Habitual sleep disturbances and migraine: a Mendelian randomization study
Funding Information
Richa Saxena was supported by grants from the National Institutes of Health, NIH/NIDDK [Grant number R01DK105072, R01DK107859] and the Phyllis and Jerome Lyle Rappaport MGH Research Scholar Award. Daniel I. Chasman was supported by grants from the NINDS/NIH (R21NS09296 and R21NS104398). The funders had no role in the study design; data collection; data analysis and interpretation; writing of the report; or the decision to submit for publication.
Abstract
Objective
Sleep disturbances are associated with increased risk of migraine, however the extent of shared underlying biology and the direction of causal relationships between these traits is unclear. Delineating causality between sleep patterns and migraine may offer new pathophysiologic insights and inform subsequent intervention studies. Here, we used genetic approaches to test for shared genetic influences between sleep patterns and migraine, and to test whether habitual sleep patterns may be causal risk factors for migraine and vice versa.
Methods
To quantify genetic overlap, we performed genome-wide genetic correlation analyses using genome-wide association studies of nine sleep traits in the UK Biobank (n ≥ 237,627), and migraine from the International Headache Genetics Consortium (59,674 cases and 316,078 controls). We then tested for potential causal effects between sleep traits and migraine using bidirectional, two-sample Mendelian randomization.
Results
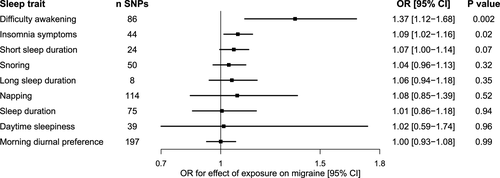
Seven sleep traits demonstrated genetic overlap with migraine, including insomnia symptoms (rg = 0.29, P < 10−31) and difficulty awakening (rg = 0.11, P < 10−4). Mendelian randomization analyses provided evidence for potential causal effects of difficulty awakening on risk of migraine (OR [95% CI] = 1.37 [1.12–1.68], P = 0.002), and nominal evidence that liability to insomnia symptoms increased the risk of migraine (1.09 [1.02–1.16], P = 0.02). In contrast, there was minimal evidence for an effect of migraine liability on sleep patterns or disturbances.
Interpretation
These data support a shared genetic basis between several sleep traits and migraine, and support potential causal effects of difficulty awakening and insomnia symptoms on migraine risk. Treatment of sleep disturbances may therefore be a promising clinical intervention in the management of migraine.
Introduction
Migraine is a debilitating and highly prevalent chronic pain condition that is a leading contributor to disability worldwide.1 By the time of clinical presentation, those with migraine are more likely to report several comorbidities, including several sleep disturbances and disorders (reviewed by Vgontzas and Pavlović).2-6 Prospective studies have found associations between insomnia and increased risk for incident migraine diagnosis7 and vice versa. Despite this epidemiologic evidence, there remain several unanswered questions about the relationship between migraine and sleep. Although both migraine (SNP-based heritability 15%)8 and sleep traits (SNP-based heritability ranging from 6.9% to 17%)9-12 are heritable, it is unknown whether this comorbidity is driven, at least partly, by shared genetic influences. It is also unknown whether causality underlies this comorbidity,4 as associations in epidemiologic studies are potentially biased by residual confounding and reverse causality. Delineating causality between sleep patterns and migraine may offer new pathophysiologic insights into these traits and inform subsequent intervention trials.
Causality can be investigated using Mendelian randomization (MR).13 MR can be conceptualized as a natural experiment whereby individuals are randomly allocated to lifelong greater exposure to a given risk factor (e.g., insomnia symptoms) based on their genetic risk, and then the risk of a disease outcome (e.g., migraine) as a function of this exposure is measured later in life.14 The validity of this approach rests on the random assortment of genetic alleles at gametogenesis, thereby rendering the alleles relatively unconfounded by environmental factors. Moreover, inherited genetic variation is fixed at birth and is therefore not modifiable by environmental factors or disease status. MR has been previously used to examine causal relationships between migraine and dementia,15 blood pressure,16 and cardiovascular disease.17, 18
The availability of large-scale genome-wide association studies (GWAS) for sleep traits9-12 (n ≤ 452,071) and migraine8 (n = 375,752) now provides an opportunity to test shared genetic predisposition and causal effects. Here, we leveraged cross-trait LD Score regression19 and MR20 using recently available data from the UK Biobank cohort and the largest GWAS of migraine8 to, respectively, assess for a shared genetic basis and for potential causal effects between sleep traits and migraine.
Methods
Data sources: sleep GWAS
Sleep traits in UK Biobank
Genetic associations for sleep traits were obtained from published9, 10, 12, 21 and unpublished GWAS summary statistics in UK Biobank (UKB) participants of European ancestry (methodologic details given in Data S1; GWAS characteristics listed in Table S1). We considered GWAS for all sleep traits ascertained in UKB: sleep duration,12 morning diurnal preference (also referred to as “chronotype”),10 daytime napping frequency,22 snoring, insomnia symptoms,9 difficulty awakening, and daytime sleepiness23 (phenotype definitions and GWAS procedures are provided in Data S1 and Table S2). We selected all available sleep traits so as to provide an unbiased survey of the relationship between sleep health and migraine. The question used to define self-reported insomnia symptoms in UKB has been shown to be sensitive and specific for clinically diagnosed insomnia disorder in an independent sample.24 Although daytime sleepiness is generally investigated as an outcome, we included it as an exposure here because the genetic architecture of daytime sleepiness suggests that the trait may partly reflect sleep fragmentation.6, 23 Genetic variants that associate with sleep traits in these GWAS also strongly associate with corresponding objective measures of sleep.21
Data sources: migraine GWAS
We obtained genetic associations with migraine from the largest available meta-analysis of genome-wide association studies (GWAS) of migraine conducted by the International Headache Genetics Consortium (IHGC).8 This study comprised 59,674 cases and 316,078 controls from 22 GWA studies (including 23andMe), conducted using data from six tertiary headache clinics (n = 20,395) and 27 population-based cohorts (n = 355,357). Characteristics of each of the contributing cohorts have been previously described.8 Migraine cases were defined using a range of different approaches across the cohorts including self-report, questionnaires assessing diagnostic criteria, and diagnosis by a trained clinician interviewer. All participants had genetically verified European ancestry.
Genetic correlation analyses
We calculated genome-wide genetic correlations (rg) using cross-trait LD Score regression with precomputed LD scores19, 25 (Data S1). A positive genetic correlation differing from 0 implies that genetic variants increasing risk for one trait tend to also increase risk for the other trait.
Mendelian randomization analyses
The design of our MR analysis is shown in Figure 1, with details of data harmonization provided in the Data S1. The primary MR method was random-effects inverse-variance weighted (IVW) regression,26 with sleep and migraine alternately used as exposure or outcome. For ordinal phenotypes (Table S1), a one-unit increase in the genetic instrument corresponds to a unit increase in the ordinal scale. For dichotomous phenotypes, a one-unit increase in the genetic instrument reflects a doubling in the odds of the exposure trait.27
Sensitivity analyses
MR provides strong evidence for causality under the following assumptions14: (1) the genetic instrument is strongly associated with the exposure, (2) the genetic instrument is not associated with confounders, and (3) the genetic instrument only affects the outcome through its effect on the exposure (i.e., no horizontal pleiotropy).14 As the second MR assumption is generally satisfied by the use of randomly allocated alleles as instrumental variables and by control for population stratification in GWAS, we focused on approaches to address assumptions 1 and 3. Broadly, to address assumption 1 we performed sensitivity analyses using stronger genetic instruments for insomnia. To assess assumption 3, we used four models robust to various forms of pleiotropy, and tested for pleiotropy between the exposures and other sleep traits, and between the exposures and psychiatric comorbidities (depression and anxiety symptoms). Technical details regarding these sensitivity analyses are provided in the Data S1.
Hypothesis testing and statistical software
The Bonferonni-adjusted threshold for MR analyses accounted for 14 forward and reverse MR tests (without double-counting short and long sleep duration, which are highly correlated with sleep duration measured continuously12), yielding an alpha threshold of 0.05/14 = 0.0036. The corrected alpha threshold in genetic correlation analyses was 0.05/7 = 0.007. P values less than these corrected alpha thresholds were considered to represent significant evidence for causal effects, and P < 0.05 was considered to represent nominal evidence for a causal effect. Analyses were performed using the LDSC software,19, 25 R version 3.5.0 and the TwoSampleMR28 package, and the GSMR29 software.30
Standard protocol approvals, registrations, and patient consents
All UKB participants provided written informed consent, and all data used in this study were deidentified. Sleep GWAS data are available at the Sleep Disorder Knowledge portal (see data links). The IGHC migraine GWAS summary statistics including data from 23andMe were provided under a Data Transfer Agreement by 23andMe.
Results
Migraine shares genetic determinants with multiple sleep patterns and disturbances
As sleep disturbances are comorbid with migraine and are also heritable, we first tested if the traits have shared genetic influences using cross-trait LD score regression.25 Migraine was genetically correlated with seven out of nine sleep patterns or disturbances after Bonferonni correction P < 0.007; Table 1). Insomnia symptoms had the strongest and most significant evidence for a shared genetic basis with migraine (rg [95% CI] 0.29 [0.25–0.33], P = 1.87 × 10−32), with weaker correlations between migraine and short sleep duration (0.18 [0.12–0.24], P = 1.69 × 10−9), difficulty awakening (0.11 [0.05–0.17], P = 2.02 × 10−5), and daytime napping (0.11 [0.05–0.17], P = 1.31 × 10−5). There was no evidence for a genetic correlation between migraine and morning diurnal preference (−0.03 [−0.07–0.01], P = 0.24) or snoring (0.01 [−0.05–0.07], P = 0.84).
| Sleep trait1 | Genetic correlation with migraine (SE) | P value |
|---|---|---|
| Morning diurnal preference | −0.03 (0.02) | 0.24 |
| Difficulty awakening | 0.11 (0.03) | 2.02 × 10−5* |
| Insomnia symptoms | 0.29 (0.02) | 1.87 × 10−32* |
| Long sleep duration (≥9h) | 0.12 (0.04) | 7.60 × 10−4* |
| Short sleep duration (<7h) | 0.18 (0.03) | 1.69 × 10−9* |
| Sleep duration (hours) | −0.08 (0.03) | 1.56 × 10−3* |
| Napping | 0.11 (0.03) | 1.31 × 10−5* |
| Daytime sleepiness | 0.09 (0.03) | 1.21 × 10−4* |
| Snoring | 0.01 (0.03) | 0.84 |
- GWAS, genome-wide association study; SE, standard error.
- 1 The LDSC intercept ranged from 1.02 (daytime sleepiness) to 1.06 (morning diurnal preference), consistent with the absence of uncontrolled confounding. Z scores for heritability were all greater than 419, supporting the validity of genetic correlation analyses.
- * P less than Bonferonni-corrected threshold of 0.05/7 = 0.007.
Mendelian randomization analyses support causal effects of difficulty awakening and insomnia symptoms on migraine
To investigate whether any of the sleep traits causally influence migraine susceptibility, we performed two-sample MR analyses using established genetic signals to proxy each of the sleep exposures (Fig. 2; Table S1). There was evidence for a significant effect of difficulty awakening on migraine (OR [95% CI] 1.37 [1.12–1.68], P = 0.002). There was also nominal evidence for an effect of liability to insomnia symptoms on migraine (1.09 [1.02–1.16], P = 0.015). Removing weakly correlated SNPs (using a stricter clumping threshold of r2 < 0.001 vs. r2 < 0.01) yielded nearly identical effect estimates for insomnia (36 SNPs; 1.09 [1.01–1.17], P = 0.019) and for difficulty awakening (71 SNPs; 1.37 [1.10–1.71], P = 0.006). MR estimates were null for the effect of all other sleep traits on migraine susceptibility (Fig. 2).
Mendelian randomization estimates are robust in sensitivity analyses
We first tested whether results were consistent when using a genetic instrument for insomnia symptoms developed from a meta-analysis of the UK Biobank and 23andme studies (n = 1.3 million24). Using this 195-SNP genetic instrument, we found a slightly stronger and more significant estimate for a causal effect of liability to insomnia symptoms on migraine (1.14 [1.11–1.16], P = 7.64 × 10−24).
We next tested whether MR results were robust to sensitivity analyses assessing the validity of the assumption of no horizontal pleiotropy. The MR estimates were largely consistent in four model-based sensitivity analyses for pleiotropy (Fig. S1; Table S6). Leave-one-out plots revealed that the rs113851554 variant in MEIS1 flipped the Egger regression effect estimate for insomnia on migraine (Fig. S2). In analyses without this variant, the Egger effect estimate was directionally concordant with the IVW estimate but had wide confidence intervals (Fig. S1). No outliers were detected in any other leave-one-out analyses (Figs. S3–S5).
We next performed sensitivity analyses to determine whether the MR estimates were biased by pleiotropy with other sleep traits or with MDD. There was no evidence for a causal effect of liability to restless legs syndrome (RLS) on migraine susceptibility, suggesting that effects of insomnia symptoms on migraine are not driven by pleiotropic effects of the variants on RLS (1.03 [0.99–1.07], P = 0.10). The effects of insomnia symptoms and difficulty awakening on migraine were consistent when excluding variants associated at genome-wide significance with other sleep traits, and in multivariable MR modeling pleiotropic effects on both exposures (Fig. S1). The MR estimates for the effect of insomnia symptoms on migraine were partly attenuated but remained significant in multivariable MR when adjusting for genetic associations with MDD or anxious symptoms (Fig. S1).
Mendelian randomization does not support causal effects of migraine on sleep patterns and disturbances
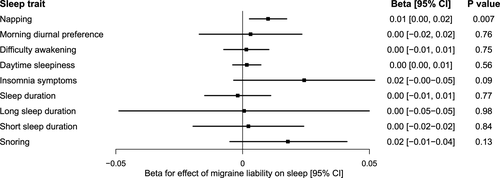
We next assessed whether genetic liability to migraine impacted habitual sleep patterns and disturbances. Genetically predicted liability to migraine did not significantly influence any sleep disturbances, with confidence intervals excluding large effects (Fig. 3; Table S8). There was suggestive evidence for a weak effect of migraine liability on increased napping (0.01 unit increase in napping frequency [0.003, 0.017], P = 0.007), with consistent estimates across sensitivity analyses (Table S9).
Discussion
We leveraged genetic methods to investigate comorbidity and causality between migraine and sleep disturbances. We found evidence for shared genetic influences between multiple sleep traits and migraine, as well as potential causal effects of insomnia symptoms and difficulty awakening on migraine. These effects were robust in sensitivity analyses for horizontal pleiotropy and there was no evidence for strong effects in the reverse direction.
We found evidence f shared genetic influences between several sleep traits and migraine, with the strongest genetic correlation found with insomnia symptoms (rg = 0.29). With the exception of a previously reported genetic correlation of migraine with MDD of 0.32, the magnitude of genetic overlap between insomnia and migraine was greater than that reported for most other common disease traits in the UK Biobank18 and in previous studies,16, 31, 32 suggesting more shared underlying biology between migraine and insomnia than migraine and other cardiometabolic, neuropsychiatric and immune phenotypes. Weaker but highly significant correlations of migraine were seen with other sleep duration and quality traits, confirming that the highly pleiotropic migraine genetic loci also influence sleep traits. As the sample size for migraine GWAS grows, future cross-phenotype analyses may identify specific loci underlying these genetic correlations. Although prior work has demonstrated that rare mutations in the casein kinase (CK Iδ) gene may simultaneously cause familial migraine and advanced sleep phase syndrome,33 our work showed no evidence for an overall shared genetic basis for migraine and morning diurnal preference. This suggests that genetic variation in circadian rhythms may not generally have an important effect on migraine etiology, but certain circadian genes (e.g., CK Iδ) may have pleiotropic roles in migraine via pathways unrelated to their circadian effects.33
Mendelian randomization analyses suggested a causal effect of insomnia symptoms on migraine, adding support to findings from prospective epidemiologic studies.7 This estimate was consistent across sensitivity analyses, and was stronger in a secondary analysis using a larger number of insomnia SNPs from a meta-analysis of UK Biobank and 23andMe. These variants were only used in sensitivity analyses because sample overlap of the insomnia symptoms GWAS (288,557 insomnia cases and 655,920 controls from 23andMe)24 with the migraine GWAS (30,465 migraine cases and 143,147 controls from 23andMe)8 may bias effect estimates away from the null. However, this bias is unlikely to be large given that the degree of case overlap is not large (up to 30,465 migraine GWAS cases included in the insomnia GWAS of n = 1,331,010; 3%) and that the genetic instrument for insomnia is strong (F-statistic > 10).34 Given the nominal statistical evidence for this finding, additional replication in independent samples with well-defined and validated diagnostic criteria for insomnia will strengthen confidence in this effect. Nevertheless, the evidence from this study supports findings from longitudinal epidemiologic studies of insomnia and migraine (reviewed by Uhlig et al.).7 One of the largest studies to date (26,197 participants from the HUNT study) reported that individuals with insomnia at baseline had a relative risk of 1.40 (95% CI 1.0–1.9; P = 0.02) for migraine after 11 years of follow up.35 Our results are also consistent with evidence from a clinical trial of cognitive behavioral therapy for insomnia in patients with migraine, in which treatment of insomnia reduced migraine frequency.36 Although insomnia symptoms are genetically correlated with short sleep duration,9, 12 there was no significant effect of genetically proxied self-reported short sleep duration on migraine. This is in contrast to prior MR analyses which found concordant effects of insomnia and short sleep duration on coronary artery disease risk,9, 37 suggesting that the short sleep component of insomnia may be less relevant to the etiology of migraine. Rather, other features of insomnia such as hyperarousal may play more prominent roles in the etiology of migraine.38
Relative to insomnia, less is known about the phenomenon of difficulty awakening, which in some settings is referred to as sleep inertia.39, 40 Difficulty awakening is inversely genetically correlated24 with morning diurnal preference (rg = −0.78) and with insomnia symptoms (rg = 0.23) and may therefore reflect a combination of circadian misalignment and interrupted sleep.24, 39 However, we did not find evidence for a causal effect of morning diurnal preference on migraine. This suggests that the effect of difficulty awakening on migraine may be driven by disturbances to sleep quality rather than through circadian mechanisms. Difficulty awakening40 may also be a consequence of psychiatric comorbidities, and prior work has highlighted genetic correlations between sleep and psychiatric comorbidities,9, 12 and between migraine and psychiatric disease.31 This motivated multivariable MR analyses adjusting MR estimates for potential pleiotropy with MDD and with anxious symptoms. We found partial attenuation of the MR estimates for both difficulty awakening and insomnia symptoms on migraine when adjusting for MDD, however the adjusted MR estimate remained significant. This finding is consistent with prior epidemiologic analyses that have shown that sleep disturbances influence migraine risk independently of MDD and anxiety.41 This suggests that sleep disturbances directly influence migraine risk independently of psychiatric comorbidities and therefore warrant intervention in their own right.
There was minimal evidence for an effect of migraine on any of the sleep patterns or disturbances. While longitudinal epidemiologic studies lasting up to 11 years have suggested potential effects of migraine on insomnia risk,7, 35 our results are in line with microlongitudinal studies that have not shown effects of migraine headaches on next-day sleep.42 We did, however, identify a small effect of migraine liability on increased napping frequency. The use of naps as an acute abortive treatment for migraine2 may be one possible mechanism mediating this effect. The generally null effects of migraine on habitual sleep patterns do not exclude an acute effect of a migraine episode on sleep. An analogy may be drawn to the relationship of caffeine with sleep, where MR analyses have not shown causal effects of caffeine on sleep patterns,43 suggesting discordance between effects of short and long-term caffeine consumption. Similarly, while a migraine headache may acutely interrupt sleep, we did not find strong evidence for effects of migraine liability on sustained sleep patterns.
There are several potential pathways by which sleep quality or insomnia symptoms may influence migraine susceptibility. Cortical excitability, a potential mechanism of migraine pathophysiology,44 may be increased by insomnia.45 Sleep disturbances2, 46 may also reduce pain thresholds47 and cause dysfunction of the glymphatic system, resulting in accumulation of nociceptive CNS waste.4, 41 Finally, difficulty awakening may reflect slow clearance of CNS adenosine,39 with the consequent increases in adenosine increasing the likelihood of headache onset.48 Additional work is necessary to determine which of these pathways, if any, are relevant to the effect of sleep disturbances on migraine.
We acknowledge limitations to this work. First, although we incorporated sensitivity analyses for horizontal pleiotropy, we cannot fully exclude the influence of this potential bias. Second, MR power calculators are not currently designed for ordinal or binary exposures, so we focused on interpretation of the confidence intervals to determine whether the bounds contained clinically relevant effects. Third, single, self-reported questions are less reliable for phenotyping than validated scales or physician-diagnosed insomnia, which were unavailable in UKB. Fourth, the known common variant contributions to migraine primarily reflect the genetic architecture of migraine without aura (MO), which is the most prevalent form of migraine.8 Our findings may therefore have greater relevance to the pain component of migraine, which is more prominent in MO.49 This limitation may be addressed in future analyses as genetic data on migraine with aura become more robust. Finally, the selection of relatively healthy individuals into UKB may limit generalizability to less healthy populations and to populations of non-European ancestry.
Conclusion
The genetic determinants of sleep and migraine are partly overlapping. Sleep disturbances may causally influence migraine etiology, and are promising targets for the treatment of migraine.
Author Contributions
ID and RS conceived and designed the study with input from all coauthors. RS and DC provided the data. ID and YG analyzed the data. ID drafted the initial manuscript. RS, AV, YG, and DC provided critical feedback to the manuscript and approved the final version. ID and RS are the guarantors. The corresponding authors attest that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Acknowledgements
This research has been conducted using the UK Biobank Resource (application 6818). We thank the staff and participants of the UK Biobank, and the members of the UK Biobank Sleep and Chronotype Genetics team. Although 23andMe provided the migraine GWAS summary statistics, they had no role in the design, conduct, or analysis of the study. We would like to thank the research participants and employees of 23andMe for making this work possible. Finally, we acknowledge the members of the International Headache Genetics Consortium below:
International Headache Genetics Consortium Members
Padhraig Gormley31−34, Verneri Anttila32,33,35, Bendik S. Winsvold36−38, Priit Palta39, Tonu Esko32,40,41, Tune H. Pers32,41−43, Kai-How Farh32,35,44, Ester Cuenca-Leon31−33,45, Mikko Muona39,46−48, Nicholas A. Furlotte30, Tobias Kurth49,9, Andres Ingason10, George McMahon50, Lannie Ligthart51, Gisela M. Terwindt52, Mikko Kallela53, Tobias M. Freilinger54,55, Caroline Ran56, Scott G. Gordon22, Anine H. Stam52, Stacy Steinberg10, Guntram Borck57, Markku Koiranen58, Lydia Quaye59, Hieab H. H. Adams6,61, Terho Lehtimäki62, Antti-Pekka Sarin39, Juho Wedenoja63, David A. Hinds30, Julie E. Buring9,64, Markus Schürks65, Paul M. Ridker9,64, Maria Gudlaug Hrafnsdottir66, Hreinn Stefansson10, Susan M. Ring50, Jouke-Jan Hottenga51, Brenda W. J. H. Penninx67, Markus Färkkilä53, Ville Artto53, Mari Kaunisto39, Salli Vepsäläinen53, Rainer Malik55, Andrew C. Heath68, Pamela A. F. Madden68, Nicholas G. Martin22, Grant W. Montgomery8, Mitja I. Kurki31−33,39,69, Mart Kals40, Reedik Mägi40, Kalle Pärn40, Eija Hämäläinen39, Hailiang Huang32,33,35, Andrea E. Byrnes32,33,35, Lude Franke70, Jie Huang34, Evie Stergiakouli50, Phil H. Lee31−33, Cynthia Sandor71, Caleb Webber71, Zameel Cader72,73, Bertram Muller-Myhsok74,75, Stefan Schreiber76, Thomas Meitinger77,78, Johan G. Eriksson79,8, Veikko Salomaa80, Kauko Heikkilä81, Elizabeth Loehrer60,82, Andre G. Uitterlinden83, Albert Hofman60, Cornelia M. van Duijn60, Lynn Cherkas59, Linda M. Pedersen36, Audun Stubhaug84,85, Christopher S. Nielsen84,86, Minna Männikkö58, Evelin Mihailov40, Lili Milani40, Hartmut Göbel87, Ann-Louise Esserlind88, Anne Francke Christensen88, Thomas Folkmann Hansen89, Thomas Werge90,91,7, Jaakko Kaprio39,63,92, Arpo J. Aromaa80, Olli Raitakari93,94, M. Arfan Ikram60,61,95, Tim Spector59, Marjo-Riitta Järvelin58,96−98, Andres Metspalu40, Christian Kubisch99, David P. Strachan100, Michel D. Ferrari52, Andrea C. Belin56, Martin Dichgans55,75, Maija Wessman39,46, Arn M. J. M. van den Maagdenberg52,101, John-Anker Zwart36−38, Dorret I. Boomsma51, George Davey Smith50, Kari Stefansson10,102, Nicholas Eriksson30, Mark J. Daly32,33,35, Benjamin M. Neale32,33,35, Jes Olesen88, Daniel I. Chasman9, Dale R. Nyholt1, Aarno Palotie31−35,103.
International Headache Genetics Consortium Affiliations
1School of Biomedical Sciences, Faculty of Health, and Institute of Health and Biomedical Innovation, Queensland University of Technology, Brisbane, Queensland, Australia. 2Department of Epidemiology and Cancer Control, St. Jude Children’s Research Hospital, Memphis, Tennessee 38105, USA. 323andMe, Inc., 899 W. Evelyn Avenue, Mountain View, California 94041, USA. 4School of Pharmacy and Biomedical Sciences, University of Central Lancashire, Preston PR1 2HE, United Kingdom. 5Department of Obstetrics and Gynecology, Niigata University Graduate School of Medical and Dental Sciences, Niigata 950-2181, Japan. 6Department of Biomedicine - Human Genetics, Aarhus University, DK-8000 Aarhus, Denmark. 7iPSYCH, The Lundbeck Foundation Initiative for Integrative Psychiatric Research, DK-2100 Copenhagen, Denmark. 8Institute for Molecular Bioscience, The University of Queensland, Brisbane, Queensland 4072, Australia. 9Divisions of Preventive Medicine, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA. 10deCODE Genetics/Amgen, 101 Reykjavik, Iceland. 11Department of Biostatistics, University of Liverpool, Liverpool L69 3GL, UK. 12Wellcome Trust Centre for Human Genetics, University of Oxford, Oxford OX3 7BN, UK.13KULeuven, Department of Development and Regeneration, Organ systems, 3000 Leuven, Belgium. 14Department of Obstetrics and Gynaecology, Leuven University Fertility Centre, University Hospital Leuven, 3000 Leuven, Belgium. 15Harvard T.H. Chan School of Public Health, Boston, Massachusetts 02115, USA. 16Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, Massachusetts 02115, USA. 17Division of Preventive Medicine, Brigham and Women’s Hospital, Boston, Massachusetts 02215, USA. 18Institute of Medicine and Public Health, Vanderbilt University Medical Center, Nashville, Tennessee 37203, USA. 19Vanderbilt Genetics Institute, Division of Epidemiology, Institute of Medicine and Public Health, Department of Medicine, Vanderbilt University Medical Center, Nashville, Tennessee 37203, USA. 20Cognitive Science Department, University of California, San Diego, La Jolla, California 92093, USA. 21Institute of Biological Psychiatry, Mental Health Centre Sct. Hans, Copenhagen University Hospital, DK-2100 Copenhagen, Denmark. 22Department of Genetics and Computational Biology, QIMR Berghofer Medical Research Institute, Brisbane, Queensland 4006, Australia. 23Endometriosis CaRe Centre, Nuffield Dept of Obstetrics & Gynaecology, University of Oxford, John Radcliffe Hospital, Oxford OX3 9DU, UK. 24Center for Integrative Medical Sciences, RIKEN, Yokohama 230-0045, Japan. 25Institute of Medical Sciences, The University of Tokyo, Tokyo 108-8639, Japan. 26Department of Obstetrics and Gynecology, Landspitali University Hospital, 101 Reykjavik, Iceland. 27Faculty of Medicine, School of Health Sciences, University of Iceland, 101 Reykjavik, Iceland. 28Vanderbilt Genetics Institute, Vanderbilt Epidemiology Center, Institute of Medicine and Public Health, Department of Obstetrics and Gynecology, Vanderbilt University Medical Center, Nashville, Tennessee 37203, USA. 29Global Medical Affairs Fertility, Research and Development, Merck KGaA, Darmstadt, Germany. 3023andMe, Inc., 899 W. Evelyn Avenue, Mountain View, California 94041, USA. 31Psychiatric and Neurodevelopmental Genetics Unit, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA. 32Medical and Population Genetics Program, Broad Institute of MIT and Harvard, Cambridge, Massachusetts, USA. 33Stanley Center for Psychiatric Research, Broad Institute of MIT and Harvard, Cambridge, Massachusetts, USA. 34Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton, UK. 35Analytic and Translational Genetics Unit, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA. 36FORMI, Oslo University Hospital, Oslo, Norway. 37Department of Neurology, Oslo University Hospital, Oslo, Norway. 38Institute of Clinical Medicine, University of Oslo, Oslo, Norway. 39Institute for Molecular Medicine Finland (FIMM), University of Helsinki, Helsinki, Finland. 40Estonian Genome Center, University of Tartu, Tartu, Estonia. 41Division of Endocrinology, Boston Children’s Hospital, Boston, Massachusetts, USA. 42Department of Epidemiology Research, Statens Serum Institut, Copenhagen, Denmark. 43Novo Nordisk Foundation Center for Basic Metabolic Research, University of Copenhagen, Copenhagen, Denmark. 44Illumina, San Diego, California, USA. 45Pediatric Neurology, Vall d’Hebron Research Institute, Barcelona, Spain. 46Folkhälsan Institute of Genetics, Helsinki, Finland. 47Neuroscience Center, University of Helsinki, Helsinki, Finland. 48Molecular Neurology Research Program, Research Programs Unit, University of Helsinki, Helsinki, Finland. 49Institute of Public Health, Charité–Universitätsmedizin Berlin, Berlin, Germany. 50Medical Research Council (MRC) Integrative Epidemiology Unit, University of Bristol, Bristol, UK. 51Department of Biological Psychology, Vrije Universiteit, Amsterdam, the Netherlands. 52Department of Neurology, Leiden University Medical Centre, Leiden, the Netherlands. 53Department of Neurology, Helsinki University Central Hospital, Helsinki, Finland. 54Department of Neurology and Epileptology, Hertie-Institute for Clinical Brain Research, University of Tuebingen, Tuebingen, Germany. 55Institute for Stroke and Dementia Research, Klinikum der Universität München, Ludwig-Maximilians-Universität München, Munich, Germany. 56Department of Neuroscience, Karolinska Institutet, Stockholm, Sweden. 57Institute of Human Genetics, Ulm University, Ulm, Germany. 58Center for Life Course Epidemiology and Systems Medicine, University of Oulu, Oulu, Finland. 59Department of Twin Research and Genetic Epidemiology, King’s College London, London, UK. 60Department of Epidemiology, Erasmus University Medical Center, Rotterdam, the Netherlands. 61Department of Radiology, Erasmus University Medical Center, Rotterdam, the Netherlands. 62Department of Clinical Chemistry, Fimlab Laboratories, School of Medicine, University of Tampere, Tampere, Finland. 63Department of Public Health, University of Helsinki, Helsinki, Finland. 64Harvard Medical School, Boston, Massachusetts, USA. 65Department of Neurology, University Duisburg–Essen, Essen, Germany. 66Landspitali University Hospital, Reykjavik, Iceland. 67Department of Psychiatry, VU University Medical Centre, Amsterdam, the Netherlands. 68Department of Psychiatry, Washington University School of Medicine, St. Louis, Missouri, USA. 69Department of Neurosurgery, NeuroCenter, Kuopio University Hospital, Kuopio, Finland. 70Department of Genetics, University Medical Center Groningen, University of Groningen, Groningen, the Netherlands. 71MRC Functional Genomics Unit, Department of Physiology, Anatomy & Genetics, Oxford University, Oxford, UK. 72Nuffield Department of Clinical Neuroscience, University of Oxford, Oxford, UK. 73Oxford Headache Centre, John Radcliffe Hospital, Oxford, UK. 74Max Planck Institute of Psychiatry, Munich, Germany. 75Munich Cluster for Systems Neurology (SyNergy), Munich, Germany. 76Institute of Clinical Molecular Biology, Christian Albrechts University, Kiel, Germany. 77Institute of Human Genetics, Helmholtz Zentrum München, Neuherberg, Germany. 78Institute of Human Genetics, Technische Universität München, Munich, Germany. 79Department of General Practice and Primary Health Care, University of Helsinki and Helsinki University Hospital, Helsinki, Finland. 80National Institute for Health and Welfare, Helsinki, Finland. 81Institute of Clinical Medicine, University of Helsinki, Helsinki, Finland. 82Department of Environmental Health, Harvard T.H. Chan School of Public Health, Boston, Massachusetts, USA. 83Department of Internal Medicine, Erasmus University Medical Center, Rotterdam, the Netherlands. 84Department of Pain Management and Research, Oslo University Hospital, Oslo, Norway. 85Medical Faculty, University of Oslo, Oslo, Norway. 86Department of Ageing and Health, Norwegian Institute of Public Health, Oslo, Norway. 87Kiel Pain and Headache Center, Kiel, Germany. 88Danish Headache Center, Department of Neurology, Rigshospitalet, Glostrup Hospital, University of Copenhagen, Copenhagen, Denmark. 89Institute of Biological Psychiatry, Mental Health Center Sct. Hans, University of Copenhagen, Roskilde, Denmark. 90Institute of Biological Psychiatry, MHC Sct. Hans, Mental Health Services Copenhagen, Copenhagen, Denmark. 91Institute of Clinical Sciences, Faculty of Medicine and Health Sciences, University of Copenhagen, Copenhagen, Denmark. 92Department of Health, National Institute for Health and Welfare, Helsinki, Finland. 93Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland. 94Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland. 95Department of Neurology, Erasmus University Medical Center, Rotterdam, the Netherlands. 96Department of Epidemiology and Biostatistics, MRC Health Protection Agency (HPE) Centre for Environment and Health, School of Public Health, Imperial College London, London, UK. 97Biocenter Oulu, University of Oulu, Oulu, Finland. 98Unit of Primary Care, Oulu University Hospital, Oulu, Finland. 99Institute of Human Genetics, University Medical Center Hamburg-Eppendorf, Hamburg, Germany. 100Population Health Research Institute, St George’s, University of London, London, UK. 101Department of Human Genetics, Leiden University Medical Centre, Leiden, the Net herlands. 102Faculty of Medicine, University of Iceland, Reykjavik, Iceland. 103Department of Neurology, Massachusetts General Hospital, Boston, Massachusetts, USA.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Sleep GWAS data are available on the Sleep Disorder Knowledge Portal: http://www.kp4cd.org/dataset_downloads/sleep. The IHGC migraine GWAS data are available upon request to 23andMe: https://research.23andme.com/dataset-access/.




