The neural network basis of altered decision-making in patients with amyotrophic lateral sclerosis
Abstract
Objective
Amyotrophic lateral sclerosis (ALS) is a multisystem disorder associated with motor impairment and behavioral/cognitive involvement. We examined decision-making features and changes in the neural hub network in patients with ALS using a probabilistic reversal learning task and resting-state network analysis, respectively.
Methods
Ninety ALS patients and 127 cognitively normal participants performed this task. Data from 62 ALS patients and 63 control participants were fitted to a Q-learning model.
Results
ALS patients had anomalous decision-making features with little shift in choice until they thought the value of the two alternatives had become equal. The quantified parameters (Pαβ) calculated by logistic regression analysis with learning rate and inverse temperature well represented the unique choice pattern of ALS patients. Resting-state network analysis demonstrated a strong correlation between Pαβ and decreased degree centrality in the anterior cingulate gyrus and frontal pole.
Interpretation
Altered decision-making in ALS patients may be related to the decreased hub function of medial prefrontal areas.
Introduction
Amyotrophic lateral sclerosis (ALS) is an adult-onset neurodegenerative disorder characterized by upper and lower motor neuron involvement.1 Although extra-motor cognitive and behavioral impairment were considered atypical clinical features in early descriptions of ALS, recent studies demonstrated that approximately 50% of patients with ALS suffer from frontotemporal dysfunction.2 Furthermore, almost all patients with sporadic ALS and more than half of those with frontotemporal dementia (FTD) had TAR DNA-binding protein 43 (TDP-43)-positive ubiquitinated cytoplasmic inclusions.1-4
While ALS and FTD are certainly on a spectrum, most ALS patients do not meet the FTD criteria and they are not consecutive in time. However, previous studies demonstrated that ALS patients who do not meet the FTD criteria show abnormalities not only in the frontotemporal cortex but also in the basal ganglia, including the head of the caudate nucleus and its networks.5-7 Although frontostriatal circuits are associated with decision-making processes8 and patients with FTD show various errors in decision-making,9-11 there is limited available evidence regarding the features of decision-making, particularly its network basis, in patients with ALS.
The probabilistic reversal learning (PRL) task is widely used to assess deficits in decision-making associated with the caudate nucleus, orbitofrontal cortex, and medial cingulate cortex.8 The PRL task is composed of two stages, that is, acquisition learning and reversal learning. Due to its stochastic nature, performance is optimally achieved by incremental learning of the action–outcome contingency over many trials. Reversal learning needs a flexible change in a previously established stimulus–response association when the prior response is no longer rewarding.
PRL also includes representative models of reinforcement learning (RL), which is a behavioral process to learn the values of actions to improve future outcomes using trial and error.12, 13 RL has two critical parameters: learning rate (alpha), which represents the extent to which old values are updated by newly acquired information, and inverse temperature (beta), known as the degree to which value estimates influence choice; high inverse temperatures indicate that individuals tend to select the higher-value option, while low inverse temperatures indicate that value differences between options govern choices to a lesser extent.
In the present study, we investigated alterations in PRL findings in patients with ALS and their underlying network basis using resting-state functional MRI (RS-fMRI). We used intrinsic connectivity contrast (ICC),14 which is a whole-brain voxel-based hypothesis-free analysis based on graph theory that provides a correlation map without prior information or assumptions.15
Methods
Participants
Patients with ALS were recruited at the Department of Neurology at Nagoya University (March 2015 to December 2018). All patients fulfilled the El Escorial revised criteria for probable laboratory-supported or probable ALS.16 A board-certificated neurologist (W.H.) and trained speech therapist (O.R.) conducted semi-structured clinical interviews concerning the FTD criteria.17, 18 Two patients with ALS met the criteria for behavioral variant FTD.
We also evaluated cognitively normal participants who did not show any cognitive impairment (Addenbrooke's Cognitive Examination Revised [ACE-R] ≥ 89) or neurological and psychological diseases. Based on the Fazekas hyperintensity rating system, we could not find white matter abnormalities characterized by hyperintensities more severe than grade 2 in T2-weighted MR images.19
All participants in the present study had adequate vision (with or without eyeglasses) to perform the PRL task.
This study was conducted according to the Ethical Guidelines for Medical and Health Research Involving Human Subjects endorsed by the Japanese government and approved by the Ethical Review Committee of Nagoya University Graduate School of Medicine. We obtained written informed consent from patients with ALS and cognitively normal participants.
Cognitive assessments
To assess general cognitive function, the Mini-Mental State Examination (MMSE), ACE-R,20, 21 Frontal Assessment Battery (FAB), Stroop test, digit span (forward and backward), and word fluency (letter and semantic) were performed to assess detailed executive function in patients with ALS.
PRL task
The PRL task comprised 120 trials. In each trial, the participants were presented with two abstract line drawings on the left and right sides of the screen. The presentation side was randomized for each trial. The participants were asked to select one drawing by pressing a key within 1000 ms, and were consequently presented with a reward or loss. If the participant did not select a stimulus within the presentation time window, the message “Time-up” appeared and the next trial was initiated. During the first 60 trials, one drawing had an advantageous option; the reward/loss frequency ratio was 80:20. The other drawing had a disadvantageous option; the reward/loss frequency ratio was 20:80. After 60 trials, the contingencies were reversed without any instruction to the participants (Supplementary Data 1). Before starting the examination, we set up a practice task to see if the participants understood the task. After the examination, we asked the participants how they had made their choice and reconfirmed that they had understood the task. The paradigm used here was based on previous studies.22
We excluded participants whose score during the acquisition phase was over 1.5 standard deviations below the mean score of cognitively normal participants because they might not have understood the task. We also excluded cognitively normal participants whose score of the reversal phase was over 1.5 standard deviations below the mean score to only select participants who performed the task successfully.
Learning models
We used Q-learning, win–stay, lose–shift (WSLS), and random choice models to classify choice behavior.8 When analyzing decision-making behavior through RL, the elimination of subjects who randomly select their choices is generally used.23, 24 In decision-making tasks, including the PRL task, it is well known that some participants make decisions based on the WSLS model instead of the RL model. The WSLS model is only sensitive to the outcome of the previous choice, and the mathematical formulas used for analysis are different.13, 25, 26 It is also necessary to exclude cases who adopt the WSLS model when analyzing decision-making behavior.
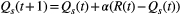
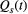 is the estimated action value for stimulus s on trial t, and R(t) is the reward value of the choice on trial t. Given the estimated values are set, the probability of choosing stimulus 1 in the next trail is determined as the soft-max function below:
is the estimated action value for stimulus s on trial t, and R(t) is the reward value of the choice on trial t. Given the estimated values are set, the probability of choosing stimulus 1 in the next trail is determined as the soft-max function below:
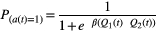
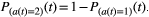
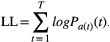
The optima function in R was employed to find the parameter set that produced the highest log-likelihood.
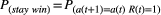
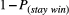 . The second parameter represented the probability of shifting to another option on the next trial if a reward was not provided:
. The second parameter represented the probability of shifting to another option on the next trial if a reward was not provided:
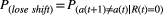
The probability of staying with an option following a loss trial was 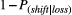 .26
.26
In the random choice model, each choice of options was made randomly and in equal frequency.
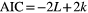
Logistic regression analysis
Logistic regression analysis of alpha and beta values showed the extent to which the participant’s choice behavior was characteristic to ALS compared with the control group. This estimated logistic regression equation was designated as “Pαβ” in the current study. By using receiver operating characteristic (ROC) analysis, we calculated the value of Pαβ that most efficiently discriminated between the ALS and control groups. Based on the value of Pαβ, patients with ALS were classified into two groups: patients with anomalous choice behavior and patients with normal choice behavior. We compared ALS patients who had anomalous choice behavior with those who had normal choice behavior, and assessed the clinical features in ALS patients with anomalous choice behavior.
Analysis of RS-fMRI
RS-fMRI was used to identify the underlying neural networks linked to specific choice behavior in patients with ALS. All MRI scans were performed using a Siemens Magnetom Verio (Siemens, Erlangen, Germany) 3.0-T scanner with a 32-channel head coil at the Brain and Mind Research Center. High-resolution T1-weighted images (T1-WI) were acquired using the following parameters: repetition time (TR) = 2.5 s, echo time (TE) = 2.48 ms, 192 sagittal slices with 1-mm thickness, field of view (FOV) = 256 mm, 256 × 256 matrix size, and an in-plane voxel resolution of 1 × 1 mm2. Eight-minute closed eyes resting-state functional images were obtained with echo-planar imaging using the following parameters: TR = 2.5 s, TE = 30 ms, 39 transversal slices with a 0.5-mm interslice interval and 3-mm thickness, FOV = 192 mm, 64 × 64 matrix dimension, flip angle = 80°, and 198 volumes.
Functional MRI data were preprocessed using the default pipeline as the standard procedure implemented in Conn Functional Connectivity Toolbox version 18b,28 using MATLAB. The first five volumes of each participant’s data were discarded to remove initial image inhomogeneity. Then, each participant’s images were realigned, unwarped to remove dynamic EPI distortions,29 slice-time corrected, co-registered to the bias corrected T1-WI, segmented and normalized to Montreal Neurological Institute coordinates, resampled to an isotropic voxel resolution of 2 × 2 × 2 mm3, and smoothed using an 8-mm FWHM Gaussian filter. Moreover, the principal component-based noise-correction CompCor approach was applied to remove the BOLD signal noise associated with white matter and cerebrospinal fluid. Band-pass filtering was performed with a frequency window of 0.008–0.09 Hz. Artifact detection tools (ART)-based outlier detection and scrubbing were performed to eliminate the effects of head motion.
ICC analysis
In graph theory, “degree centrality” represents the number of direct connections between a given region and the rest of the brain. By measuring degree centrality, ICC analysis detects hub regions.14 We calculated the ICC-power (ICC-p) value that could be interpreted as a “weighted” degree centrality, a network measure representing the number of connections between a given voxel and the rest of the brain, obtained with the connectivity threshold set to 0. We performed correlation analysis between the ICC-p value and Pαβ using the CONN toolbox considering age, sex, and education history as nuisance covariates. Statistical significance was set at a height threshold of P < 0·005, FDR-corrected cluster-size threshold of P < 0·05, and one-sided negative contrast to reveal network dysfunction. We calculated the mean ICC-p value of the cluster with significantly different ICC-P values.
We subsequently performed seed-to-voxel analysis to identify the origin of the alterations in degree centrality. First, all regions with significantly different ICC-p values were extracted as the regions of interest for seeds. The average filtered BOLD signal in each seed was evaluated, and its bivariate correlation with the BOLD signal in all other voxels in the brain was computed. Then, we conducted correlation analysis on the calculated connectivity and Pαβ. The results were assessed with a cluster-forming height threshold of P < 0.005 and FDR-corrected cluster-size threshold of P < 0.05.
Statistical analyses
Clinical backgrounds and cognitive examinations were compared using the Mann–Whitney or chi-squared test. The threshold of statistical significance was set at P < 0.05. Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) version 24 (SPSS, Inc., Chicago, IL, USA).
Results
Specific choice behavior in patients with ALS
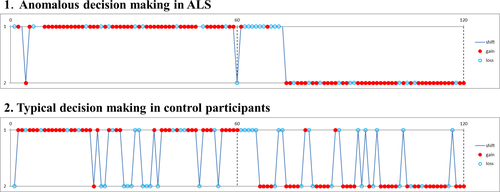
We performed the PRL task in 127 cognitively normal people and 90 patients with ALS. Clinical backgrounds and cognitive examinations are shown in Supplementary Data 2. Although there were no significant differences in the scores of the PRL tasks and ratio of strategies classified by AIC, we found some ALS patients demonstrated a unique choice behavior (Figure 1), that is, they rarely changed their choice, with few switches in the early stage of the acquisition and reversal phases. Few cognitively normal participants showed such a pattern. Thus, we selected cognitively normal participants with typical choice behavior as control subjects and compared them with ALS patients, to reveal the choice behavior features of ALS patients with unique choice behavior.
First, we excluded 19 cognitively normal participants who scored below 1.5 standard deviations from the mean score during the acquisition and/or reversal phase (score at acquisition phase < 28, score at reversal phase < 20) since they could not perform the PRL task appropriately. Second, we classified their strategies of choice behavior into Q-learning, WSLS, and random choice models, using AIC. Since the unique choice behavior of patients with ALS had the lowest AIC value of Q-learning among the three models, we selected 62 from 90 patients with ALS and 66 of 108 control participants who adopted RL (Q-learning model) and evaluated the parameters in the Q-learning model. Finally, we excluded three control participants who showed abnormal inverse temperature with scores over 3 standard deviations from the mean score (Supplementary Data 3).
Demographic and cognitive features of participants with the Q-learning model
Choice data from 62 patients with ALS and 63 control participants were best fitted using the Q-learning model. Clinical backgrounds and results of the PRL task are shown in Table 1. Although there was no significant difference in age, the ALS group exhibited significantly lower education and MMSE and ACE-R scores. In the PRL task, there were no significant differences in total score, score at acquisition phase, or score at reversal phase between the ALS and control participants. Although the alpha value tended to be lower in the ALS group, it was not significantly different from the control group. The mean PRL beta value was significantly higher in patients with ALS compared to controls.
| Control | ALS | P-value | |
|---|---|---|---|
| N | 63 | 62 | - |
| Age (y) | 63.4 (9.8) | 66.0 (8.9) | N.S. (0.152) |
| Sex (men: women) | 24:39 | 40:21 | 0.002 |
| Education (years) | 14.1 (2.2) | 12.1 (2.6) | <0.001 |
|
Clinical phenotype (spinal: bulbar: others) |
- | 44:16:2 | - |
| Disease duration (years) | - | 1.9 (1.4) | - |
| ALSFRS-R | - | 40.9 (4.1) | - |
| MMSE | 29.2 (1.1) | 27.7 (2.1) | <0.001 |
| ACE-R Total score | 97.3 (2.6) | 89.0 (8.8) | <0.001 |
| ACE-R Orientation/attention | 17.9 (0.3) | 17.4 (1.3) | 0.002 |
| ACE-R Memory | 24.7 (1.6) | 20.4 (4.3) | <0.001 |
| ACE-R Fluency | 13.7 (0.6) | 12.2(2.4) | <0.001 |
| ACE-R Language | 25.2 (1.0) | 23.8 (2.1) | <0.001 |
| ACE-R Visuospatial abilities | 15.7 (0.7) | 15.2 (1.0) | 0.004 |
| Total score in PRL task | 74.3 (7.6) | 72.4 (9.4) | N.S. (0.229) |
| Score at acquisition phase | 41.7 (4.2) | 40.9 (6.4) | N.S. (0.980) |
| Score at reversal phase | 32.6 (5.8) | 31.5 (8.2) | N.S. (0.692) |
| Alpha | 0.4 (0.3) | 0.3 (0.3) | N.S. (0.197) |
| Beta | 4.0 (1.7) | 7.0 (7.1) | 0.007 |
- Data are shown as mean ± standard deviation (SD). Age, years of education, and scores in cognitive examinations and the PRL task were compared by Mann–Whitney analysis. Sex was compared by the chi-squared test. The statistical significance threshold was set at P < 0.05. ACE-R, Addenbrooke’s Cognitive Examination revised; ALSFRS-R, ALS Functional Rating Scale Revised; MMSE, Mini-Mental State Examination; N.S., not significant.
Features of choice behavior in patients with ALS
Figure 1 shows a representative specific choice pattern, with low exploration and high exploitation, observed in patients with ALS, with high beta values, but relatively variable alpha values. However, ALS patients who had a mild to moderate increase in beta values (from 5 to 10) showed lower alpha values than controls. Besides, there was no significant correlation between alpha and beta values in patients with ALS. Thus, we applied logistic regression analysis to predict the probability of ALS (Pαβ) based on the two parameters concerning choice behavior. According to AIC, Pαβ yielded stronger explanatory power than those using alpha or beta values alone (AIC alpha = 174.69, AIC beta = 163.78, AIC alpha and beta = 162.12). A higher Pαβ well represented the unique choice pattern observed in patients with ALS (Figure 2). A Pαβ cutoff value of 0.512 provide optimal separation between ALS patients and controls in ROC analysis (area under the ROC curve, 0.666 [95% confidence interval 0.571–0.761]; sensitivity, 0.565; and specificity, 0.762). There were no significant correlations between Pαβ and age, education, disease type, disease duration, disease severity (ALS Functional Rating Scale Revised [ALSFRS-R]), and other scores of cognitive tests in patients with ALS (Supplementary Data 4).
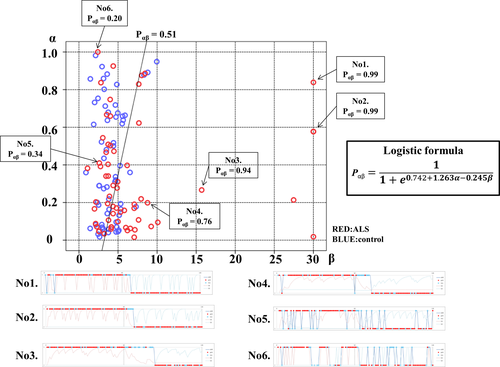
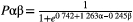 . A higher Pαβ was associated with more ALS-specific choice behavior. Examples of choice behavior are shown in 1–6 (1–5 showed anomalous choice behavior; 6 showed typical choice behavior). The line (P = 0.512) demonstrates ROC analysis results, which determined the most effective division between the ALS and control groups.
. A higher Pαβ was associated with more ALS-specific choice behavior. Examples of choice behavior are shown in 1–6 (1–5 showed anomalous choice behavior; 6 showed typical choice behavior). The line (P = 0.512) demonstrates ROC analysis results, which determined the most effective division between the ALS and control groups.Clinical characteristics and ALS-specific choice behavior
Using a Pαβ cutoff value of 0.512, 35 out of 62 ALS patients were classified into the ALS anomalous choice behavior group. In a comparison of ALS patients who had anomalous choice behavior with those who had typical choice behavior, there were no significant differences in clinical backgrounds and conventional cognitive examinations. In the PRL task, ALS patients with anomalous choice behavior exhibited significantly higher scores at the acquisition phase, lower alpha values, and higher beta values (Supplementary Data 5).
In the 35 patients with ALS who had anomalous choice behavior, the total score of the PRL task was significantly correlated with alpha, MMSE, and ACE-R. Furthermore, the score at the acquisition phase was significantly correlated with MMSE. The score at the reversal phase was significantly correlated with alpha and MMSE (Supplementary Data 6).
Functional connectivity changes associated with ALS-specific choice behavior
We performed RS-fMRI in 34 patients with ALS and 33 age- and sex-matched control participants. There were no significant differences in clinical backgrounds, PRL scores, parameters, and Pαβ of ALS patients who underwent MRI and those who did not undergo MRI. ALS patients who underwent MRI had higher ACE-R scores than those who did not (Supplementary Data 7).
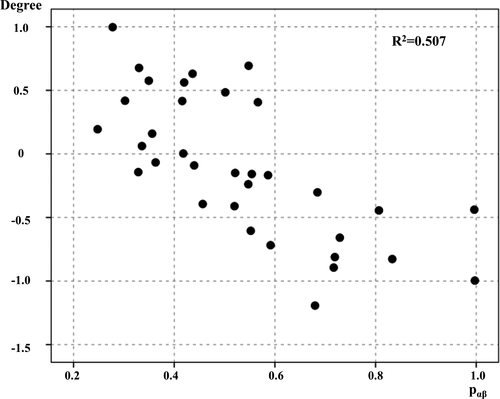
ICC analysis demonstrated that Pαβ was associated with decreased degree centrality in the region of the anterior cingulate gyrus and frontal pole (Figure 3). Only the ALS group showed a significant correlation between the degree centrality Z-score in this region and Pαβ score (r = −0.712, p < 0.001; Figure 4). Seed-based analysis from the region of the anterior cingulate gyrus and frontal pole revealed that patients with ALS had decreased functional connectivity with the paracingulate gyrus, frontal medial cortex, anterior cingulate gyrus, frontal pole, subcallosal cortex, and superior frontal gyrus (Supplementary Data 8).
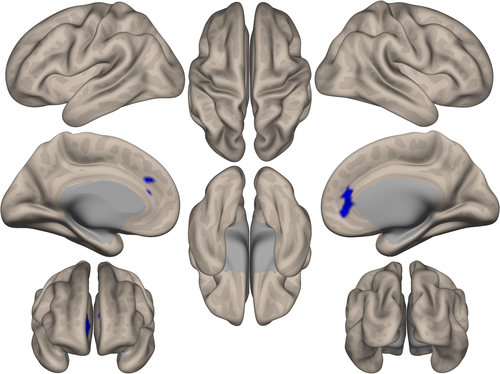
Discussion
This is the first study to demonstrate that ALS patients could have an anomalous decision-making pattern, in which there is little exploration and infrequent shifts in their choice to other PRL task alternatives. ALS patients barely chose the option they deemed disadvantageous, but often changed their choice when they thought the value of the two alternatives was equal. Pαβ calculated by the Q-learning model and logistic regression analysis well represented the unique choice pattern of patients with ALS. Pαβ was not correlated with age, education, other conventional cognitive tests, and disease severity. Network analysis using the ICC method demonstrated a significant correlation between Pαβ and decreased degree centrality in the regions associated with decision-making (including the anterior cingulate gyrus and frontal pole). Altered decision-making in ALS patients may be related to the decreased hub function of medial prefrontal areas.
RL, Q-learning model, inverse temperature, and learning rate
The PRL task was designed to assess RL capacity, but as Worthy et al30 demonstrated, participants often use heuristic-based models, including the WSLS model, during these kinds of tasks. In particular, when working memory load was high, participants were more likely to adopt WSLS strategies than RL.31 In the WSLS model, they were more likely to stay-with or shift-to options with higher expected values than options with lower expected values (i.e., RL). The combined WSLS-RL dual-process model may provide a superior fit to the data relative to the WSLS model alone.32 When different learning parameters could be associated with positive and negative prediction errors33, 34 and participants had a high learning rate and high inverse temperature, decision-making based on Q-learning was close to that based on the WSLS model.
However, individual choices in the Q-learning model are not made in isolation, but are embedded in a series of past experiences, decisions, and outcomes. Conversely, the WSLS model is only sensitive to the outcome of the previous choice. The mathematical formulas used for analysis are also different.13, 25, 26 We also thought the WSLS model was a different strategy to RL. Random choice behavior must also be treated as a different strategy from the RL strategy because random choice behavior indicates the failure of learning. Since the WSLS model and random choice are different strategies to the RL model, and the Q-learning model should be applied to the participants who adopted RL, we used AIC to clarify which strategy (i.e., the RL, WSLS, and random choice models) was the most appropriate model and excluded participants classified as WSLS and random choice model. There were no significant differences between the proportion of WSLS to random choice between patients with ALS and control participants. We found that the unique choice behavior of ALS patients could be classified using the Q-learning model. These findings supported the idea that the choice model is determined independently of disease state.
The Q-learning model is the most commonly used RL algorithm for model-free analysis of choice behavior. In the PRL task, the Q-learning model provides two crucial parameters, that is, inverse temperature (beta) and learning rate (alpha). The beta value determines the randomness of choice behaviors. Patients with ALS had high beta values, indicating that they hardly shifted their choice to another alternative, especially in a good situation.12, 13 A significantly higher beta value could indicate that they were likely to select the higher value option, which might reflect stereotypic and compulsive behavior.
The alpha value has a complex relationship with performance in RL tasks; different learning environments afford distinct optimal learning rates. However, a low alpha value indicates difficulty in updating old values despite newly acquired information, and subsequently no shift in choice. In ALS patients with the unique choice pattern, the alpha value was significantly correlated with the total score and score at the reversal phase (Supplementary Data 6). This could be attributed to optimization of their internal model, indicating more flexible, goal-directed actions due to representation of the contingencies of the task and less accelerated exploration, in addition to lower attention to the alternative choice after the situation changed.35, 36
Pαβ indicates anomalous decision-making behavior in patients with ALS
The balance between “exploration” for searching for a better choice and “exploitation” for obtaining a large reward is said to be one of the major issues in RL. ALS patients did not change their choice as frequently as controls (Figure 1), which means the change of the balance of exploration vs. exploitation trade-off. ALS patients barely chose the option that they thought was disadvantageous, although they often changed their choice when they thought the value of the two alternatives had become equal. Their choice behavior seemed to be strictly guided by action values that are estimated through RL (such as Q-learning). This anomalous choice behavior is the key point in the change in choice behavior in patients with ALS.
In the current study, we used Pαβ, which was a better measure of anomalous PRL behavior in patients with ALS. The use of only alpha or beta values could not completely explain the uniqueness of choice behavior in patients with ALS. AIC showed Pαβ had stronger explanatory power than alpha or beta values alone. Interestingly, ICC analysis showed that Pαβ was significantly correlated with decreased degree centrality in the anterior cingulate gyrus and frontal pole in patients with ALS. This region was reportedly associated with the decision-making process based on perceptual cues and reward values.37 Furthermore, patients with ALS frequently show pathological changes in these regions.3, 38 These findings support the idea that Pαβ, induced by both alpha and beta values, can represent the anomalous decision-making behavior related to the involvement of the prefrontal cortex in patients with ALS.
Network alterations and altered decision-making in patients with ALS
Seed-based analysis from the anterior cingulate gyrus and frontal pole revealed changes in functional connectivity to regions that consist of the paracingulate gyrus, frontal medial cortex, anterior cingulate gyrus, frontal pole, subcallosal cortex, and superior frontal gyrus. The medial prefrontal cortex monitors whether choice behavior is reliable and enforces the switch from exploitation to exploration.37, 39 The functional connectivity of the frontal polar cortex alters when someone decides to switch to the alternative behavior, since the frontal pole plays a role in monitoring the alternative course of action.37, 40 The reorganization of these networks might lead to a change in the balance between exploitation and exploration, or influence the switch to the alternative behavior, subsequently producing the anomalous choice behavior in patients with ALS.
Influence of motor dysfunction in patients with ALS on the PRL task
Upper and lower motor neurons are affected in patients with ALS, which could influence the selections made by pressing a key within a short period of time. Motor deficits might make ALS patients have a stronger choice perseverance tendency than control participants. However, we carefully assessed whether ALS patients performed the PRL task appropriately. We postulated that motor deficits could not significantly influence the uniqueness of choice behavior in ALS patients. There were no significant differences in the scores and strategies of the PRL task between ALS and control participants. Furthermore, the anomalous choice behavior linked with a high Pαβ required the selection of the advantageous stimulus, which was randomly presented on either the left or right side in each trial. Moreover, there was no significant correlation between Pαβ and disease duration, type, and severity (ALSFRS-R). Thus, changes in choice behavior were not mainly due to motor dysfunction.
Limitations
We classified the participants’ strategies into Q-learning, WSLS, or random choice using only standard models. In standard RL models, the action values are assumed to be updated according to the reward prediction error. Numerous studies have noted that the magnitude of the update is biased depending on the sign of the reward prediction error.33, 34 The bias is represented in RL models by differential learning rates for positive and negative reward prediction errors.
Regarding the sensitivity of the choice probabilities shown by inverse temperature, choice perseverance, shown by choice trace weight “ϕ”,25, 41 more closely fitted ALS symptoms. Thus, in future work, it is recommended that other mathematical schemes be compared to reveal the anomalous choice behavior of ALS patients.
We found there were significant differences in cognitive function, especially memory, fluency, and language, between ALS patients and cognitively normal controls (Supplementary Data 2 and Table 1). Previous studies also showed ALS patients had frontotemporal dysfunction.3, 42 Our results supported the findings of these studies. However, the participants performed a practice task before the PRL task to ensure they knew how to perform the task. Besides, we could not find a significant correlation between Pαβ and conventional cognitive examinations. We postulated that cognitive dysfunction assessed by conventional examinations could not explain the anomalous choice behavior of patients with ALS.
Another limitation was that we assessed ICC with a cluster-forming height threshold of P < 0.005. However, the contributing neural systems in our analysis overlapped with those found in task-fMRI studies. Thus, it is best to perform RS-fMRI analysis with more participants in a future study to obtain more reliable results.
Conclusion
ALS patients showed anomalous changes in choice behavior during the PRL task. The Q-learning model and logistic regression analysis were useful for quantifying the decision-making process. Analysis of RS-fMRI by ICC suggested that the anomalous changes in choice behavior in patients with ALS were associated with decreased degree centrality of medial prefrontal areas. Altered decision-making in ALS patients may be related to the decreased hub function of these areas.
Acknowledgments
This study was partially supported by a Grant-in-Aid from the Research Committee of Central Nervous System Degenerative Diseases by the Ministry of Health, Labour, and Welfare, Integrated Research on Neuropsychiatric Disorders project carried out Strategic Research Program for Brain Sciences (SRPBS), a Grant-in-Aid for Scientific Research on Innovative Areas (Brain Protein Aging and Dementia Control 26117002) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, as well as Integrated Research on neuropsychiatric disorders carried out under the SRPBS, Scientific Research on Innovative Areas (Comprehensive Brain Science Network), and Integrated Research on Depression, Dementia, and Development Disorders by SRPBS from Japan Agency for Medical Research and development.
Conflict of Interests
The authors declare no competing financial interests.




