Validation of differentially expressed brain-enriched microRNAs in the plasma of PD patients
Abstract
Objective
There is a pressing need to identify and validate, minimally invasive, molecular biomarkers that will complement current practices and increase the diagnostic accuracy in Parkinson’s disease (PD). Brain-enriched miRNAs regulate all aspects of neuron development and function; importantly, they are secreted by neurons in amounts that can be readily detected in the plasma. Τhe aim of the present study was to validate a set of previously identified brain-enriched miRNAs with diagnostic potential for idiopathic PD and recognize the molecular pathways affected by these deregulated miRNAs.
Methods
RT-qPCR was performed in the plasma of 92 healthy controls and 108 idiopathic PD subjects. Statistical and in silico analyses were used to validate deregulated miRNAs and pathways in PD, respectively.
Results
miR-22-3p, miR-124-3p, miR-136-3p, miR-154-5p, and miR-323a-3p levels were found to be differentially expressed between healthy controls and PD patients. miR-330-5p, miR-433-3p, and miR-495-3p levels were overall higher in male subjects. Most of these miRNAs are clustered at Chr14q32 displaying CREB1, CEBPB, and MAZ transcription factor binding sites. Gene Ontology annotation analysis of deregulated miRNA targets revealed that “Protein modification,” “Transcription factor activity,” and “Cell death” terms were over-represented. Kyoto Encyclopedia of Genes and Genome analysis revealed that “Long-term depression,” “TGF-beta signaling,” and “FoxO signaling” pathways were significantly affected.
Interpretation
We validated a panel of brain-enriched miRNAs that can be used along with other measures for the detection of PD, revealed molecular pathways targeted by these deregulated miRNAs, and identified upstream transcription factors that may be directly implicated in PD pathogenesis.
Introduction
Parkinson’s disease (PD), the second most common neurodegenerative disease after Alzheimer’s disease, affects about 1% of people over the age of 60. It is characterized by resting tremor, rigidity, and bradykinesia. In addition, patients with PD display a variety of non-motor symptoms including autonomic disturbances, impaired cognition, depression, and sleep disorders.1 The neuropathological hallmark of PD is the loss of dopaminergic neurons and the presence of fibrillary aggregates known as Lewy bodies and Lewy neurites in the substantia nigra pars compacta and other brain regions.2 The etiology of PD is complex and is believed to arise from the interaction of genes, environmental factors, and aging. While the genetic causes have provided invaluable insights into the pathogenesis, they represent the minority of cases with the remaining resulting from epigenetic changes caused by environmental risk factors and aging. Intracellular processes that are affected in PD include mitochondrial function, protein folding, proteasomal, and autophagy clearance pathways.3
Diagnosis of PD is currently based on clinical diagnostic criteria and neuroimaging and is monitored by rating scales related to motor and non-motor features.4 Rating scales are frequently subjective and influenced by periodic fluctuations in symptoms and effective symptomatic therapies, while neuroimaging techniques, such as DAT-SPECT, offer a quantifiable measure of disease progression but are limited by practicality and costs.5 In addition, protein biomarkers, such as those based on alpha-synuclein (SNCA) and dopamine (DA) metabolic products have yielded mixed results, do not reflect disease progression and require an invasive lumbar puncture.6
MicroRNAs (miRNAs) are conserved, ~22 nucleotide RNA molecules that inhibit protein expression by hybridizing to complementary sequences in the 3' untranslated region (UTR) of target RNA transcripts.7 Each miRNA is estimated to regulate multiple target mRNAs, and the combinatorial action of miRNAs is expected to regulate the expression of thousands of mRNAs. Although miRNAs act primarily intracellularly, they are secreted by cells for intercellular communication8 and can be detected in all peripheral fluids including saliva, serum, plasma, CSF, and urine. The latter property together with their role in overseeing the cellular process makes miRNAs an invaluable tool for disease diagnosis and understanding pathology. Additional advantages of miRNAs over other biomarkers, such as proteins, is that they are stable in bodily fluids,9 many have restricted expression in the tissues they regulate,10 and they are accurately quantified by routine and fast laboratory methods (such as RT-qPCR) that are available in most clinical laboratories. Moreover, clear-cut interpretations can be drawn, as mature miRNA levels generally correlate with miRNA activity, whereas post-translational modifications of proteins confer a complex correlation between activity and expression levels. With respect to PD, the expression levels of many miRNAs are altered in different regions of PD brain or target PD-associated proteins including α-synuclein (SNCA, miR-7, and miR-153)11 and β-glucocerebrosidase (GBA, miR-22).12 Previously, several studies, yet not of a critical mass, have identified deregulated miRNAs in the different biofluids of PD patients. Generally, there is limited overlap between findings due to the source of biofluid used, sample size, methodology, and lack of appropriate controls.13 Here, we established the validity of our previous findings which had a specific focus on brain-derived miRNAs,14 using an independent idiopathic PD cohort, and in-depth analyzed current and previous data together to reveal not only a panel of deregulated miRNAs but also the upstream and downstream mediators of their effect.
Patients and Methods
Study population
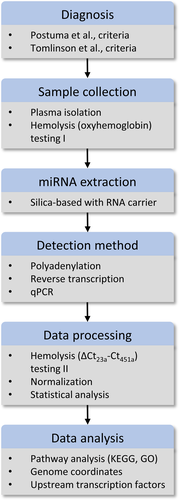
Figure 1 provides a schematic representation of the workflow. The present study included 109 idiopathic PD patients and 92 healthy individuals in two separate cohorts. Patients were assessed with brain magnetic resonance imaging (MRI) or computed tomography (CT) and no relevant brain vascular lesions explaining the clinical phenotype were detected. The control group included spouses or unrelated companions of patients who had no known neurological disease, comorbidities or PD family history. Individuals with concurrent malignant tumors, psychiatric disorders, collagen diseases, endocrine and cardiovascular diseases, or infections were excluded from this study, since these conditions are expected to alter the expression profile of circulating miRNAs. In addition, patients affected by atypical Parkinsonism were also excluded. All patients and controls were recruited from the National and Kapodistrian University of Athens’ neurological unit at the Eginition hospital between 2018 and 2019. PD was diagnosed by two neurologists according to Postuma et al., criteria.4 In all cases, essential demographic and clinical information, including study questionnaire for motor and non-motor manifestations of the disease, rating scales [Hoehn & Yahr (H&Y) stage, mini-mental state examination (MMSE, cognitive impairment score < 2615), and Unified Parkinson's Disease Rating Scale part III (UPDRS III) on or off state] were collected and documented. The demographic and clinical features of patients and controls are summarized in Table 1. Levodopa equivalent daily dose (LEDD) was calculated for the patient groups according to Tomlinson et al., criteria.16 The Hospital and BRFAA ethics committees approved the study and all participants provided written consent.
| Variables | Healthy controls | iPD | P value |
|---|---|---|---|
| No of subjects | 92 | 109 | Ν/Α |
| Age1 | 57.10 ± 12.01 | 64.22 ± 10.41 | <0.0001 |
| Sex (M/F) | 33/59 | 57/52 | <0.0001 |
| Age of onset1 | Ν/Α | 58.94 ± 11.74 | Ν/Α |
| Disease duration1, 2 | Ν/Α | 5.404 ± 5.78 | Ν/Α |
| UPDRS III1, 3 | Ν/Α | 24.19 ± 16.61 | Ν/Α |
| MMSE1, 4 | Ν/Α | 26.87 ± 4.01 | Ν/Α |
- 1 ± SD.
- 2 Disease duration is counted in years.
- 3 Unified Parkinson’s Disease Rating Scale.
- 4 Mini-Mental State Examination.
Plasma collection
Blood processing was carried out according to the Parkinson Progression Marker Initiative (PPMI) protocol. Venous blood was collected in EDTA-treated tubes (BD Vacutainer), followed by immediate centrifugation at 1500× g for 5 minutes at 4°C. The supernatant plasma was aliquoted to 1 mL per tube and stored at −80°C until further use.
miRNA isolation from plasma and RT-qPCR analysis
Plasma samples were thawed at room temperature, centrifuged to pellet any cell debris, and spectrophotometrically analyzed for oxyhemoglobin absorbance at 414 nm. The cut-off level was set at 0.22 against water. Approximately 8.7% and 11.9% of healthy controls and PD plasma samples, respectively, were found hemolyzed and were discarded. miRNA extraction was performed using the NucleoSpin® miRNA plasma kit from Macherey-Nagel. About 1 μg of MS2 (Roche) RNA was added to improve miRNA yield during the extraction method. Polyadenylation and reverse transcription reactions were performed in triplicate for every sample. Similarly, qPCR was performed in triplicate on the Roche Lightcycler® 96 using the SYBR FAST Universal 2X qPCR Master Mix from Kapa Biosystems. Stable reference (miR-103a-3p, miR-191-5p, miR-425-5p, miR-223-3p, miR-423-3p) and hemolysis (miR-23a, miR-451a) miRNA controls were included in the plasma analysis and were identical to previous study.14 Primer sequences can be found in Table S1. The relative expression level of miRNAs was calculated using the 2−ΔΔCt method. Samples with a cycle threshold difference Δ(Ct23α-3p-Ct451α)> 5.5, a PCR-based indicator of hemolysis, were excluded from the final analysis.
List of miRNAs
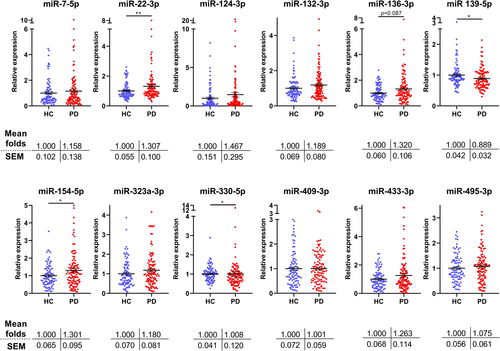
Previously, eight brain-enriched miRNAs were found to be differentially expressed between healthy controls and PD patients.14 These were miR-7-5p, miR-22-3p, miR-124-3p, miR-136-3p, miR-139-5p, miR-330-5p, miR-433-3p, and miR-495-3p. Four miRNAs that were found to differ in their expression between the two groups, but in a marginal non-significant manner, were also included in the analysis. These were miR-132-3p (P = 0.12), miR-154-5p (P = 0.09), miR-323a-3p (P = 0.13), and miR-409-3p (P = 0.10).
Statistical analysis
Statistical analysis was performed using GraphPad PRISM v5.0 and R v3.5.3. All data underwent a normality test (Shapiro–Wilk) and were found to be non-normally distributed. The non-parametric Mann-Whitney U test was used to observe differences between healthy controls and PD patients. Spearman’s method, with Βonferroni correction for multiple comparisons, was used to correlate miRNA expression levels with participants’ demographic and clinical characteristics. The threshold for significance was set to p-values less than 0.05.
To assess the possibility that sex is a confounding factor, a two-way ANOVA model was applied to the log-transformed data (normally distributed) with sex as an additional factor. No difference in the miRNAs that were statistically significant was found.
Receiver operating characteristic (ROC) curves were constructed and the area under the curve (AUC) was calculated to evaluate the predictive accuracy/power of plasma miRNAs for PD diagnosis. The cutoff for the ROC analysis was determined using the Youden Index. Data are presented as means ± SEM. miRNA selection was based on the stepwise removal approach. A logistic regression statistical model containing all available miRNAs as independent variables and PD status as the dependent variable was built. Then, the miRNAs with the least contribution in the model (as determined by an F test) were removed. This process continued until no further removals were possible.
Pathway analysis
The DIANA mirPath v.3 software suite was used to identify miRNA-regulated pathways. This software renders possible the functional annotation of one or more miRNAs using standard hypergeometric distributions, unbiased empirical distributions, and meta-analysis statistics.17 Here, predicted targets from the DIANA microT-CDS algorithm with high quality experimentally supported interactions were used to identify the Kyoto Encyclopaedia of Genes and Genomes (KEGG) molecular pathways, as well as Gene Ontology (GO) terms targeted by each miRNA. The combinatorial effect of deregulated miRNAs was identified by simultaneously selecting multiple miRNAs in the software. The default values (p-value threshold 0.05, microT-CDS threshold 0.8) were used for the analysis.
Chromosomal location of miRNAs
Human coordinates for each miRNA were obtained from the miRbase release 22.1 website (http://www.mirbase.org/). To visualize the data, the coordinates were uploaded on the PhenoGram software which was used to create the chromosomal ideogram.18
Transcription factors regulating miRNAs
The TransmiR v2.0 literature-curated database of experimentally validated transcription factors (TF) -miRNA regulations was searched to identify the TFs located 300 bp upstream and 100 bp downstream of each miRNA transcription start site (TSS).19
Results
miRNAs are differentially expressed in the plasma of idiopathic PD patients
The demographic and clinical characteristics of 92 healthy controls and 109 idiopathic PD (iPD) patients are summarized in Table 1. RT-qPCR was used to analyze the differential expression of 11 brain-enriched miRNAs14 and the ubiquitous miR-22-3p that targets GBA mRNA12 in the plasma of control and PD cohorts. Eight of these miRNAs have been previously identified as significantly deregulated in PD, while four approached statistical significance.14
Data revealed that 4 out of 12 miRNAs were significantly altered in the plasma obtained from PD patients compared to healthy controls (Figure 2). These were miR-22-3p, miR-139-5p, miR-154-5p, and miR-330-5p. Table 2 compares the results obtained from the previous14 and current analyses as well as the data when both studies were pooled together. Except miR-139-5p and miR-433-3p that showed reverse expression, the remaining 10 miRNAs displayed a very similar expression pattern between the two studies. Pooling the data together, to increase accuracy and statistical power, it was revealed that miR-22-3p, miR-124-3p, miR-136-3p, miR-154-5p, and miR-323a-3p are differentially expressed between healthy controls and PD patients.
| miRNA expression |
Initial study1 (N: 101 HC, 99 iPD) |
Current study (Ν: 92 HC, 109 iPD) |
Pooled data2 (N: 193 HC, 208 iPD) |
|||
|---|---|---|---|---|---|---|
| miRNA |
Fold change towards healthy controls (mean ± SEM) |
Mann-Whitney |
Fold change towards healthy controls (mean ± SEM) |
Mann-Whitney |
Fold change towards healthy controls (mean ± SEM) |
Mann-Whitney |
| miR-7-5p | 1.276 ± 0.114 | P = 0.039 | 1.158 ± 0.138 | P = 0.811 | 1.214 ± 0.090 | P = 0.205 |
| miR-22-3p | 1.180 ± 0.066 | P = 0.039 | 1.307 ± 0.100 | P = 0.007 | 1.246 ± 0.061 | P = 0.0003 |
| miR-124-3p | 1.587 ± 0.194 | P = 0.006 | 1.467 ± 0.295 | P = 0.198 | 1.524 ± 0.180 | P = 0.018 |
| miR-132-3p | 1.201 ± 0.214 | P = 0.392 | 1.189 ± 0.080 | P = 0.171 | 1.195 ± 0.110 | P = 0.823 |
| miR-136-3p | 1.378 ± 0.115 | P = 0.004 | 1.320 ± 0.106 | P = 0.088 | 1.347 ± 0.078 | P = 0.006 |
| miR-139-5p | 1.342 ± 0.075 | P = 0.0001 | 0.889 ± 0.032 | P = 0.021 | 1.104 ± 0.042 | P = 0.307 |
| miR-154-5p | 1.223 ± 0.116 | P = 0.111 | 1.312 ± 0.095 | P = 0.038 | 1.270 ± 0.074 | P = 0.014 |
| miR-323a-3p | 1.293 ± 0.107 | P = 0.018 | 1.180 ± 0.081 | P = 0.147 | 1.234 ± 0.066 | P = 0.045 |
| miR-330-5p | 1.206 ± 0.080 | P = 0.042 | 1.008 ± 0.120 | P = 0.028 | 1.101 ± 0.074 | P = 0.977 |
| miR-409-3p | 1.026 ± 0.092 | P = 0.814 | 1.001 ± 0.059 | P = 0.620 | 1.013 ± 0.053 | P = 0.707 |
| miR-433-3p | 0.987 ± 0.250 | P = 0.964 | 1.263 ± 0.114 | P = 0.438 | 1.132 ± 0.133 | P = 0.187 |
| miR-495-3p | 1.301 ± 0.303 | P = 0.424 | 1.075 ± 0.061 | P = 0.459 | 1.183 ± 0.147 | P = 0.845 |
Association between miRNA levels and clinical features
Spearman correlation test was used to relate miRNA levels to idiopathic PD patients’ clinical features. We found no correlation between age-at-onset, disease duration, UPDRS III, MMSE, LEDD and miRNA levels, or patients’ on/off state and miRNA levels (Table 3). Furthermore, no difference in the results was observed when data from both studies were pooled together; there was only a weak positive correlation of miR-22-3p with age-at-onset, but this was not significant (P = 0.0006, Fig. S1). Finally, correcting clinical scores with LEDD did not reveal any more associations (data not shown).
| Clinical features |
Age Rho (P-value) |
Age at onset Rho (P-value) |
PD duration Rho (P-value) |
UPDRS Rho (P-value) |
MMSE Rho (P-value) |
LEDD Rho (P-value) |
|---|---|---|---|---|---|---|
| miR-7-5p | 0.121 (0.082) | 0.142 (0.041) | −0.035 (0.617) | 0.089 (0.203) | −0.038 (0.582) | −0.106 (0.127) |
| miR-22-3p | 0.285 (<0.001) | 0.235 (0.001) | 0.039 (0.577) | 0.147 (0.034) | −0.138 (0.047) | −0.021 (0.765) |
| miR-124-3p | 0.263 (<0.001) | 0.202 (0.004) | 0.112 (0.107) | 0.115 (0.098) | −0.074 (0.286) | 0.018 (0.8) |
| miR-132-3p | 0.036 (0.608) | 0.057 (0.415) | 0.01 (0.886) | 0.061 (0.381) | −0.109 (0.118) | 0.028 (0.686) |
| miR-136-3p | 0.123 (0.077) | 0.072 (0.304) | 0.036 (0.61) | 0.01 (0.892) | −0.038 (0.583) | 0.039 (0.581) |
| miR-139-5p | 0.123 (0.076) | 0.019 (0.791) | 0.167 (0.016) | 0.134 (0.054) | −0.049 (0.48) | 0.07 (0.316) |
| miR-154-5p | 0.1 (0.149) | 0.062 (0.37) | −0.012 (0.861) | −0.001 (0.988) | −0.01 (0.882) | 0.059 (0.393) |
| miR-323a-3p | 0.11 (0.114) | 0.102 (0.142) | −0.036 (0.606) | 0.008 (0.905) | −0.045 (0.52) | 0.004 (0.957) |
| miR-330-5p | 0.113 (0.106) | −0.016 (0.818) | 0.177 (0.011) | 0.073 (0.30) | −0.043 (0.537) | 0.152 (0.029) |
| miR-409-3p | 0.084 (0.233) | 0.078 (0.268) | −0.039 (0.579) | −0.054 (0.441) | −0.02 (0.776) | 0.043 (0.537) |
| miR-433-3p | 0.091 (0.192) | 0.122 (0.079) | 0.027 (0.7) | 0.079 (0.257) | −0.135 (0.052) | 0.084 (0.229) |
| miR-495-3p | −0.054 (0.439) | 0.004 (0.95) | −0.058 (0.402) | −0.073 (0.296) | −0.048 (0.491) | 0.024 (0.734) |
- * Significance level is set at P < 0.0005 following Bonferonni correction.
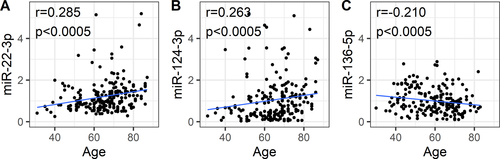
Association between miRNA levels and age
There was no significant correlation between miRNA expression and age in either healthy controls or PD patients. When data from both studies were pooled together, miR-22-3p and miR-124-3p showed a positive correlation with age in PD, while miR-136-3p showed a negative correlation with age in healthy controls (Fig. 3A-C). Since both miR-22-3p and miR-124-3p were found upregulated in patients with PD, we performed an analysis of covariance (ANCOVA) with the PD group as a factor and age as a covariate to explore whether these alterations were associated with aging. No differences in these miRNAs between the ANCOVA and the ordinary analysis of variance (ANOVA) were found. Hence, even in the case where age correlates with miRNA levels, PD pathology has an additional effect on miRNA expression.
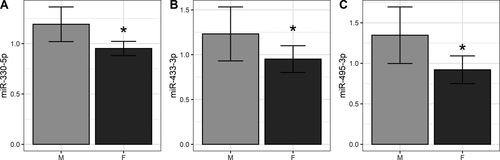
Association between miRNA levels and sex
We found no significant differences between miRNA relative expression and sex in the present cohort. However, when data from both studies were combined, miR-330-5p, miR-433-3p, and miR-495-3p showed higher expression in male subjects (Fig. 4A-C).
Discriminant analysis
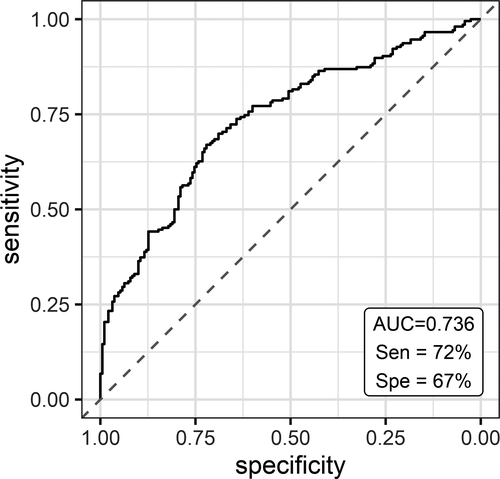
To evaluate the utility of plasma miRNA levels in discriminating subjects with PD from healthy controls, ROC curve analysis was performed after merging the data from both studies. The diagnostic sensitivity and specificity of a three-miRNA panel (miR-7-5p, miR-136-3p, miR-409-3p), when age and sex are taken into account, were 72% and 67%, respectively, and the AUC was 0.736 (Fig. 5).
Pathway analysis
In order to explore the biological pathways affected by the deregulated brain-enriched miRNAs (pooled data), the DIANA mirPath v3 tool was used to align miRNA predicted targets with KEGG pathways and GOslim categories. Gene union of the deregulated miRNA targets revealed eight KEGG categories as significantly enriched; they included “Long-term depression” (P < 0.00024, 15 genes), “TGF-beta signaling pathway” (P < 0.0017, 17 genes), “FoxO signaling pathway” (P < 0.0059, 29 genes), “Estrogen signaling pathway” (P < 0.011, 18 genes), and “ErbB signaling pathway” (P < 0.011, 20 genes) (Table 4A, Fig. S2). Thirteen GOslim categories that are controlled by the gene union of the deregulated miRNA targets were enriched; these included “Cellular protein modification” (P < 2.6e-31, 324 genes), “Nucleic acid binding transcription factor activity” (P < 7.4e-18, 149 genes), “Cell death” (P < 3.2e-08, 115 genes), “Catabolic process” (P < 7.9e-8, 206 genes), and “Response to stress” (P < 0.0003, 221 genes) (Table 4B).
| A | |||
|---|---|---|---|
| KEGG pathway | P-value | # genes | miRNAs |
| Long-term depression | 0.00024 | 15 | miR-22-3p, miR-124-3p, miR-136-3p, miR-154-5p, miR-323a-3p |
| TGF-beta signaling pathway | 0.00166 | 17 | miR-22-3p, miR-124-3p, miR-136-3p, miR-323a-3p |
| FoxO signaling pathway | 0.00594 | 29 | miR-22-3p, miR-124-3p, miR-136-3p, miR-154-5p, miR-323a-3p |
| Estrogen signaling pathway | 0.01093 | 18 | miR-22-3p, miR-124-3p, miR-323a-3p |
|
ErbB signaling pathway |
0.01171 | 20 | miR-22-3p, miR-124-3p, miR-136-3p, miR-323a-3p |
| Neurotrophin signaling pathway | 0.01517 | 25 | miR-22-3p, miR-124-3p, miR-136-3p, miR-154-5p, miR-323a-3p |
| Amphetamine addiction | 0.02492 | 12 | miR-22-3p, miR-124-3p, miR-323a-3p |
| Prolactin signaling pathway | 0.03655 | 16 | miR-22-3p, miR-124-3p, miR-323a-3p |
| B | |||
|---|---|---|---|
| GOslim category | P-value | # genes | miRNAs |
| Cellular protein modification process | 2.63E-31 | 324 | miR-22-3p, miR-124-3p, miR-136-3p, miR-154-5p, miR-323a-3p |
| Nucleic acid binding transcription factor activity | 7.43E-18 | 149 | miR-22-3p, miR-124-3p, miR-136-3p, miR-154-5p, miR-323a-3p |
| Nucleoplasm | 2.40E-08 | 142 | miR-22-3p, miR-124-3p, miR-136-3p, miR-154-5p, miR-323a-3p |
| Cell death | 3.24E-08 | 115 | miR-22-3p, miR-124-3p, miR-136-3p, miR-154-5p, miR-323a-3p |
| Catabolic process | 7.95E-08 | 206 | miR-22-3p, miR-124-3p, miR-136-3p, miR-154-5p, miR-323a-3p |
| Membrane organization | 2.14E-07 | 74 | miR-22-3p, miR-124-3p, miR-136-3p, miR-154-5p, miR-323a-3p |
| Cytoskeletal protein binding | 7.21E-05 | 87 | miR-22-3p, miR-124-3p, miR-136-3p, miR-154-5p, miR-323a-3p |
| Response to stress | 0.0003 | 221 | miR-22-3p, miR-124-3p, miR-136-3p, miR-154-5p, miR-323a-3p |
| RNA binding | 0.0016 | 187 | miR-22-3p, miR-124-3p, miR-136-3p, miR-154-5p, miR-323a-3p |
| Endosome | 0.0032 | 77 | miR-22-3p, miR-124-3p, miR-136-3p, miR-154-5p, miR-323a-3p |
| Cell-cell signaling | 0.0091 | 66 | miR-22-3p, miR-124-3p, miR-136-3p, miR-154-5p, miR-323a-3p |
| Vesicle-mediated transport | 0.0152 | 105 | miR-22-3p, miR-124-3p, miR-136-3p, miR-154-5p, miR-323a-3p |
| Immune system process | 0.0152 | 150 | miR-22-3p, miR-124-3p, miR-136-3p, miR-154-5p, miR-323a-3p |
Chromosomal distribution and transcription factors
Identifying the gene location of the differentially expressed miRNAs enables a better understanding of their regulation of expression and association with putative chromosomal abnormalities previously linked with these regions. To assess this parameter, the chromosomal coordinates of the deregulated miRNAs in PD obtained from the pooled data (miR-22-3p, miR-124-3p, miR-136-3p, miR-154-5p, and miR-323a-3p) and the miRNAs that showed higher expression in male subjects (miR-330-5p, miR-433-3p, and miR-495-3p) were collected from the miRbase 22.1 and visualized using Phenogram (Fig. 6A). Interestingly, five of these miRNAs (miR-136-3p, miR-154-5p, miR-323a-3p, miR-433-3p, and miR-495-3p) were clustered at the same location on chromosome 14q32, suggesting that they are co-regulated by specific transcription factors or methylation.
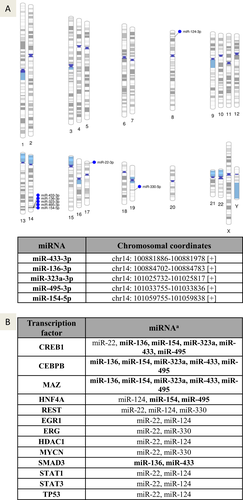
To identify the transcription factors regulating these differentially expressed miRNAs, the manually curated TransmiR v2.0 experimental database was used with highly stringent parameters to locate TF sites downstream of each miRNA transcription start site. The analysis revealed that TF binding sites for cAMP-responsive element-binding protein 1 (CREB1), CCAAT enhancer-binding protein beta (CEBPB) and MYC associated zinc finger protein (MAZ) were found in over half of the miRNAs, including all five miRNAs located on Chr14 (Fig. 6B).
Discussion
We previously reported that brain-enriched miRNAs can differentiate healthy controls from genetic and idiopathic PD subjects.14 Here, we report results of an equally large validation study conducted on twelve miRNAs that were significantly or marginally non-significantly altered in our original study. Except two miRNAs, miR-139-5p and miR-433-3p, all other miRNAs displayed very similar expression patterns and differences between healthy control and PD subjects in both studies. Combining the data, it was revealed that miR-22-3p, miR-124-3p, miR-136-3p, miR-154-5p, and miR-323a-3p are differentially expressed between healthy controls and PD subjects. Of these, miR-22-3p,20-22 miR-124-3p,23 and miR-136-3p21, 24 have been identified as deregulated in other PD biomarker studies, further validating current findings and their replicability at different neurological centers.
There is considerable information regarding the biological role of some of these deregulated miRNAs. miR-22-3p targets GBA, which encodes for β-glucocerebrosidase, a lysosomal enzyme involved in sphingolipid degradation; mutations in GBA are the single largest risk factor for PD.25 In addition, miR-22 is neuroprotective in multiple neurodegeneration and traumatic brain injury models, has regenerative capabilities, and is involved in several aspects of neuronal development including cell cycle length, polarization of migrating neurons, and long-term synaptic plasticity.26-28 miR-124-3p is the most abundant neuronal miRNA in the nervous system, and is considered indispensable for neuronal fate determination, differentiation, and plasticity.29, 30 It is anti-inflammatory and protects dopaminergic neurons from MPTP- and 6-OHDA -induced toxicity via multiple pathways.31 Less is known about miR-136-3p, miR-154-5p, and miR-323a-3p. miR-136-3p expression is upregulated in synaptoneurosomes at the preclinical stage of prion disease and its overexpression protects cells from neuroinflammation following ischemic insults.32, 33 miR-154-5p is differentially regulated during morphine self-administration and is predicted to have an important role in dopaminergic neuron differentiation and mu-opioid receptor regulation.34 miR-323a-3p is upregulated in mild cognitive impairment and differentially regulated by 6-OHDA and ischemia/reperfusion injury.35, 36 Importantly, we found that all five deregulated miRNAs are significantly increased in PD; the fact that most of these miRNAs appear to have strong neuroprotective properties at multiple settings may suggest that they are regulated as a compensatory response to brain impairment.
Men have 50 percent higher incidence of PD37 and, interestingly, we found that the expression of three brain-enriched miRNAs, miR-330-5p, miR-433-3p, and miR-495-3p was significantly higher in male subjects. Considering that the ratio of males to women was different in our two studies, we think that this partly explains the inconsistency in miR-433-3p expression between them. Little is known of their biological roles. miR-330-5p targets mRNAs involved in activity-dependent synaptic plasticity in the hippocampus.38 miR-433-3p targets follicle-stimulating hormone (FSH) expression in the anterior pituitary and HIF1α levels in the brain during hypoxia inhibiting neuron proliferation and migration.39, 40 miR-495-3p targets are enriched for addiction and pro-survival genes, including BDNF, CAMK2, and ARC.41 Furthermore, miR-495-3p expression is upregulated following deep brain stimulation.42 Collectively, it is documented that these three miRNAs have a rather negative impact on neuronal processes affected in PD, however, further work is required to better delineate their roles, and if/how their higher expression in males affects vulnerability to PD.
Examining the chromosomal coordinates of the differentially expressed brain miRNAs, we found that miR-136-3p, miR-154-5p, miR-323a-3p, miR-433-3p, and miR-495-3p are located on Chr14q32, suggesting that they are co-deregulated by transcription factors or methylation. This area is inherently unstable and a 1.1 Mb microdeletion (14q32.2.q32.3, coordinates Chr14: ~100 400 000-101 500 000) has been reported in a number of patients displaying motor delay, hypotonia, and feeding problems.43, 44 This genomic rearrangement is thought to be generated after the 500 bp expanded repeats flanking the deletion boundaries undergo either non-allelic homologous recombination (NAHR) or form secondary structures that interfere with normal DNA replication and chromosome condensation.43 It should be noted that the particular location also hosts 15 protein-coding genes, some of which are imprinted.43, 44 More recently, miRNAs of this cluster were found to be among the most longitudinally stable miRNAs, indicating that they are ideal biomarkers to monitor the progression pathophysiological states including PD, reiterating the importance of the current findings.45 Moreover, we have used the TransmiR v2 experimental, manually curated, database to probe the TFs that regulate the expression of these deregulated brain miRNAs. Transcription binding sites for CREB1, CEBPB, and MAZ were found in all miRNAs located at Chr14q32. In addition, a CREB1 site was found at the miR-22 gene locus. CREB1 is essential for neuronal survival and axonal growth via both transcription of neurotrophins and neurotrophin-dependent CREB1-mediated transcription of pro-survival genes.46 CREB1 is also required for adult neurogenesis, synaptic plasticity, and memory formation.47, 48 Similarly to CREB1, CEBPB has been implicated in the control of neuronal development and survival, including processes such as cell fate determination, synthesis, and response to trophic factors, learning, and memory.49, 50 MAZ, moreover, has been linked to neural stem cell differentiation toward the glial cell lineage51 and the induction of the NMDA receptor subunit type 1 (NR1) gene after neuronal differentiation.52 Overall, these data indicate that epigenetic deregulation at Chr14q32 locus maybe responsible or contribute to neuronal miRNA differential expression in PD.
To explore the molecular pathways controlled by the deregulated miRNAs, in silico analysis of KEGG pathways and GO terms was performed. KEGG categories such as “Long-term depression,” “TGF-beta signaling pathway,” “FoxO signaling pathway,” “Estrogen signaling pathway,” “ErbB signaling pathway,” and “Neurotrophin signaling pathway” were over-represented. “Long-term depression” (LTD) is particularly affected in PD, as one of the most important functions of the nigrostriatal dopaminergic pathway is the induction of striatal LTD. Synaptic plasticity at corticostriatal glutamatergic synapses of striatal medium spiny neurons requires DA receptor activation by endogenous DA.53, 54 This form of synaptic plasticity is lost following dopaminergic denervation in PD and has been confirmed in multiple PD models (6-OHDA, MPTP, A53T-SNCA, G2019S-LRRK2).55-58 “TGF-beta signaling pathway” has been shown to control dopaminergic neuron development and survival.59 Tgfb2 or Tgfb3 knockout mouse embryos display a significant reduction of dopaminergic neurons embryonically.60, 61 These mice also show postnatal or age-dependent loss of dopaminergic neurons, while TGF-β activation by overexpressing constitutively active TβR1 aborts degeneration of these neurons in the MPTP-induced PD mouse model.62, 63 The “FoxO family” of transcription factors is implicated in stress-resistance and apoptotic programs 64. One of the prime regulators of FoxO activity is the PI3K/AKT pathway. In response to a wide variety of growth factors (insulin, neurotrophins), AKT is activated which then phosphorylates FoxOs at different sites rendering them unable to regulate gene expression.65, 66 In agreement with other neuronal types, FOXO3 overexpression induces acute apoptosis of dopaminergic neurons in vivo. However, the downregulation of FOXO3 is also harmful to neurons as it is accompanied by oxidative damage, indicating that FOXO3 is required for basal neuronal function.67 Further, FOXO3 downregulation completely protects dopaminergic neurons following α-synuclein overexpression, suggesting that it is indispensable for neuronal cell death. Interestingly, mild FOXO3 activity is also protective against the accumulation of human α-synuclein by inducing autophagy.67 In addition, it has been shown that FOXO1 and its target genes are increased in the frontal cortex of PD patients but the implications of this are still unclear.68 “ErbB signaling,” mediated by the ErbB1-4 receptor tyrosine kinases ligands epidermal growth factor (EGF) and neuregulins, are important regulators of brain development and function. With respect to PD-associated processes, EGF is a growth factor for dopaminergic neurons and its levels as well as ErbB1 levels are diminished in the postmortem PD brains.69 Further, ErbB4 signaling in midbrain dopaminergic axonal projections regulates extracellular DA levels and spatial/working memory behaviors.70 Overall, the top deregulated KEGGS pathways identified here, are strongly associated with PD.
“Cellular protein modification,” “Nucleic acid binding transcription factor activity,” “Cell death,” “Catabolic process,” were overrepresented among the biological processes affected. Cellular protein modifications such as phosphorylation, ubiquitination, truncation, acetylation, nitration, and sumoylation of PD-linked proteins have emerged as important modulators of pathogenic mechanisms in PD.71, 72 Transcription factor changes are also of particular interest, as they indicate that there is not only misexpression at the mRNA translation level by miRNA deregulation, but that there exists a second wave of en masse deregulation involving transcription-wide changes. Recently, we have integrated the data across 24 PD biomarker studies and identified 25 miRNAs reported deregulated in at least two studies.13 Five of the currently reported deregulated miRNAs are found in that list. Interestingly, following stringent bioinformatic analysis and different bioinformatic tools to the ones used in the current manuscript [TargetScan 7.2 for miRNA target prediction in conjunction with DAVID v6.8 for pathway analysis as opposed to DIANA mirPath v.3 used here], revealed that all top four GO categories controlled by the 25 deregulated miRNAs were likewise associated with transcription factors.13 Hence, it appears that transcription factor misexpression is an important component of miRNA deregulation in PD.
To conclude, we performed an RT-qPCR analysis of brain-derived miRNAs in the plasma of a relatively large cohort of patients with PD and matched controls to replicate and validate previous findings. The identified miRNAs form a robust set of deregulated brain-associated miRNAs in PD, that can now be further evaluated, along with other measures, as diagnostic and therapeutic tools for PD. Importantly, existing information on their neurological functions provides a clue of the processes they regulate during PD. In silico analysis provided a comprehensive guide of the pathways and processes they control, improving current understanding of their biological role. Further, Chr14q32 turns out to be a hotspot of these deregulated miRNAs. Importantly, they are co-transcribed by specific transcription factors that regulate neuronal survival, memory, and plasticity in the adult brain. The impact of the latter findings will now await further exploration.
Acknowledgments
The authors are grateful to patients, relatives, and volunteer healthy controls for their participation in this study. This work was supported by the Michael J. Fox Foundation for Parkinson’s Research (Grant ID 13353). This research is also co-financed by Greece and European Union (European Social Fund-ESF) through the Operational Program «Human Resources Development, Education and Lifelong Learning 2014-2020» in the context of the project “Development of diagnostic biomarker tests for Parkinson’s disease” (MIS 5049385)(Fig. 7).

Authors’ Contributions
Conceived the study: ED. Organized the study: ED, LS. Collected blood samples and neurologically examined patients: LS, AB, CK, AMS, IP, MB. Designed and optimized protocols: ED. Performed RNA extraction and PCR experiments: SR. Analyzed data: SR, NP, ED. In silico analyses: ED. Wrote the manuscript: ED. All authors read, edited, and approved the final manuscript.
Conflict of Interests
The authors declare that they have no competing interests.
Open Research
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.




