse than those in attack-NMOSD group (p = 0.038).
| Groups | Attack-NMOSD | Remission-NMOSD | p-value |
|---|---|---|---|
| Follow-up cases after sampling, n (%) | 38 (95) | 26 (100) | 0.247b |
| Follow-up duration after sampling, monthsa (38 vs. 26) | 28.1 (25.1–34.4) | 26.7 (22.5–34.0) | 0.104c |
| Attack after sampling, n (%) (38 vs. 26) | 13 (34.2) | 5 (19.2) | 0.191b |
| Time from sampling to the latest attack, monthsa (38 vs. 26) | 9.3 (7.7–19.7) | 4.9 (3.2–7.9) | 0.038c |
| Attack within 1 year after sampling, n (%) (38 vs. 26) | 7 (18.4) | 5 (19.2) | 0.935b |
| Attack within 2 year after sampling, n (%) (35 vs. 17) | 13 (37.1) | 5 (29.4) | 0.583b |
| Preventive treatment after sampling, n (%) (38 vs. 26) | 29 (76.3) | 19 (73.1) | 0.769b |
| RTX, n (%) (29 vs. 19) | 18 (62.1) | 15 (78.9) | 0.217b |
| MMF, n (%) (29 vs. 19) | 11 (37.9) | 4 (21.1) | 0.217b |
- MMF, mycophenolate mofetil; NMOSD, neuromyelitis optica spectrum disorders; RTX, rituximab.
- a Median (Q1, Q3);
- b Pearson chi-squared test;
- c Mann–Whitney U test.
In the whole NMOSD cohort, (1) patients with relapse within 2 years after sampling had higher sPD-1 level (p = 0.021) and sPD-1/sPD-L1 ratio (p = 0.008) than those without; (2) the risk of relapses within 2 years after sampling was associated with an increased sPD-1/sPD-L1 ratio (p = 0.019; Exp(B) = 1.357, 95% CI: 1.050–1.752). Based on the cutoff value of sPD-1/sPD-L1 ratio, patients were divided into two groups: high group (sPD-1/sPD-L1 ≥ 4.09) and low group (sPD-1/sPD-L1 < 4.09). Patients in high group had shorter time elapse from sampling to relapse than those in low group (p = 0.026, Fig. 5A). Cytokines or inflammatory markers (WBC/ lymphocyte count, CRP, hsCRP, ESR) detected in this study were not predictive of future relapses.
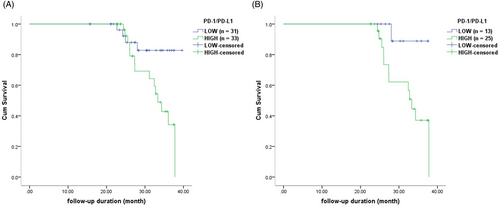
In attack-NMOSD group, we observed similar results: (1) patients with relapse within 2 years after sampling had higher sPD-1 level (p = 0.038), sPD-1/sPD-L1 ratio (p = 0.006) and IL-22 level (p = 0.048) than those without; (2) an increased sPD-1/sPD-L1 ratio was identified to be a predictor for relapse within 2 years after sampling (p = 0.022; Exp(B) = 1.589, 95% CI: 1.070–2.360); however, 10 cytokines and inflammatory markers (WBC/ lymphocyte count, CRP, hsCRP, ESR) were not predictive of future relapses; (3) patients in HIGH group had shorter time elapse from sampling to relapse than those in LOW group (p = 0.029, Fig. 5B). There was no indicator tested in this study that predicted relapse after sampling in remission-NMOSD group.
Discussion
In this study, we investigated the association of plasma sPD-1 and sPD-L1 levels with disease status, future relapse, as well as cytokines levels with application of ultrasensitive Simoa technology in AQP4-IgG (+) NMOSD. The results demonstrated that (1) sPD-1 level and sPD-1/sPD-L1 ratio could differentiate attack-NMOSD from remission-NMOSD; (2) levels of sPD-1 and sPD-L1 were associated with parameters indicating current diseases severity and activity; an increased sPD-1 level was identified to be a predictor for severe disability in attack-NMOSD; (3) higher sPD-1/sPD-L1 ratio was identified to be a predictor for relapse within 2 years after sampling; (4) sPD-1 and sPD-L1 levels correlated with expressions of various pro-inflammatory cytokines including IL-6, IFN-γ, and TNF-α, suggesting that their close association with pro-inflammatory profiles and superior evaluative and predictive value in clinical settings of AQP4-IgG (+) NMOSD.
Most previous efforts to address the immunoregulatory functions and mechanisms of PD-1/PD-L1 pathway have mainly focused on mPD-1/mPD-L1. mPD-1, belonging to the CD28 family is mainly expressed on T lymphocytes, B lymphocytes, and other immune cells. mPD-L1, belonging to the B7 family is the ligands of mPD-1 and mainly expressed on antigen-presenting cells (APCs) including B cells, dendritic cells, monocytes, and macrophages, and parenchymal tissues including vascular endothelial cells. The mPD-1/mPD-L1 pathway has been found to provide negative regulatory signals that effectively inhibit T-cell proliferation and function, thereby becoming an important target in tumor therapy. However, in recent years, a more readily measurable form, sPD-1/sPD-L1, has received extensive attention from researchers in the context of oncology, infectious diseases, and especially autoimmune disorders.19, 25 sPD-1, with an IgV-type extracellular domain of membrane bound form is encoded by PD-1Dex3 (lacks only exon 3) and reported to manifest independently functional antagonism, thereby inhibiting the immunomodulatory effects of mPD-1/ mPD-L1 and leading to aberrant T-cell activation and proliferation.25, 26 sPD-L1 is mainly produced by two mechanisms: (1) protease cleavage of the membrane-bound form; (2) alternative splicing of the PD-L1 pre-mRNA to produce an mRNA which then produces a soluble isoform lacking the transmembrane domain.19 Accordingly, there is no unique sPD-L1 species, but rather a pool of circulating proteins. Therefore, whether sPD-L1 exerts an immunosuppressive or immunopromotional function remains controversial. Recent series of studies suggested that the function of sPD-L1 is dependent on its specific conformation (dimerization), as well as the affinity for binding to mPD-1 (the suppressive capacity of mPD-1/mPD-L1 pathway could be inhibited with enhanced affinity of sPD-L1 and mPD-1 interaction).27, 28 In this study, we found that sPD-1 and sPD-L1 levels were positively correlated with each other, and both sPD-1 and sPD-L1 levels were positively correlated with pro-inflammatory cytokines (IL-6, IFN-γ, TNF-α) levels in attack-NMOSD group, suggesting that there is a dynamic equilibrium between sPD-1 and sPD-L1, and they may also reflect the cumulative burden of peripheral inflammation in AQP4-IgG (+) NMOSD in the attack phase. Meanwhile, previous studies have demonstrated that some cytokines, such as IL-6, IFN-γ, IL-7, IL-12, IL-21, and TNF-α, could regulate the expression of mPD-1/mPD-L1,18 which may also contribute to the correlation between levels of sPD-1 and sPD-L1 and levels of cytokines above. Moreover, we revealed that sPD-1 level and sPD-1/sPD-L1 ratio, but not levels of cytokines or other inflammatory markers (e.g., CRP, ESR) were significantly higher in patients with attack-NMOSD than those with remission-NMOSD and HC, and sPD-1 level and sPD-1/sPD-L1 ratio could differentiate attack-NMOSD from remission-NMOSD, which was similar with findings in RA, SLE, and antineutrophil cytoplasmic antibody-associated vasculitis.20, 21, 29 More importantly, these results indicated that compared to other inflammatory indicators, sPD-1 level and sPD-1/sPD-L1 ratio may provide a favorable differential diagnosis between the attack phase and remission phase in AQP4-IgG (+) NMOSD, which demonstrated that they may serve as potential indicator for the assessment of disease status and direct timely interventions. To be noted, levels of sPD-1 and sPD-L1 positively correlated with indicators indicating the current disease activity and severity (EDSS score, number of attacks, and segments of spinal cord involvement), and sPD-1 level and sPD-1/sPD-L1 ratio (but not levels of sPD-L1 or cytokines or inflammatory markers) could predict the attack severity and future relapses. These findings suggested that sPD-1 and sPD-1/sPD-L1 ratio are better predictors of disease activity & severity and future relapses compared to cytokines or inflammatory parameters tested in this study. They also confirmed the pro-inflammatory effects of sPD-1 and implied the possibly immunosuppressive effect of sPD-L1 in AQP4-IgG (+) NMOSD, and higher sPD-1 level and sPD-1/sPD-L1 ratio illustrated the immune balance is tilted in a pro-inflammatory direction. Since the pathogenesis and progression of NMOSD is closely related to the abnormal activation of immune cells and the release of large quantities of autoantibodies and cytokines, an even more pronounced pro-inflammatory state may further lead to severe and frequent relapses in this disorder.
In the present study, we found that the patients with attack-NMOSD had a significantly reduced level of C3 than those with remission-NMOSD, suggesting a potential role for the complement system in regulating or reflecting the disease states of AQP4-IgG (+) NMOSD. The pathogenic effects of AQP4-IgG binding to astrocytes are primarily thought to activate complement-dependent cytotoxicity via the classical pathway by binding to C1q, which is followed by C3 convertase enzymes converting C3 into C3a and C3b.30, 31 This process will cause subtle consumption of C3, and multiple studies have found that an obvious declined serum/plasma level of C3 in patients with NMOSD, especially AQP4-IgG (+) NMOSD than healthy controls.32, 33 In the attack phase, AQP4-IgG initiates a more severe attack on astrocytes, leading to the activation of the complement system and a massive depletion of C3, which may explain why patients in the acute phase have significantly lower levels of C3 compared to the remission phase. However, plasma C4 levels did not differ significantly between patients in the attack and remission phases in this study, similar to the results of a related study in SLE, which showed that C3 level was associated with disease activity and lupus nephritis, but C4 level was not.34 We proposed that the difference between C3 and C4 is due to the central role of C3 in the complement cascade and the fact that complement components or complement-activating molecules are released and play different roles in autoimmune diseases. Although IL-10 was commonly reported as an anti-inflammatory cytokine, our results supported some previous reports that a higher level of IL-10 was found in NMOSD than HC.35, 36 It is well-established that IL-10 was mainly secreted by regulatory B cells and T cells, and Cho et al reported that the IL-10-producing regulatory B cells and regulatory T-cell subsets were also significantly increased in NMOSD at attack than HC.37 These may be due to the compensatory elevation of anti-inflammatory cells and factors to maintain immune homeostasis in an acute and severe inflammatory setting.
Recently, it is well-established that optic nerve lesion length measured at the acute phase of ON was associated with retinal neuro-axonal loss and visual impairment at a chronic stage, suggesting that it was an important predictor of the visual prognosis and help to stratify future therapeutic strategies.24, 38, 39 Among sequences to detect optic nerve lesion with MRI, 3D double-inversion recovery (3D-DIR) sequence is one of the most sensitive with good intraobserver and interobserver agreement for optic nerve DIR hypersignal length measurement.40 Unfortunately, patients in this study had not yet undergone MRI of the brain or optic nerve that included 3D-DIR sequences, which prevented us from directly and precisely measuring the lesion length of optic nerve using the methods described so far.40 Alternatively, we approximated the length of optic nerve lesion by utilizing the optic nerve lesion sub-segment score, which also exhibited a good feasibility.24 In addition, as the number of patients with complete optic MRI recordings that can be further analyzed in our hospital was very limited in this study, we did not find a correlation between sPD-1, sPD-L1, and cytokines levels and the optic neuritis lesion length. Therefore, considering the close association between levels of sPD-1 and sPD-L1 and disease status and prognosis, further studies on a large number of samples by applying brain/optic MRI including 3D-DIR sequences are undoubtedly needed to clarify the relationship between the plasma sPD-1 and sPD-L1 levels with optic neuritis lesion length and visual prognosis.
There are some limitations needed to be pointed out in this study. First, due to the relative rarity of NMOSD, the number of patients included was not large enough, and we did not detect the sPD-1 and sPD-L1 levels in AQP4-IgG (−) NMOSD patients, whose differential diagnosis and clinical evaluation may be more difficult. Second, we did not perform longitudinal testing of sPD-1/sPD-L1 and cytokines levels to assess their dynamics as the disease progression. Third, analysis of sPD-1/sPD-L1 in plasma without prior isolation of exosomes may not distinguish between vesicular and soluble forms. However, we believe that this study is valuable as a pilot investigation to elucidate the clinical significance of sPD-1 and sPD-L1 in AQP4-IgG (+) NMOSD. Future studies including larger cohort of NMOSD patients with both seropositive and seronegative for AQP4-IgG, especially from multiple centers with serial and component-specific measurement of sPD-1 and sPD-L1 will substantially help physicians apply these circulatory indicators to the real clinical settings.
Conclusion
This study investigated the clinical relevance and predictive value of sPD-1 and sPD-L1 in AQP4-IgG (+) NMOSD. The results showed that levels of sPD-1 and sPD-L1, correlating with the cumulative burden of peripheral inflammation, reflected the current disease severity and activity, and predicted future relapses in AQP4-IgG (+) NMOSD, which suggested that they are valuable in assessing and predicting the disease status, and are expected to guide timely interventions and serve as an important target for the management of this disorder.
Acknowledgments
None.
Author Contributions
Jia Liu contributed with: conception and design, acquisition, analysis and interpretation of data, statistical analysis, and drafting and revision of the manuscript. Xi Shao, Jingya Fan, and Ying Wang contributed with acquisition of data, analysis and interpretation of data, and revision of the manuscript. Yuanbo Cao and Guojun Tan contributed with acquisiton of data and revision of the manuscript. Kazuo Sugimoto, Zhen Jia, and Bin Li contributed with study supervisor.
Disclosures
The authors declare no conflicts of interest regarding this work.
Funding Information
This study was supported by the National Natural Science Foundation of China (Grant No. 82205034), the Beijing Natural Science Foundation (Grant No. 7222114), the National Natural Science Foundation of China (Grant No. 81904131), and the Basic scientific research operation cost project of Beijing University of Chinese Medicine (2023-JYB-JBQN-017).
Open Research
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.




