Topiramate dosage optimization for effective antiseizure management via population pharmacokinetic modeling
Abstract
Objective
Despite the suggested topiramate serum level of 5–20 mg/L, numerous institutions have observed substantial drug response at lower levels. We aim to investigate the correlation between topiramate serum levels, drug responsiveness, and adverse events to establish a more accurate and tailored therapeutic range.
Methods
We retrospectively analyzed clinical data collected between January 2017 and January 2022 at Seoul National University Hospital. Drug responses to topiramate were categorized as “insufficient” or “sufficient” by reduction in seizure frequency ≥ 50%. A population pharmacokinetic model estimated serum levels from spot measurements. ROC curve analysis determined the optimal cutoff values.
Results
A total of 389 epilepsy patients were reviewed having a mean dose of 178.4 ± 117.9 mg/day and the serum level, 3.9 ± 2.8 mg/L. Only 5.6% samples exhibited insufficient response, with a mean serum level of 3.6 ± 2.5 mg/L while 94.4% demonstrated sufficient response, with a mean 4.0 ± 2.8 mg/L, having no statistical significance. Among the 69 reported adverse events, logistic regression analysis identified a significant association between ataxia and serum concentration (p = 0.04), with an optimal cutoff value of 6.5 mg/L.
Interpretation
This study proposed an optimal therapeutic concentration for topiramate based on patients' responsiveness to the drug and the incidence of adverse effects. We recommended serum levels below 6.5 mg/L to mitigate the risk of ataxia-related side effects while dose elevation was found unnecessary for suboptimal responders, as the drug's effectiveness plateaus at minimal doses.
Introduction
Epilepsy stands as one of the most prevalent neurological disorders worldwide, impacting approximately 50 million individuals, with an annual influx of around 5 million new diagnoses.1, 2 Central to the treatment of epilepsy are antiseizure medications (ASMs), offering a diverse range of options based on various mechanisms of action. Among these, topiramate, a second-generation ASM, exerts its influence through a multifaceted approach, encompassing sodium channel blockade, enhancement of gamma-aminobutyric acid (GABA) receptors, and antagonism of glutamate receptors.3 As such, topiramate finds application as both monotherapy and adjunct therapy for focal and generalized epilepsy.4 However, the potency of topiramate's effects often brings about an increased susceptibility to adverse events, particularly notable in elderly patients. Hence, the judicious prescription of an appropriate dosage within the defined therapeutic range emerges as a critical aspect in topiramate administration.
Currently, the International League Against Epilepsy (ILAE) recommends a topiramate therapeutic range of 5–20 mg/L.5 This recommendation was heavily influenced by a dose escalation add-on study that demonstrated seizure improvement within the range of 5-25 mg/L, coupled with an augmented incidence of side effects beyond the 20 mg/L threshold.6 However, in real-world practice, topiramate serum levels are frequently maintained at much lower levels. A study conducted in Italy reported a mean topiramate serum level of 4.5 mg/L, falling beneath the ILAE's proposed lower therapeutic threshold of 5 mg/L, while institutions in Norway base their protocols on a 2–10 mg/L range derived from local data.7 This scenario propels the necessity for a reevaluation of the presently endorsed therapeutic range for topiramate.
In this retrospective investigation, we delve into topiramate dosages and serum levels within an epilepsy patient cohort. Employing population pharmacokinetic (PK) modeling, we extrapolate trough and maximum serum levels. Armed with actual and estimated data, we scrutinize the interplay between topiramate concentration and adverse events, drawing comparisons between groups showcasing sufficient and insufficient drug responses. Our aim is to propose a pragmatic therapeutic range for topiramate, capable of furnishing valuable guidance in clinical decision-making.
Method
Patient enrollment and samples
We obtained serum samples from patients with epilepsy who were prescribed topiramate between January 2017 and January 2022 at Seoul National University Hospital. ASM regimens were maintained for at least 1 month before sampling in order to assume a steady state. To have precise estimates of the PK model, we obtained the time of sample collection and calculated the interval between sampling and final dosing for each sample. Topiramate serum tests performed during hospital admission were excluded from our analysis due to the variable conditions (such as infection or surgery) that patients may have experienced while hospitalized. Other variables including body weight and laboratory results were obtained and further analyzed.
ASM responses were evaluated from a retrospective review of medical records and dichotomized as follows: no change of seizure frequency or less than 50% reduction as the insufficient response group and seizure frequency reduction over 50% as the sufficient response group. The sufficient response group was again subdivided into the tolerable seizure group which had residual seizures and the seizure-free group, by reviewing the occurrence of seizures between the two latest visits before serum sampling. If a patient underwent serum sampling more than once, each test was listed separately with its own regimen and ASM response at the time of sampling.
In addition, patients who were prescribed topiramate as monotherapy were tracked of their seizure frequency and ASM prescription until April 2023 to see the progression into drug-resistant epilepsy. Their type of epilepsy syndrome and etiology were further investigated using brain magnetic resonance imaging, electroencephalography (EEG), video EEG monitoring, genetic tests, and medical charts.
The patients' free recalled experience of somnolence, dizziness, diplopia/nystagmus, tremor, ataxia, dysarthria, psychic symptoms, headache, hyperammonemia, gastrointestinal symptoms, weight gain, weight loss, hair loss, encephalopathy, rash, abnormal liver function test, paresthesia, renal stone, visual field defect, osteoporosis, gingival hypertrophy, and limb edema was collected by retrospective chart review. Adverse events were considered topiramate-induced when they appeared when the topiramate was started or increased in dosage. This study was approved by the Institutional Review Board of Seoul National University (IRB No.2209-081-1359).
Determination of serum topiramate concentration
Serum concentrations of topiramate were quantified using a validated ultra-performance liquid chromatography (ACQUITY UPLC I-Class, Waters Corporation, USA) coupled with tandem quadrupole mass spectrometry (Xevo TQ-XS IVD, Waters Corporation, USA). The 3PLUS1® Multilevel Plasma Calibrator Set Antiepileptic Drugs (Chromsystems, Germany) was used to measure topiramate. The lower limit of quantification was 0.25 μg/mL, having a linear calibration curve from 0.25 to 50 μg/mL. The accuracy and precision of the assay ranged from 102.9%–108.6% and 3.64%–4.06%, respectively.
Pharmacokinetic analysis of topiramate
A population PK analysis was performed using nonlinear mixed-effects modeling approach by NONMEM version 7.5.1 (Icon Development Solutions, Ellicott City, MD, USA) compiled with Perl-speaks-NONMEM (PsN) version 5.3.0, and interface with Pirana version 2.9.7. Serum topiramate concentrations below the quantification limit (BQL) were handled by the M1 method, which discard BQL observations and estimate using the remaining observations.8, 9 The previous population PK model structured in adult epilepsy patients with topiramate was used to estimate PK parameters in the current data.10 The model performance was evaluated through basic goodness-of-fit (GOF) plots, prediction-corrected visual predictive checks (pcVPC), and bootstrap resampling method. A total of 1000 datasets were simulated for pcVPC, and the median, 5th and 95th percentiles of the prediction-corrected observations were plotted with the simulation-based 95% confidence intervals (CIs) for the corresponding values. For the bootstrap approach, 1000 repeated sampling datasets were generated from the original dataset, and the final population PK model was fitted to each resampled dataset. The median and 95% CIs of PK parameters obtained from the bootstrap analysis were compared with the corresponding estimates of the final model.
Individual patient's serum topiramate concertation-time profiles were estimated using the final model. The following PK parameters were calculated using Phoenix WinNonlin software version 8.3.4 (Certara, USA); the maximum concentration at steady state (Cmax), the minimum concentration at steady state (Cmin), the area under the curve for 24 hours at steady state (AUC24h), apparent total clearance at steady state (CL/F), and apparent volume of distribution at steady state (Vd/F).
Statistics
Clinical data and PK parameters were presented as the mean ± standard deviation in continuous variables and as the median with interquartile range. Group comparisons were performed using the Student's t-test or Mann–Whitney U test, depending on the data normality, for continuous variables. The chi-square test was employed for categorical variables. Logistic regression analysis of ASM response and adverse events was performed for spot serum level and estimated PK parameters, including Cmax, Cmin, and AUC24h. To determine the optimal cutoff value of the topiramate level for predicting adverse events, we utilized the receiver operating characteristic (ROC) curve analysis, considering the point where the sum of sensitivity and specificity was maximized. The ROC curve analysis was performed using the pROC package in R. Statistical analysis was conducted using R version 4.2.2 (R Foundation for Statistical Computing, Vienna, Austria), and a p-value of less than 0.05 was considered statistically significant.
Results
Demographics and characteristics of the patients who tested Topiramate serum level
Of the 389 patients included in the analysis, a total of 555 samples were collected to measure the level of topiramate in serum (Table 1). The average age of the patients at the time of test was 46.4 ± 10.9 years, and approximately half of the samples were taken from male patients (n = 280, 50.5%). The mean body weight was 64.6 ± 17.7 kilograms, based on measurements from 237 samples. The median number of ASM prescriptions was 2 [interquartile range, 1–3], and 55.3% of the samples (n = 307) were collected when the patients were simultaneously prescribed enzyme-inducing ASMs (EIASMs), including carbamazepine (CBZ), oxcarbazepine (OXC), phenytoin (PHT), and phenobarbital (PB). The analyzed patients were categorized into two groups based on their response to topiramate: sufficient response and insufficient response. The majority of the samples (524, 94.4%) were collected when the patients showed a sufficient response to topiramate, and more than half of the samples (320, 57.7%) were obtained while the patients were in a seizure-free status.
| Demographics | Total | Monotherapy | Polytherapy | p-value | |
|---|---|---|---|---|---|
| Number of patients | 389 | 53 | Total | 340 | – |
| w/o EIASM | 130 | ||||
| w EIASM | 213 | ||||
| Tested samples | 555 | 76 | Total | 479 | – |
| w/o EIASM | 172 | ||||
| w EIASM | 307 | ||||
| Demographics | |||||
| Age (yrs) | 46.4 ± 10.9 | 46.0 ± 9.6 | 46.5 ± 11.1 | 0.68 | |
| Sex, male | 280 (50.5%) | 24 (31.6%) | 256 (53.4%) | <0.001*** | |
| Body weights (kg)a | 64.6 ± 17.7 | 59.8 ± 12.4 | 65.2 ± 18.3 | 0.06 | |
| Total ASMs | 2 [1–3] | – | 3 [2–4] | – | |
| Insufficient response | 31 (5.6%) | 1 (1.3%) | 30 (6.2%) | 3.04 | |
| Sufficient response | 524 (94.4%) | 75 (98.7%) | 449 (93.8%) | ||
| Tolerable seizure | 204 (36.8%) | 15 (19.7%) | 189 (39.5%) | <0.001*** | |
| Seizure-free | 320 (57.7%) | 60 (78.9%) | 260 (54.3%) | ||
| Pharmacokinetics | |||||
| Dose (mg/day) | 178.4 ± 117.9 | 121.4 ± 59.4 | Total | 187.5 ± 122.3 | <0.001*** |
| w/o EIASM | 176.5 ± 121.1 | <0.001*** | |||
| w EIASM | 193.6 ± 122.7 | <0.001*** | |||
| Serum level (mg/L) | 3.9 ± 2.8 | 3.7 ± 2.3 | Total | 4.0 ± 2.8 | 0.39 |
| w/o EIASM | 4.8 ± 3.3 | <0.01** | |||
| w EIASM | 3.5 ± 2.4 | 0.56 | |||
| Concentration/dose ratio [(μg/L)/(mg/day)] | 24.4 ± 16.1 | 30.5 ± 11.6 | Total | 23.4 ± 16.5 | <0.001*** |
| w/o EIASM | 30.1 ± 13.0 | 0.80 | |||
| w EIASM | 19.7 ± 17.0 | <0.001*** | |||
| Cmax (mg/L) | 4.2 ± 2.5 | 4.0 ± 1.9 | Total | 4.3 ± 2.5 | 0.20 |
| w/o EIASM | 5.1 ± 2.9 | <0.001*** | |||
| w EIASM | 3.8 ± 2.2 | 0.67 | |||
| Cmin (mg/L) | 3.0 ± 1.9 | 3.0 ± 1.5 | Total | 3.0 ± 2.0 | 0.98 |
| w/o EIASM | 3.8 ± 2.3 | <0.01** | |||
| w EIASM | 2.6 ± 1.6 | <0.05* | |||
| AUC24h (h∙mg/L) | 87.7 ± 52.7 | 84.4 ± 41.4 | Total | 88.2 ± 54.2 | 0.48 |
| w/o EIASM | 107 ± 62.9 | <0.001*** | |||
| w EIASM | 77.7 ± 45.6 | 0.24 | |||
| CL/F (L/h) | 2.1 ± 0.8 | 1.5 ± 0.3 | Total | 2.2 ± 0.8 | <0.001*** |
| w/o EIASM | 1.6 ± 0.4 | <0.05* | |||
| w EIASM | 2.6 ± 0.7 | <0.001*** | |||
| Vd/F (L) | 82.4 ± 13.3 | 79.9 ± 9.6 | Total | 82.8 ± 13.8 | 0.08 |
| w/o EIASM | 81.6 ± 16.2 | 0.30 | |||
| w EIASM | 83.5 ± 12.3 | <0.05* | |||
- ASM, antiseizure medication; AUC24h, area under the concentration–time curve for 24 hours at steady state; Cmax, maximum concentration at steady state; Cmin, minimum concentration at steady state; CL/F, apparent total clearance at steady state; EIASM, enzyme-inducing ASM; Vd/F, apparent volume of distribution at steady state.
- a Body weight had been documented in 237 samples. Data are presented as the mean ± standard deviation. Pharmacokinetic parameters (Cmax, Cmin, AUC24h, CL/F, and Vd/F) were estimated by the population pharmacokinetic model. p-values were calculated to compare patient groups prescribed with monotherapy and polytherapy (total) using two sample t-test or Mann–Whitney U test depending on data normality.
- * p < 0.05;
- ** p < 0.01;
- *** p < 0.001.
Among the 555 samples, 76 (13.7%) were collected from 53 patients who were prescribed topiramate as monotherapy, while 479 (86.3%) samples were obtained from 340 patients who were prescribed other ASMs in addition to topiramate. The patients receiving ASM-polytherapy were prescribed a median of 3 [2–4] ASMs, including topiramate. In comparison with the samples from patients receiving ASM-polytherapy, the samples from patients on topiramate monotherapy were less frequently collected from males (24/76 [31.6%] vs 256/479 [53.4%], p < 0.001), and more commonly associated with a seizure-free status (60/76 [78.9%] vs 260/479 [54.3%], p < 0.001). In both the monotherapy and the polytherapy groups, the proportion of the samples collected in an insufficient response to topiramate was low, having only 1 sample (1.3%) in the monotherapy and 30 (6.2%) in the polytherapy, respectively.
From the analysis of topiramate serum levels, the mean dosage of topiramate was found to be 178.4 ± 117.9 mg/day, while the mean spot serum level was 3.9 ± 2.8 mg/L (Table 1, Fig. 1). The mean spot serum levels in the topiramate monotherapy and polytherapy groups were similar (3.7 ± 2.3 vs 4.0 ± 2.8, p = 0.39), but the concentration/dose ratio in the polytherapy group was significantly lower than that in the monotherapy group (30.5 ± 11.6 vs 23.4 ± 16.5, p < 0.001).

Topiramate pharmacokinetics
The PK parameters of our population data with spot serum levels of topiramate were well estimated based on the previously structured one-compartment model with first-order absorption and elimination.10 The topiramate clearance was affected by creatinine clearance (CLcr), topiramate daily dose (DOSE), and co-administration of EIASMs. Overall, the typical value of topiramate CL/F was increased while the concomitant drug effect was decreased compared to the previous model: CL/F (L/h) =(1.45 + 1.02 × PHT + 0.703 × CBZ + 0.419 × OXC + 0.376 × PB) × (CLcr/90)0.277 × (DOSE/100)0.193 (Table S1). Other co-medications (lacosamide, perampanel, valproate, rufinamide, lamotrigine, pregabalin, zonisamide, levetiracetam, clobazam, vigabatrin, and/or lorazepam) were assessed as potential covariates on topiramate CL/F, but adding those effects was not clinically significant. The topiramate Vd/F was increased with body weight, where allometric scaling was applied with the exponent of 1. The estimated Vd/F was approximately 30% decreased than that of the previous model, but it was a reasonable value compared to the reported Vd/F of topiramate.11, 12 The final parameter estimates for CL/F and Vd/F were 2.1 ± 0.8 L/h and 82.4 ± 13.3 L for the total samples. The estimated level of topiramate showed a consistent pattern with the spot serum level (Table 1).
For the estimated Cmin of topiramate was a mean of 3.0 ± 1.9 mg/L in the total samples and means of 3.0 ± 1.5 mg/L and 3.0 ± 2.0 mg/L in the monotherapy and polytherapy groups, respectively, showing no significant difference (p = 0.98).
Effect of enzyme-inducing ASM
Within the polytherapy group, our results demonstrated that samples from patients receiving EIASMs generally had lower serum levels of topiramate (Table 1, Fig. 1). In the polytherapy group, the prescribed daily dose of topiramate was significantly higher than that of the monotherapy group, regardless of concurrent EIASM (p < 0.001). The mean spot serum level, however, was significantly higher only in the polytherapy group without EIASMs, (4.8 ± 3.3 mg/L vs 3.7 ± 2.3 mg/L, p < 0.01) but not in those with EIASM (3.5 ± 2.4 mg/L, p = 0.56), which was also reflected the effect of EIASM in 1.5-fold difference in serum concentration/dose ratio and the CL/F.
Therapeutic range of Topiramate serum level
To investigate the relationship between serum levels and the antiseizure response to topiramate, we compared the dose and serum level of topiramate between samples collected from patients who exhibited an insufficient response to topiramate and those who showed a sufficient response (Fig. 1 and Table 2). For a total of 555 samples, 31 samples were collected from 30 patients who showed insufficient response to topiramate at that time. In the insufficient response group, the mean dose of topiramate was 203.2 ± 129.1 mg/day and the spot serum level was 3.6 ± 2.5 mg/L. The comparison with the sufficient response group was statistically insignificant by having 177.0 ± 117.1 mg/day (p = 0.23) and 4.0 ± 2.8 mg/L (p = 0.45), respectively.
| Insufficient response | Sufficient response | p-value | |||
|---|---|---|---|---|---|
| Total | Tolerable seizure | Seizure-free | |||
| Total | |||||
| Number of patients | 30 | 371 | 171 | 222 | |
| Tested samples | 31 | 524 | 204 | 320 | – |
| Dose (mg/day) | 203.2 ± 129.1 | 177.0 ± 117.1 | 201.7 ± 124.3 | 161.2 ± 109.7 | 0.23 |
| Serum level (mg/L) | 3.6 ± 2.5 | 4.0 ± 2.8 | 4.3 ± 2.8 | 3.8 ± 2.7 | 0.45 |
| Concentration/dose ratio [(μg/L)/(mg/day)] | 18.9 ± 9.2 | 24.7 ± 16.3 | 23.5 ± 18.0 | 25.5 ± 15.2 | 0.05 |
| Cmax (mg/L) | 4.5 ± 2.4 | 4.2 ± 2.5 | 4.5 ± 2.4 | 4.0 ± 2.5 | 0.57 |
| Cmin (mg/L) | 3.2 ± 1.9 | 3.0 ± 1.9 | 3.2 ± 1.9 | 2.9 ± 1.9 | 0.70 |
| AUC24h (h∙mg/L) | 92.0 ± 51.0 | 87.4 ± 52.8 | 93.4 ± 52.4 | 83.6 ± 52.8 | 0.64 |
| CL/F (L/h) | 2.4 ± 1.1 | 2.1 ± 0.7 | 2.2 ± 0.7 | 2.0 ± 0.8 | 0.05 |
| Vd/F (L) | 84.3 ± 14.5 | 82.3 ± 13.3 | 83.6 ± 15.3 | 81.5 ± 11.7 | 0.43 |
| Monotherapy | |||||
| Number of patients | 1 | 52 | 14 | 42 | |
| Number of patients ≥ 3 ASMsa | 0 | 10 | 7/13 | 3/39 | <0.001***,b |
| Tested samples | 1 | 75 | 15 | 60 | |
| Dose (mg/day) | 100.0 | 121.7 ± 59.7 | 151.7 ± 61.6 | 114.2 ± 57.4 | <0.05b, * |
| Serum level (mg/L) | 2.8 | 3.7 ± 2.3 | 4.8 ± 2.0 | 3.4 ± 2.3 | <0.01**,b |
| Serum level/dose ratio [(μg/L)/(mg/day)] | 28.0 | 30.5 ± 11.7 | 33.6 ± 10.0 | 29.8 ± 12.0 | 0.27b |
| Cmax (mg/L) | 2.9 | 4.0 ± 1.9 | 4.8 ± 1.6 | 3.8 ± 1.9 | <0.01**,b |
| Cmin (mg/L) | 2.3 | 3.1 ± 1.6 | 3.7 ± 1.5 | 2.9 ± 1.5 | <0.05*,b |
| AUC24h (h∙mg/L) | 62.4 | 84.7 ± 41.6 | 101.9 ± 37.5 | 80.4 ± 41.7 | <0.05*,b |
| CL/F (L/h) | 1.6 | 1.5 ± 0.3 | 1.5 ± 0.3 | 1.5 ± 0.4 | 0.94b |
| Vd/F (L) | 82.2 | 79.9 ± 9.6 | 80.4 ± 13.8 | 79.8 ± 8.4 | 0.81b |
| Polytherapy | |||||
| Number of patients | |||||
| Total | 29 | 323 | 158 | 182 | |
| w/o EIASM | 10 | 124 | 52 | 82 | |
| w EIASM | 19 | 201 | 108 | 100 | |
| Tested samples | |||||
| Total | 30 | 449 | 189 | 260 | – |
| w/o EIASM | 10 | 162 | 55 | 107 | – |
| w EIASM | 20 | 287 | 134 | 153 | – |
| Dose (mg/day) | |||||
| Total | 206.7 ± 129.8 | 186.2 ± 121.8 | 205.7 ± 127.2 | 172.0 ± 115.9 | 0.38 |
| w/o EIASM | 195.0 ± 121.2 | 175.3 ± 121.4 | 193.2 ± 129.9 | 166.1 ± 116.4 | 0.49 |
| w EIASM | 212.5 ± 136.6 | 192.3 ± 121.8 | 210.8 ± 126.2 | 176.1 ± 115.8 | 0.47 |
| Serum level (mg/L) | |||||
| Total | 3.6 ± 2.5 | 4.0 ± 2.8 | 4.2 ± 2.9 | 3.9 ± 2.8 | 0.45 |
| w/o EIASM | 4.9 ± 2.8 | 4.8 ± 3.3 | 5.0 ± 3.5 | 4.7 ± 3.2 | 0.98 |
| w EIASM | 3.0 ± 2.2 | 3.5 ± 2.5 | 3.9 ± 2.5 | 3.2 ± 2.4 | 0.31 |
| Concentration/dose ratio [(μg/L)/(mg/day)] | |||||
| Total | 18.6 ± 9.2 | 23.7 ± 16.8 | 22.7 ± 18.2 | 24.5 ± 15.7 | 0.10 |
| w/o EIASM | 25.7 ± 8.9 | 30.3 ± 13.2 | 28.6 ± 13.8 | 31.2 ± 12.8 | 0.27 |
| w EIASM | 15.1 ± 7.2 | 20.0 ± 17.5 | 20.2 ± 19.3 | 19.8 ± 15.8 | 0.22 |
| Cmax (mg/L) | |||||
| Total | 4.5 ± 2.4 | 4.3 ± 2.5 | 4.5 ± 2.5 | 4.1 ± 2.6 | 0.57 |
| w/o EIASM | 5.5 ± 2.8 | 5.0 ± 2.9 | 5.3 ± 2.9 | 4.9 ± 2.9 | 0.66 |
| w EIASM | 4.1 ± 2.1 | 3.8 ± 2.2 | 4.2 ± 2.2 | 3.5 ± 2.1 | 0.63 |
| Cmin (mg/L) | |||||
| Total | 3.2 ± 1.9 | 3.0 ± 2.0 | 3.2 ± 1.9 | 2.9 ± 2.0 | 0.65 |
| w/o EIASM | 4.1 ± 2.4 | 3.8 ± 2.3 | 4.0 ± 2.3 | 3.7 ± 2.3 | 0.66 |
| w EIASM | 2.7 ± 1.5 | 2.6 ± 1.6 | 2.9 ± 1.7 | 2.4 ± 1.6 | 0.71 |
| AUC24h (h∙mg/L) | |||||
| Total | 93.0 ± 51.6 | 87.9 ± 54.5 | 92.7 ± 53.4 | 84.4 ± 55.1 | 0.62 |
| w/o EIASM | 115.8 ± 62.9 | 106.4 ± 63.0 | 111.9 ± 63.2 | 103.6 ± 63 | 0.65 |
| w EIASM | 81.6 ± 42.1 | 77.4 ± 45.9 | 84.8 ± 46.9 | 71.0 ± 44.2 | 0.56 |
| CL/F (L/h) | |||||
| Total | 2.4 ± 1.1 | 2.2 ± 0.7 | 2.3 ± 0.7 | 2.1 ± 0.8 | 0.17 |
| w/o EIASM | 1.7 ± 0.3 | 1.6 ± 0.5 | 1.7 ± 0.4 | 1.6 ± 0.5 | 0.73 |
| w EIASM | 2.8 ± 1.2 | 2.5 ± 0.7 | 2.5 ± 0.6 | 2.5 ± 0.7 | 0.61 |
| Vd/F (L) | |||||
| Total | 84.3 ± 14.7 | 82.7 ± 13.7 | 83.9 ± 15.4 | 81.9 ± 12.3 | 0.53 |
| w/o EIASM | 77.6 ± 17.9 | 81.9 ± 16.1 | 86.9 ± 17.9 | 79.3 ± 14.5 | 0.42 |
| w EIASM | 87.7 ± 12.0 | 83.2 ± 12.2 | 82.7 ± 14.2 | 83.6 ± 10.2 | <0.05* |
- ASM, antiseizure medication; AUC24h, area under the concentration–time curve for 24 hours at steady state; Cmax, maximum concentration at steady state; Cmin, minimum concentration at steady state; CL/F, apparent total clearance at steady state; EIASM, enzyme-inducing ASM; Vd/F, apparent volume of distribution at steady state.
- a The number of patients who had been prescribed with more or equal to three ASMs until the last clinic were described, out of the number of patients in each response group depending on patients' first sample. Data are presented as the mean ± standard deviation. Pharmacokinetic parameters (Cmax, Cmin, AUC24h, CL/F, and Vd/F) were estimated by the population pharmacokinetic model. p-values were calculated to compare patient groups of insufficient and sufficient response except.
- b Where it indicates comparison between tolerable seizure and seizure-free group, using two sample t-test or Mann–Whitney U test depending on data normality.
- * p < 0.05;
- ** p < 0.01;
- *** p < 0.001.
Logistic regression analysis also proved insignificant association of spot serum level and response to topiramate (p = 0.45), as was also observed with estimated parameters (Tables 3 and S2). The probability curve based on the logistic regression analysis demonstrated that the probability of showing sufficient response to topiramate was saturated in the lowest spot serum level and Cmin (Fig. 2).
| Response | Events | Spot serum level | Estimated Cmax | ||
|---|---|---|---|---|---|
| Odds ratio | p-value | Odds ratio | p-value | ||
| ASM response | 555 | 1.06 [0.93–1.23] | 0.45 | 0.96 [0.84–1.11] | 0.57 |
| Total adverse events | 69 | 0.96 [0.87–1.06] | 0.44 | 0.94 [0.84–1.05] | 0.29 |
| TPM-induced adverse events | 56 | 0.96 [0.86–1.06] | 0.47 | 0.93 [0.82–1.04] | 0.23 |
| Somnolence | 8 | 1.15 [0.92–1.37] | 0.16 | 1.13 [0.86–1.41] | 0.32 |
| Dizziness | 16 | 0.91 [0.72–1.10] | 0.40 | 0.86 [0.65–1.07] | 0.23 |
| Diplopia, nystagmus | 1 | 1.23 [0.64–1.84] | 0.37 | 1.1 [0.41–1.92] | 0.79 |
| Tremor | 5 | 1.14 [0.85–1.42] | 0.29 | 1.12 [0.78–1.47] | 0.48 |
| Ataxia | 3 | 1.3 [0.97–1.65] | <0.05* | 1.38 [0.98–1.87] | <0.05* |
| Dysarthria | 2 | 1.18 [0.74–1.59] | 0.37 | 1.21 [0.7–1.77] | 0.39 |
| Psychic symptoms | 2 | 1.2 [0.76–1.61] | 0.37 | 1.32 [0.82–1.90] | 0.17 |
| Fatigue | 5 | 0.5 [0.19–0.93] | 0.09 | 0.36 [0.12–0.79] | <0.05* |
| Cognitive decline | 17 | 0.86 [0.67–1.05] | 0.19 | 0.87 [0.67–1.08] | 0.24 |
| Headache | 2 | 0.04 [0.00–0.54] | 0.09 | 0.06 [0.00–0.51] | <0.05* |
| Hyperammonemia | 1 | 0.20 [0–1.17] | 0.30 | 0.87 [0.21–1.72] | 0.77 |
| GI symptoms | 5 | 1.03 [0.72–1.33] | 0.82 | 0.96 [0.61–1.33] | 0.83 |
| Body weight Gain | 5 | 0.73 [0.38–1.10] | 0.23 | 0.79 [0.44–1.18] | 0.35 |
| Body weight Loss | 5 | 0.94 [0.61–1.26] | 0.73 | 0.94 [0.59–1.31] | 0.75 |
| Hair loss | 1 | 0.85 [0.2–1.55] | 0.73 | 1.02 [0.33–1.85] | 0.95 |
| Etc† | 0 | ||||
- Etc†: encephalopathy, rash, abnormal liver function test, paresthesia, renal stone, visual field defect, osteoporosis, gingival hypertrophy, limb edema.
- ASM, antiseizure medication; Cmax, maximum concentration at steady state; GI, gastrointestinal; TPM, topiramate.
- * p < 0.05;
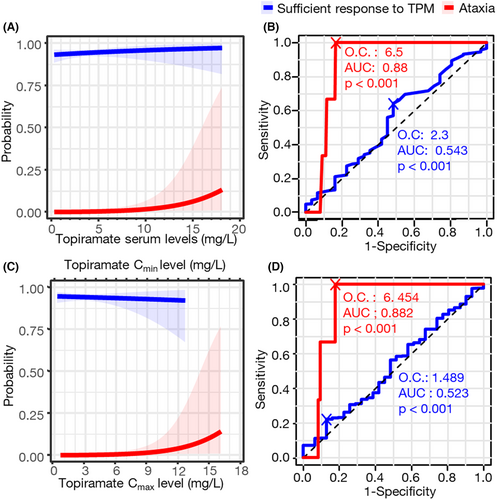
Meanwhile, the adverse effects were associated with the serum level of topiramate (Tables 3 and S2). Ataxia was associated with a high serum level of topiramate (p = 0.037 in the spot serum level, 0.041 in Cmax, and 0.048 in AUC24h). On the contrary, the protective effect of topiramate was shown in the symptoms of fatigue and headache with estimated Cmax and AUC24h by the PK model. According to the ROC curves, the optimal cutoff value by ataxia occurrence was 6.5 mg/L by spot serum level (specificity 83.5%, sensitivity 100%, p < 0.001) and 6.454 mg/L by Cmax (specificity 82.2%, sensitivity 100%, p < 0.001), which could be a reasonable upper limit of topiramate therapeutic range (Fig. 2).
Detailed analysis of patients with monotherapy group
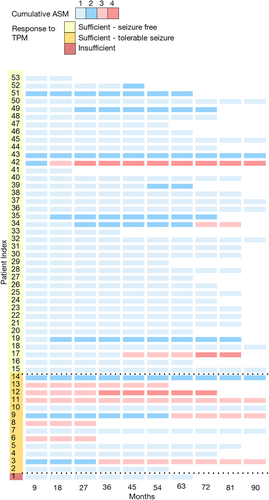
To exclude the effects of other ASMs on seizure reduction, we investigated the dose–response association solely within the monotherapy group. However, only one sample was collected from a patient having insufficient response. Instead, we compared the samples collected from patients who achieved seizure freedom (60 samples from 42 patients) and those who experienced tolerable seizures (15 samples from 14 patients) within the group showing a sufficient response (Table 2). The samples from seizure freedom showed significantly lower doses and serum levels of topiramate compared to those from tolerable seizures (114.2 ± 57.4 vs 151.7 ± 61.6 mg/day, p = 0.02, 3.4 ± 2.3 vs 4.8 ± 2.0 mg/L, p < 0.01).
We delved into the detailed epilepsy profiles of the 55 patients on factors that could affect the outcome of topiramate treatment. (Fig. 3, Table S3). During the follow-up period, patients who experienced residual seizures while on topiramate monotherapy had a higher likelihood of being prescribed more than 3 ASMs, ultimately leading to the progression of drug-resistant epilepsy (7/13 [53.8%] vs 3/39 [7.7%], p < 0.001).
Discussion
In our study, the optimal therapeutic concentration was suggested based on the responsiveness of patients with topiramate and the occurrence of adverse effects, and we developed a population PK model based on the measurement of topiramate serum levels. Our analysis showed that the antiseizure effect of topiramate is saturated at a level much lower than current recommendation so that dose–response relationship does not appear in usual dosages. We also observed that the use of EIASMs lead to an increase in topiramate clearance.
We used the previously structured population PK model of topiramate to estimate the population PK parameters of topiramate from our data, and the results were consistent with previously reported findings.10 The typical value of topiramate CL/F (θ1) was estimated to be higher compared to the previous model, but the percentage increase with each co-medication was lower: topiramate CL/F increased by 70%, 48%, 29%, and 26% in patients co-administered with PHT, CBZ, OXC, and PB, respectively, compared to topiramate monotherapy. However, these results were still consistent with previously reported findings that concomitant medications, particularly EIASMs, increased topiramate clearance by 1.3- to 2-fold.13-15 Therefore, patients who are concomitantly using topiramate and EIASMs should be mindful of the approximately 1.5-fold difference in the concentration/dose ratio. Given the linear PKs and low inter-individual variability of topiramate,11 we suggest the therapeutic drug monitoring of topiramate for specific conditions such as the lack of response, addition of concomitant medications, or occurrence of adverse events.16, 17
According to our analysis, topiramate exhibits a sufficient antiseizure effect at doses and serum concentration levels lower than previously suggested. The ILAE currently recommends a topiramate concentration range of 5–20 mg/L.5 However, our analysis of data indicated that an average concentration of approximately 4 mg/L was sufficient for the desired antiseizure effect in 94.2% of patients. Notably, serum level exceeding 6 mg/L was associated with a significant increase in the side effect of ataxia. The ILAE's recommended concentration range is based on a compilation of various studies, each yielding different results.18-24 The results of the Reife et al. study, which involved three double-blind placebo-controlled add-on trials, demonstrated seizure control improvement within the range of 3.5–5 mg/L, similar to our findings.18 Another triple-blind, concentration-controlled, parallel-group trial indicated that the group with a serum concentration of 10 mg/L showed the best effectiveness, with a corresponding increase in adverse events as concentrations rose.24 Our results align with these studies, suggesting that lower doses and concentrations can be effectively employed in real-world clinical practice. In particular, patients who did not achieve seizure freedom while using topiramate monotherapy were more likely to progress to drug-resistant epilepsy requiring three or more ASMs compared to those who achieved seizure freedom. These results suggest that if the desired level of seizure control is not achieved even after reaching a concentration of 4 mg/L, adding another ASM may be more effective for seizure control than increasing the dose of topiramate to a higher level.
Moreover, the upper therapeutic range of topiramate may be lower than previously reported. In the case of ataxia, a clear increase in concentration was correlated with its occurrence, suggesting that a lower level than 6 mg/L, if feasible, should be considered. Although only three ataxia events were observed in 389 patients over 5 years, the probability is very small, and 6 mg/L cannot be considered the upper therapeutic range. Previous studies have set the upper therapeutic range of topiramate serum levels at 8 mg/L or higher23, 24 but in conjunction with the aforementioned results, targeting 6 mg/L or higher may not be justified.
Additionally, fatigue and headache showed a protective effect as Cmax increased. Given that chronic fatigue is a common comorbidity of migraineurs,25, 26 topiramate might be beneficial for attenuating the migraine. Meanwhile, the most common adverse effects of topiramate, such as cognitive impairment, dizziness, and somnolence,27 were not significantly associated with serum concentration levels in our data. On the contrary, the association between topiramate dose and cognitive side effects has been well-established in carefully designed studies.28 Since these symptoms are subjective experiences reported by individual patients and were assessed while patients were taking various ASMs concurrently in an outpatient setting, it is possible that the association of side effects to topiramate went under-recognized. Therefore, caution should be exercised regarding cognitive-related adverse events with dose usage, and further research on the relationship with serum concentration should be explored in subsequent studies.
Our retrospective study has certain limitations that need to be considered. Adverse events are typically observed during the initial introduction of a drug; however, in our study, adverse events were recorded at the time of topiramate serum level measurement. Additionally, the timing of topiramate serum level assessment was not random but determined based on clinical judgment, such as suspicion of noncompliance or potential changes in drug metabolism. Consequently, the time points at which topiramate serum levels were measured may not be representative of epilepsy in general. Our analysis treated each sample as an individual unit rather than considering the data for each patient. Patients experiencing aggravated symptoms tended to undergo multiple topiramate serum level tests. These factors may have resulted in an underestimation of the serum levels and the response to ASMs. Furthermore, we did not account for the variability in serum levels due to topiramate partition effects by red blood cells,29, 30 and this aspect also warrants further investigation in subsequent studies.
In recent years, the utilization of topiramate monotherapy has become less common in South Korea due to the development of next-generation ASMs.31, 32 However, topiramate remains a highly effective option when used as an add-on therapy. Our study highlights the importance of using lower doses than previously suggested. Also, when the desired antiseizure effect is not adequately achieved with topiramate monotherapy, addition of another ASM instead of escalating the dose of topiramate is prudent. Moreover, when using topiramate as a part of polytherapy with EIASMs, clinicians should be aware that the clearance of topiramate is elevated 1.5-fold. Further research is warranted to investigate a more homogeneous group of patients and explore a broader range of doses, especially at very low doses, in order to determine the lower limit of the therapeutic range and optimize treatment strategies.
Acknowledgements
This research was supported by a grant from ILDONG Pharmaceuticals (Grant No. 0620232180) and SAMJIN Pharmaceuticals (Grant No. 0620212810).
Author Contributions
S.L., H.C.K., and Y.J. drafted the manuscript. Y.J. and S.L. revised the manuscript. S.L. and H.C.K. prepared tables. S.L. and H.C.K. prepared figures. S.L., Y.J., and H.S.L. reviewed patients' medical records. H.C.K., S.L., and J.O. analyzed the population PK model data. K.C. and S.K.L. collected clinical data. K.C. and S.K.L. provided study concepts. K.C., S.K.L., J.O., S.L., K-S.Y., I-J.J., and S.L. supervised the study. All authors reviewed the manuscript.
Disclosures
The authors declare no conflict of interests.




