Neuropsychiatric symptoms and cognitive abilities over the initial quinquennium of Parkinson disease
Funding Information
PPMI, a public–private partnership, is funded by the Michael J. Fox Foundation for Parkinson’s Research and funding partners (see www.ppmi-info.org/fundingpartners).
Abstract
Objective
To determine the evolution of numerous neuropsychiatric symptoms and cognitive abilities in Parkinson disease from disease onset.
Methods
Prospectively collected, longitudinal (untreated, disease onset to year 5), observational data from Parkinson's Progression Markers Initiative annual visits was used to evaluate prevalence, correlates, and treatment of 10 neuropsychiatric symptoms and cognitive impairment in Parkinson disease participants and matched healthy controls.
Results
Of 423 Parkinson disease participants evaluated at baseline, 315 (74.5%) were assessed at year 5. Eight neuropsychiatric symptoms studied increased in absolute prevalence by 6.2–20.9% at year 5 relative to baseline, and cognitive impairment increased by 2.7–6.2%. In comparison, the frequency of neuropsychiatric symptoms in healthy controls remained stable or declined over time. Antidepressant and anxiolytic/hypnotic use in Parkinson disease were common at baseline and increased over time (18% to 27% for the former; 13% to 24% for the latter); antipsychotic and cognitive-enhancing medication use was uncommon throughout (2% and 5% of patients at year 5); and potentially harmful anticholinergic medication use was common and increased over time. At year 5 the cross-sectional prevalence for having three or more neuropsychiatric disorders/cognitive impairment was 56% for Parkinson disease participants versus 13% for healthy controls, and by then seven of the examined disorders had either occurred or been treated at some time point in the majority of Parkinson disease patients. Principal component analysis suggested an affective disorder subtype only.
Interpretation
Neuropsychiatric features in Parkinson disease are common from the onset, increase over time, are frequently comorbid, and fluctuate in severity.
Introduction
Parkinson disease (PD) is diagnosed based on the presence of motor symptoms, but the high prevalence of numerous neuropsychiatric symptoms (NPS) and cognitive impairment suggests that it is more accurately conceptualized as a neuropsychiatric disorder.1 It has only been relatively recently, with the introduction of levodopa and other PD medications, the increasing life span of patients, and through increased awareness and research, that NPS and cognitive impairment have gained recognition as being common, often disabling, and associated with poor long-term outcomes and caregiver burden.2
In addition to cognitive impairment (both mild cognitive impairment [MCI] and dementia), depression, and psychosis, other relatively common psychiatric complications include impulse control disorders (ICDs), anxiety symptoms, disorders of sleep and wakefulness, apathy, and fatigue.
Although cognitive impairment and decline in early PD has been studied,3-7 less is known8-11 about the evolution of a broader range of NPS in newly diagnosed patients and compared with healthy controls (HC), point prevalence versus cumulative prevalence, comorbidity of symptoms, and their stability over time. In addition, the use or introduction of psychiatric medications has not been well studied, nor the impact of medications with anticholinergic properties on cognitive performance. Finally, there is an interest in subtyping of non-motor symptoms in PD,12 but this has not been well studied in de novo and early PD.13, 14
The Parkinson’s Progression Markers Initiative (PPMI) study is a large, multi-site, international, observational, biomarker-discovery program of de novo, untreated (at baseline) PD patients and a matched group of HC.15 We previously reported PPMI baseline NPS data7 and biomarker predictors of the 3-year cognitive data.16 Now we report NPS outcomes over the initial lustrum (i.e. 5 years) of PD.
Methods
Participants
Parkinson disease patients (N = 423) and HC (N = 196) were enrolled in PPMI. At baseline PD participants were required to: (1) have an asymmetric resting tremor or asymmetric bradykinesia, or two of bradykinesia, resting tremor, and rigidity; (2) have a recent PD diagnosis (mean duration of PD diagnosis = 7 months); (3) be untreated; (4) have a dopamine transporter (DAT) deficit on neuroimaging; and (5) be non-demented. Healthy controls were required to have: (1) no significant neurologic dysfunction; (2) no first-degree family member with PD; and (3) a Montreal Cognitive Assessment (MoCA) score ≥27. The aims and methodology of the study have been published elsewhere15 (www.ppmi-info.org/study-design). The study was approved by the institutional review board at each site, and participants provided written informed consent.
Assessments
Cognitive abilities
Global cognition was assessed with the MoCA.17 As no MoCA cutoff was applied for PD patients, a direct comparison of PD patients and HCs on cognitive assessments was not conducted. The following cognitive tests were administered: memory: Hopkins Verbal Learning Test-Revised (HLVT-R);18 visuospatial function: Benton Judgment of Line Orientation (JOLO)19 15-item (split-half) version; processing speed-attention: Symbol-Digit Modalities Test (SDMT);20 and executive function and working memory: Letter-Number Sequencing (LNS)21 and semantic (animal) fluency.22 Published norms (referenced above) were applied.
Cognitive impairment (CI) was defined at two levels: (1) at the screening level, the recommended MoCA cutoff of <26 was applied;17, 23 and (2) using psychometric tests, CI categorization was reached through a cognitive test-based classification, requiring impairment (>1.5 standard deviations below the standardized mean score, in the range recommended to establish a MCI diagnosis24) on any two cognitive test scores (using both total immediate recall and recognition recall from the HVLT-R, and single scores from each of the other tests). Within 2 years of study initiation, an additional cognitive determination was added, the site investigator’s clinical diagnosis of normal cognition, MCI or dementia at each annual visit based on clinical judgment and option to review any clinical data.
Neuropsychiatric symptoms
The following instruments (cutoff scores to indicate clinically significant symptoms) were administered: 15-item Geriatric Depression Scale (GDS-15),25 score ≥ 5 for depression;26 State-Trait Anxiety Inventory (STAI),27 state subscale, score ≥ 40 for anxiety;28 short version of the Questionnaire for Impulsive-Compulsive Disorders in Parkinson's Disease (QUIP) for ICDs (gambling, sexual, buying, and eating), related behaviors (punding, hobbyism, and walkabout), and compulsive medication use, hereafter together called ICDs;29 REM Sleep Behavior Disorder Screening Questionnaire (RBDSQ),30 score ≥ 6 for RBD symptoms; Epworth Sleepiness Scale,31 score ≥ 10 indicating daytime sleepiness; in addition, psychosis, apathy, insomnia and fatigue were assessed with single items from the Movement Disorder Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS)32 Part I. A score ≥ 2 was considered presence of a given symptom for apathy, insomnia, and fatigue, and a score ≥ 1 was considered presence of psychosis, in order to capture mild psychosis.
Analyses
Statistical analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC) on data downloaded from the PPMI data portal at the Laboratory of Neuroimaging (LONI) on July 1, 2019. Summary statistics for demographics, clinical characteristics, and NPS were presented by year separately in PD and HC. Cumulative prevalence and number of NPS were also presented by year and group. Kaplan–Meier survival curves were generated for each NPS in PD participants. Time was calculated from the baseline visit date to the first visit date where NPS, or NPS treatment, was present. Participants who did not experience NPS within 5 years from baseline were censored at their last completed visit date. Principal component analysis (PCA) was performed to examine clustering (i.e. potential subtypes) among NPS at baseline and year 5 in PD participants. In addition, the difference in cognition was examined in PD patients who dropped out of the study early compared to those who completed the year 5 visit. At a significance level of 0.05, Mann–Whitney U tests or Fisher’s exact tests were used to compare the last observed MoCA scores in PD patients who dropped out versus MoCA scores in patients who completed the study, separately at year 2, 3, and 4.
Neuropsychiatric symptoms over time in PD participants versus HC
Changes in NPS over time in PD patients compared to HC were assessed using Generalized Estimating Equations (GEEs) under the first order autoregressive correlation (AR1) structure. Under the assumption that the log odds of a positive response is linear in time, each model was fit with effects for group, time, and an interaction between group and time. Odds Ratios (ORs) were presented for the time and interaction effects. A family wise error rate (Bonferroni correction) was applied to account for multiple comparisons.
Predictors of neuropsychiatric symptoms in PD participants
Similarly, GEEs were also used to examine demographics and PD medication use as univariate predictors of each NPS over time in PD patients. Age, sex, and education level were assessed as baseline predictors, while total levodopa equivalent daily dose (LEDD), dopamine agonist (DA) use, and Anticholinergic Cognitive Burden (ACB) Scale33 (an assessment of anticholinergic burden of prescribed medications) score were assessed as time-varying predictors. All models treated time as linearly associated with the log odds and were fit under the AR1 correlation structure. ORs were presented for the interaction effect between the predictor and time. Again, a Bonferroni correction was applied.
Results
Participant characteristics
Baseline characteristics for PD patients and HC are presented in Table 1; the groups were similar in age, race and sex distribution, and education level. Clinical characteristics of PD participants over the 5-year period are presented in Table 2. Antidepressants were prescribed in 26.7% of patients at year 5 visit, and anxiolytics/sedative-hypnotic agents in 24.1%, while antipsychotic and cognitive-enhancing medication use was uncommon even after 5 years. The percentage of patients taking a medication with possible or definite anticholinergic properties (i.e. ACB score ≥ 1) increased from 33.6% at baseline to 51.4% at year 5, and the mean ACB scale score for patients taking an anticholinergic medication increased by 32.4% from baseline to year 5.
| Variable | PD | Healthy |
|---|---|---|
| Participants | Controls | |
| (N = 423) | (N = 196) | |
| Age | ||
| Mean (SD) | 61.66 (9.7) | 60.82 (11.2) |
| (Min, Max) | (33.5, 84.9) | (30.6, 83.7) |
| Gender | ||
| Male | 277 (65.5%) | 126 (64.3%) |
| Female | 146 (34.5%) | 70 (35.7%) |
| Education | ||
| <13 Years | 75 (17.7%) | 29 (14.8%) |
| ≥13 Years | 348 (82.3%) | 167 (85.2%) |
| Race | ||
| White | 391 (92.4%) | 182 (92.9%) |
| Black/African-American | 6 (1.4%) | 9 (4.6%) |
| Asian | 8 (1.9%) | 1 (0.5%) |
| Other | 18 (4.3%) | 4 (2.0%) |
| Duration of disease (months) | ||
| Mean (SD) | 6.65 (6.5) | NA |
| (Min, Max) | (0.4, 35.8) | NA |
| Variable | Baseline | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 |
|---|---|---|---|---|---|---|
| (N = 423) | (N = 395) | (N = 378) | (N = 366) | (N = 346) | (N = 315) | |
| MDS-UPDRS Part III OFF1 | ||||||
| N | 423 | 335 | 285 | 257 | 250 | 230 |
| Mean (SD) | 20.89 (8.9) | 25.16 (11.1) | 27.19 (11.3) | 29.23 (12.2) | 31.46 (12.3) | 31.31 (12.6) |
| (Min, Max) | (4.0, 51.0) | (2.0, 67.0) | (3.0, 68.0) | (4.0, 80.0) | (6.0, 80.0) | (6.0, 90.0) |
| MDS-UPDRS Part III ON1 | ||||||
| N | 423 | 380 | 358 | 342 | 326 | 299 |
| Mean (SD) | 20.89 (8.9) | 23.37 (10.9) | 23.16 (11.3) | 24.15 (12.2) | 24.31 (13.1) | 24.67 (13.4) |
| (Min, Max) | (4.0, 51.0) | (1.0, 67.0) | (0.0, 68.0) | (0.0, 65.0) | (1.0, 70.0) | (0.0, 85.0) |
| TD/PIGD Classification ON1 | ||||||
| N | 422 | 380 | 358 | 342 | 326 | 299 |
| TD | 299 (70.9%) | 241 (63.4%) | 217 (60.6%) | 207 (60.5%) | 167 (51.2%) | 134 (44.8%) |
| PIGD | 76 (18.0%) | 96 (25.3%) | 102 (28.5%) | 101 (29.5%) | 117 (35.9%) | 126 (42.1%) |
| Indeterminate | 47 (11.1%) | 43 (11.3%) | 39 (10.9%) | 34 (9.9%) | 42 (12.9%) | 39 (13.0%) |
| PD medication use | ||||||
| Any PD medication | 0 (0.0%) | 224 (56.7%) | 299 (79.1%) | 316 (86.3%) | 306 (88.4%) | 279 (88.6%) |
| Levodopa | 0 (0.0%) | 92 (23.3%) | 157 (41.5%) | 210 (57.4%) | 235 (67.9%) | 239 (75.9%) |
| Dopamine agonist | 0 (0.0%) | 90 (22.8%) | 123 (32.5%) | 138 (37.7%) | 133 (38.4%) | 117 (37.1%) |
| MAO-B inhibitor | 0 (0.0%) | 103 (26.1%) | 138 (36.5%) | 144 (39.3%) | 136 (39.3%) | 120 (38.1%) |
| Total LEDD | ||||||
| N | 0 | 224 | 299 | 316 | 306 | 279 |
| Mean (SD) | NA | 306.1 (235.3) | 397.7 (311.4) | 481.7 (338.2) | 556.6 (343.3) | 645.0 (345.7) |
| (Min, Max) | NA | (30.3, 1600.0) | (50.0, 2268.0) | (50.0, 2474.0) | (50.0, 3020.0) | (120.0, 3184.0) |
| DBS treatment | ||||||
| Yes | 0 (0.0%) | 0 (0.0%) | 1 (0.3%) | 2 (0.5%) | 4 (1.2%) | 12 (3.8%) |
| Other medication use | ||||||
| Antidepressants | 77 (18.2%) | 84 (21.3%) | 94 (24.9%) | 95 (26.0%) | 98 (28.3%) | 84 (26.7%) |
| Anxiolytics/Sed.-Hypnotics | 55 (13.0%) | 64 (16.2%) | 67 (17.7%) | 70 (19.1%) | 80 (23.1%) | 76 (24.1%) |
| Antipsychotics | 0 (0.0%) | 0 (0.0%) | 2 (0.5%) | 8 (2.2%) | 7 (2.0%) | 7 (2.2%) |
| Stimulants | 3 (0.7%) | 2 (0.5%) | 2 (0.5%) | 4 (1.1%) | 4 (1.2%) | 7 (2.2%) |
| Cognitive-enhancing | 0 (0.0%) | 2 (0.5%) | 5 (1.3%) | 10 (2.7%) | 16 (4.6%) | 16 (5.1%) |
| Anticholinergic cognitive burden scale | ||||||
| N 2 | 142 (33.6%) | 168 (42.5%) | 170 (45.0%) | 183 (50.0%) | 178 (51.4%) | 162 (51.4%) |
| Mean (SD)3 | 2.16 (1.4) | 2.49 (1.5) | 2.71 (1.7) | 2.83 (1.7) | 2.76 (1.8) | 2.86 (1.8) |
| (Min, Max)3 | (1.0, 7.0) | (1.0, 8.0) | (1.0, 9.0) | (1.0, 11.0) | (1.0, 11.0) | (1.0, 11.0) |
- 1 OFF scores include untreated + treated OFF scores; ON scores include untreated + treated ON scores.
- 2 Number of patients on a medication with anticholinergic properties.
- 3 Includes only those patients on a medication with anticholinergic properties at that visit.
Psychiatric and cognitive outcomes over time
Parkinson disease
Baseline and year 5 neuropsychiatric outcome in PD patients is presented in Figure 1. Comparing year 5 with baseline, absolute prevalence rates increased by 6.2–20.9%; specifically, they increased by 20.9% for insomnia, 20.6% for fatigue, 15.2% for daytime sleepiness, 12.5% for RBD, 9.6% for psychosis, 9.5% for apathy, 6.6% for ICDs, and 6.2% for depression. In contrast, the anxiety rate decreased by 3.1% over this time period. Individual primary ICD rates at year 5 were 11.2% (eating), 7.1% (sexual behaviors), 5.4% (buying), and 1.3% (gambling). At year 5, cross-sectional prevalence rates of >25% occurred for insomnia, RBD, fatigue, daytime sleepiness, and ICDs; only RBD crossed this threshold at baseline.
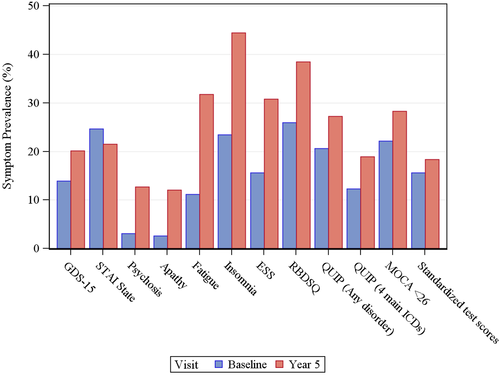
For cognitive abilities, mean MoCA score declined by only 2% over the 5-year period; applying MoCA cutoff scores, any cognitive impairment (i.e. MoCA score < 26) had an absolute increase of 6.2%, and significant cognitive impairment (i.e. MoCA score < 21) of 4.8%. Using detailed cognitive testing the rate of impairment increased by only 2.7%. By site investigator diagnosis the prevalence of any cognitive impairment at the year 5 visit was 19.8% (MCI = 16.2%, dementia = 3.6%).
Given the relatively low rates of cognitive impairment, we assessed whether study dropouts were more likely to have cognitive impairment. By year 5, data were not available for 25.3% (108/423) of PD participants with a baseline visit. Those PD participants who were not assessed starting with the year 3 visit had evidence for lower MoCA scores at their last observed visit compared with PD patients who completed the year 5 visit (Table 3).
| MOCA Score | 2 | 3 | 4 |
|---|---|---|---|
| PD subjects | |||
| Completed | Discontinued study | P-value | |
| Year 2 | |||
| N | 302 | 14 | |
| Mean (SD) | 26.49 (2.7) | 24.07 (6.0) | 0.2187 |
| MoCA < 26 | 91 (30.1%) | 8 (57.1%) | 0.0416 |
| MoCA < 21 | 10 (3.3%) | 3 (21.4%) | 0.0153 |
| Year 3 | |||
| N | 305 | 15 | |
| Mean (SD) | 26.60 (2.8) | 25.07 (3.6) | 0.0876 |
| MoCA < 26 | 93 (30.5%) | 7 (46.7%) | 0.2519 |
| MoCA < 21 | 8 (2.6%) | 2 (13.3%) | 0.0743 |
| Year 4 | |||
| N | 302 | 18 | |
| Mean (SD) | 26.59 (3.4) | 23.06 (6.0) | 0.0148 |
| MoCA < 26 | 87 (28.8%) | 10 (55.6%) | 0.0309 |
| MoCA < 21 | 15 (5.0%) | 6 (33.3%) | 0.0004 |
- Column definitions: 2, MoCA scores at each visit in PD subjects who completed the year 5 visit; 3, Last observed MoCA scores in PD subjects who later discontinued study participation; 4, P-values from Mann–Whitney U test or Fisher's exact test.
Healthy controls
Unlike in PD, cross-sectional prevalence rates for HC decreased, or remained the same or negligible, for depression, anxiety, psychosis, daytime sleepiness, RBD, and ICDs. For cognition, mean MoCA score declined only 2% over the 5-year period; applying MoCA cutoff scores, by entry criteria no HC met criteria for cognitive impairment at baseline, and 18% did so by year 5.
Comorbidity
Accounting for psychiatric or cognitive treatment (i.e. counting a disorder as present if treated, regardless of the assessment outcome), cross-sectional prevalence of ≥3 psychiatric/cognitive disorders increased in PD participants from 29% at baseline to 56% by year 5, whereas in HC the rates went from 12% to just 13% over this time period (Fig. 2). Examining psychiatric symptoms only (i.e. excluding cognition), rates increased from 24% to 50% over time in PD patients, compared with decreasing from 12% to 10% in HC. Thus, rates of multimorbid psychiatric disorders approximately doubled in PD patients over the 5-year period, and by year 5 were approximately five times more common in PD patients than HC.
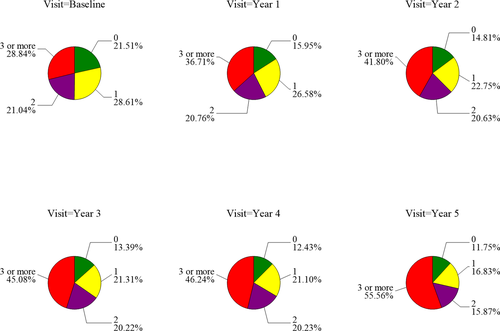
Cumulative prevalence
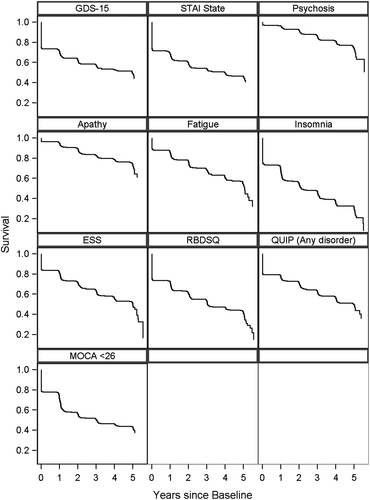
Again accounting for symptomatic treatment in addition to presence of symptoms, the Kaplan–Meier estimates for cumulative prevalence of NPS/cognitive impairment in PD participants is presented in Figure 3. By year 5, seven of the 10 disorders studied had either been present or treated at some time point in >50% of patients, with the highest frequency for insomnia and RBD. All 10 symptoms had occurred or been treated in at least 25% of patients.
Persistence of symptoms
Persistence of NPS in PD participants is presented in Table 4. For psychiatric symptoms, the highest likelihood of still having a symptom 1 year later was for depression (60–79%), insomnia (60–78%) and RBD (64–81%). Less persistent were anxiety (55–60%), psychosis (33–58%), apathy (25–54%), and ICDs and related disorders (33–68%). Of those participants who had cognitive impairment at any time point based on a MoCA score < 26, the percentage still meeting this criterion 1 year later was 62–70%.
| Variable | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Year 1 | Year 1 | Year 2 | Year 2 | Year 3 | Year 3 | Year 4 | Year 4 | Year 5 | |
| GDS-15 | ||||||||||
| ≥5 | 57 | 34 (59.6%) | 59 | 37 (62.7%) | 61 | 37 (60.7%) | 56 | 35 (62.5%) | 47 | 37 (78.7%) |
| STAI state | ||||||||||
| ≥40 | 97 | 54 (55.7%) | 85 | 48 (56.5%) | 75 | 45 (60.0%) | 63 | 37 (58.7%) | 61 | 35 (57.4%) |
| MDS-UPDRS psychosis | ||||||||||
| ≥1 | 9 | 3 (33.3%) | 17 | 8 (47.1%) | 24 | 13 (54.2%) | 36 | 21 (58.3%) | 36 | 15 (41.7%) |
| MDS-UPDRS apathy | ||||||||||
| ≥2 | 11 | 5 (45.5%) | 27 | 7 (25.9%) | 32 | 11 (34.4%) | 24 | 10 (41.7%) | 28 | 15 (53.6%) |
| MDS-UPDRS fatigue | ||||||||||
| ≥2 | 42 | 26 (61.9%) | 63 | 37 (58.7%) | 69 | 35 (50.7%) | 68 | 48 (70.6%) | 71 | 57 (80.3%) |
| MDS-UPDRS insomnia | ||||||||||
| ≥2 | 92 | 55 (59.8%) | 114 | 76 (66.7%) | 112 | 73 (65.2%) | 119 | 90 (75.6%) | 127 | 99 (78.0%) |
| Epworth sleepiness scale | ||||||||||
| ≥10 | 57 | 23 (40.4%) | 65 | 45 (69.2%) | 78 | 58 (74.4%) | 94 | 67 (71.3%) | 86 | 65 (75.6%) |
| RBDSQ | ||||||||||
| ≥6 | 100 | 64 (64.0%) | 95 | 77 (81.1%) | 116 | 86 (74.1%) | 117 | 88 (75.2%) | 105 | 83 (79.0%) |
| QUIP | ||||||||||
| ≥1 disorders1 | 84 | 28 (33.3%) | 52 | 24 (46.2%) | 73 | 44 (60.3%) | 76 | 52 (68.4%) | 83 | 49 (59.0%) |
| MOCA | ||||||||||
| <26 | 89 | 55 (61.8%) | 126 | 88 (69.8%) | 110 | 74 (67.3%) | 103 | 72 (69.9%) | 85 | 58 (68.2%) |
| <21 | 4 | 3 (75.0%) | 13 | 8 (61.5%) | 17 | 8 (47.1%) | 12 | 8 (66.7%) | 14 | 8 (57.1%) |
- Column definitions: 2,4,6,8,10, Number of subjects with symptoms at the visit (only includes subjects with data available 1 year later); 3,5,7,9,11, Number (percentage) of subjects with symptoms at previous visit who remained symptomatic 1 year later, out of subjects with data available at both years.
- 1 Defined as any of four main ICDs OR other behaviors OR compulsive medication use.
Models comparing NPS over time in PD participants versus HC
Nearly all (except anxiety) of the nine assessed psychiatric symptoms increased significantly in prevalence in the PD group over time. Comparing PD patients with HC, insomnia, EDS, RBD, and ICD and related symptoms all increased significantly in prevalence over time in PD patients relative to HC (Table 5).
| Outcome | Year | Year*Group1 Interaction | ||
|---|---|---|---|---|
| OR2 (95% CI) | P-value3 | OR4 (95% CI) | P-value3 | |
| GDS-15 | 1.10 (1.03, 1.17) | 0.0021 | 0.83 (0.70, 0.98) | 0.0318 |
| STAI state | 0.96 (0.91, 1.01) | 0.1106 | 0.99 (0.86, 1.14) | 0.8926 |
| MDS-UPDRS psychosis | 1.33 (1.23, 1.44) | <0.0001 | 0.50 (0.27, 0.92) | 0.0247 |
| MDS-UPDRS apathy | 1.26 (1.16, 1.37) | <0.0001 | 0.96 (0.71, 1.30) | 0.8139 |
| MDS-UPDRS fatigue | 1.27 (1.20, 1.35) | <0.0001 | 0.89 (0.74, 1.07) | 0.2184 |
| MDS-UPDRS insomnia | 1.22 (1.15, 1.28) | <0.0001 | 0.87 (0.80, 0.94) | 0.0006 |
| Epworth sleepiness scale | 1.20 (1.14, 1.27) | <0.0001 | 0.86 (0.78, 0.95) | 0.0027 |
| RBDSQ | 1.13 (1.08, 1.19) | <0.0001 | 0.81 (0.73, 0.91) | 0.0003 |
| QUIP5 | 1.11 (1.05, 1.18) | 0.0003 | 0.84 (0.76, 0.93) | 0.0011 |
- 1 PD is the reference group.
- 2 Year effect OR indicates the change in odds of the symptom in PD participants for every year increase. OR >1 indicates an increase in odds of the symptom over time.
- 3 Bonferroni-corrected significant P value is < 0.005.
- 4 Year*Group Interaction OR indicates the change in estimated OR between HC relative to PD for every year increase. OR < 1 indicates the odds of the symptom increase faster in PD over time compared to the change in odds in HC over time.
- 5 Defined as any of four main ICDs OR other behaviors OR compulsive medication use.
Models assessing predictors of NPS over time in PD participants
There were no significant effects of demographic variables on change in symptoms in PD participants over time. Regarding impact of PD treatments, lower total LEDD was associated with cognitive impairment. There were no significant impacts of DA use or ACB scale score on symptoms over time, including cognitive impairment.
Principal component analysis in PD
A PCA was conducted in PD patients for the 10 NPS/cognitive impairment, using first baseline and then year 5 data. At both baseline and year 5, seven principal components were needed to explain at least 80% of total variance. The first component explained only 21% and 26% of the total variance at baseline and year 5 and had highest and positively associated loadings for depression and anxiety at baseline, and depression, anxiety, and fatigue at year 5. Examining the Pearson correlation matrix for all 10 variables, only depression-anxiety (0.49) and fatigue-apathy (0.37) had an r value >0.30.
Discussion
In this analysis of the 5-year course in de novo PD patients enrolled in the PPMI study, of the nine non-cognitive NPS we examined, cross-sectional prevalence rates increased by 6.2–20.9% over the 5-year period for eight of the disorders, with only anxiety decreasing in prevalence over time. This is consistent with previous research done in smaller samples, and with different assessments, that a range of non-motor symptoms are common in early PD8 and increase over time.10 Although we can only speculate based on the data presented herein, possible explanations for the increase in NPS over time in early PD include the spread of disease pathology, impact of PD treatments on some symptoms, and psychological factors.
At baseline, we found that only one disorder (RBD) occurred in >25% of patients, while by year 5 this threshold was crossed by five of the disorders. Within this overall increase in NPS over time, there are subtle differences. For instance, depression and anxiety, both considered features of prodromal PD,34 were common at disease onset but did not increase markedly over the next 5 years. Anxiety actually decreased over time; possible explanations for this include that some anxiety at baseline may be related to the recent PD diagnosis, anxiety that occurs in the context of non-motor fluctuations is not common in early disease, and that the nearly doubling in frequency of anxiolytic/sedative-hypnotic medication use over 5 years may have effectively treated anxiety symptoms in some patients. Psychosis and ICDs are both known to be associated with PD medication use, but only psychosis increased significantly over the 5-year period, perhaps suggesting a greater disease contribution for psychosis35 or a longer lag time after initiation of PD medications for ICD development.36 The greatest increase in and highest prevalence of symptoms at year 5 occurred for disorders of sleep and wakefulness, as well as fatigue.
In contrast, cognition in PD patients was relatively unimpaired at baseline and declined less over time than reported in other longitudinal studies in early PD (3, 5, 6). Factors contributing to this may include a cohort that was relatively younger at baseline, highly educated, and highly motivated given the demands of study participation. Another possible factor, not typically examined, was evidence that study discontinuation was associated with worse cognitive performance, thus eliminating these participants from subsequent analyses. Variability in cognitive performance around the MoCA cutoff score of 26 may have to do as much with instrument reliability as with true variation in patient performance.
To put the results in context, cross-sectional prevalence rates increased over time for nearly every NPS/cognitive impairment in PD patients, while they changed little in HC over time. This helps confirm that disease or PD treatment effects, not increasing age, is the primary driving force to NPS in PD, although the fact that the healthy control cohort all had normal cognition at baseline may also have made them less likely to have NPS as well.
When examining prevalence of NPS in PD, studies typically don’t account for initiation of symptomatic therapy for the studied disorders. In additional analyses we decided to include initiation of psychopharmacology as indicating the presence of a disorder (ICDs excepted, given lack of approved treatments), regardless of the assessment score, as a clinical decision had been made to initiate symptomatic therapy. Then again examining cross-sectional, as well as cumulative prevalence, the burden of NPS was even higher. For instance, the cumulative prevalence of all 10 NPS/cognitive impairment in PD was >25% by year 5, and seven of the NPS/cognitive impairment had a cumulative prevalence of >50%.
Given the high cumulative prevalence of nearly all the examined NPS, high disorder comorbidity is expected. Considering the presence of three or more NPS/cognitive impairment at any time point as significant comorbidity, this prevalence doubled over the 5-year period, going from approximately one-quarter to over one-half of patients.
Fluctuations in cognitive performance and diagnosis over time have been reported in PD, and we extend the findings to include other NPS too. Given that not all patients who screened positive for a neuropsychiatric symptom were receiving treatment for that disorder, this indicates some natural variability in NPS from year to year, and also highlight the limitation in using a screening instrument with a single cutoff point to indicate possible presence of a disorder.
Regarding treatment of NPS/cognitive impairment, use of antidepressants and anxiolytics/sedative-hypnotic agents were common even at disease onset, and rose to approximately one-quarter of patients by year 5 for each medication class. In contrast, antipsychotic and cognitive-enhancing medications were uncommon throughout this period. The latter finding could represent either that psychosis or cognitive deficits are often not determined to be clinically significant early in the disease course, or that NPS symptoms may be under-recognized and undertreated in PD.37
Medications can also have an adverse impact on NPS/cognitive abilities in PD. A range of PD medications are associated with both psychosis and ICDs, but neither total LEDD nor DA use predicted the presence of either symptom in this study. This may in part be due to the low overall prevalence or increase in the symptoms in the first 5 years of the illness, or the lower doses of DAs and levodopa used when initiated in early disease. In addition, the use of medications with anticholinergic properties increased over time, and although there was no impact of ACB score on meeting a MoCA cutoff score in these analyses, it is possible that with more long-term ACB exposure and examination of other cognitive outcomes, an effect may be detected, as previously reported in PD.38
There is increasing interest in subtyping non-motor symptoms in PD, which can be done at the symptom or patient level, with some previous research showing broad clusters including non-motor symptoms in early PD,13, 39 with subtypes also predicting long-term course.14, 39 Examining clustering of only NPS symptoms using PCA in our PD cohort, only the first component (depression and anxiety symptoms) accounted for >20% of the variance in NPS at either baseline or year 5, indicating limited clustering of symptoms in this cohort.
Limitations of the study include different inclusion criterion for PD patients and HC related to cognitive performance (i.e. HC were required to have normal cognition at baseline), which precluded comparison of the two cohorts over time, and may also have impacted the frequency of NPS in the two cohorts. In addition, approximately 25% of patients seen at baseline did not have a 5-year visit, with evidence for differential dropout based on worse cognitive performance. All instruments were self-rated, and detailed questionnaires or rating scales were not available for some of the NPS examined. Regarding use of psychiatric medications, we were not able to determine the specific indication for medication use. Finally, the cohort is relatively young (at baseline), overwhelmingly white, and highly educated, which limits generalizability.
Longitudinal studies have demonstrated that the cumulative prevalence of most psychiatric and cognitive complications are far higher than earlier cross-sectional studies suggested, and the results reported herein extend many of these findings to even early PD. From a research standpoint, this indicates that NPS are a potential outcome measure for disease progression clinical trials. Clinically, under-recognition of NPS remains,37 and management options for most NPS remain quite limited.40 From disease onset, the common, accumulating, and frequently comorbid occurrence of numerous NPS makes imperative investments into improving their recognition and treatment. Parkinson disease is not only a motor disorder, but also a neuropsychiatric disease from the beginning, and needs to be treated as such.
Conflict of Interest
Weintraub reports receiving salary support from the Michael J Fox Foundation during the conduct of the study (Weintraub serves on the Steering Committee of the PPMI Study). Ms. Caspell-Garcia has nothing to disclose. Simuni reports receiving grant funding from NINDS, MJFF, as well as the Parkinson's Foundation. Additionally, Simuni receives grants from Biogen, Roche, Neuroderm, Sanofi, Sun Pharma, Abbvie, IMPAX, as well as other funding from Acadia, Abbvie, Accorda, Adamas, Allergan, Amneal, Anavex, Aptinyx, Denali, General Electric (GE), Neuroderm, Neurocrine, Sanofi, Sinopia, Sunovion, TEVA, Takeda, Voyager, and US WorldMeds. Cho has nothing to disclose. Coffey received grant funding from the Michael J. Fox Foundation, NHLBI, and NINDS as well as consulting fees from the Michael J. Fox Foundation. Aarsland reports research support or honoraria from AstraZeneca, H. Lundbeck, Novartis Pharmaceuticals and GE Health; served as paid consultant for H. Lundbeck, Biogen, Eisai, Heptares, Sanofi and Mentis Cura; is partly funded by the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King's College London; the views expressed are those of the author and do not necessarily those of the NHR or the Department of Health and Social Care. Alcalay reports receiving personal fees from Sanofi, Roche, Restorbio, Uanssen, and Biogen. Barrett has nothing to disclose. Chahine has nothing to disclose. Eberling has nothing to disclose. Espay reports receiving grants from NIH, Great Lakes Neurotechnologies, the Michael J Fox Foundation, as well as personal fees from Abbvie, TEVA, lmpax, Acadia, Acorda, Cynapsus/Sunovion, Lundbeck, US WorldMeds, UCB, Lippincott Williams & Wilkins, Cambridge University Press, Springer, and lnTrance. Hamilton has nothing to disclose. Hawkins has nothing to disclose. Leverenz reports receiving grants from the Michael J. Fox Foundation, National Institute of Health, as well as other funding from the Veterans Affairs (VA) during the conduct of the study. Additionally, Dr. Leverenz receives grants from the Lewy Body Dementia Association, grants and personal fees from GE 'Healthcare, grants from Genzyme, grants from Alzheimer's Drug Discovery Foundation, personal fees from Eisai, grants and personal fees from Biogen, personal fees from Acadia, personal fees from Aptinyx, grants and personal fees from Sanofi, personal fees from Takeda, and grants from Avid Biopharmaceuticals. Litvan reports receiving grants from the Michael J Fox Foundation during the conduct of the study; grants from the National Institutes of Health, grants from the Parkinson Study Group, grants from the Lewy Body Association, as well as Abbvie, Biogen, Roche, and other funding from the Lundbeck Advisory Board and Sunovion. Richard has nothing to disclose. Rosenthal received support from NIH/NINOS P50NS038377, the Marilyn and Edward Macklin Foundation, and the Michael J. Fox Foundation. She also received an honorarium from the Edmond J. Safra Foundation and from Functional Neuromodulation. Siderowf has nothing to disclose. York has nothing to disclose.
Author Contributions
Daniel Weintraub (corresponding author): Substantial contributions to the conception or design of the work; the acquisition, analysis, or interpretation of data for the work; drafting the work or revising it critically for important intellectual content; final approval of the version to be published; and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Chelsea Caspell-Garcia: Substantial contributions to the conception or design of the work; the acquisition, analysis, or interpretation of data for the work; and drafting the work or revising it critically for important intellectual content. Tanya Simuni: Substantial contributions to the conception or design of the work; the acquisition, analysis, or interpretation of data for the work; and drafting the work or revising it critically for important intellectual content. Hyunkeun Ryan Cho: Substantial contributions to the conception or design of the work; the acquisition, analysis, or interpretation of data for the work; and drafting the work or revising it critically for important intellectual content. Christopher C. Coffey: Substantial contributions to the conception or design of the work; the acquisition, analysis, or interpretation of data for the work; and drafting the work or revising it critically for important intellectual content. Dag Aarsland: Drafting the work or revising it critically for important intellectual content. Roy N. Alcalay: Drafting the work or revising it critically for important intellectual content. Matthew J. Barrett: Drafting the work or revising it critically for important intellectual content. Lana M. Chahine: Substantial contributions to the conception or design of the work; the acquisition, analysis, or interpretation of data for the work; and drafting the work or revising it critically for important intellectual content. Jamie Eberling: Substantial contributions to the conception or design of the work; and drafting the work or revising it critically for important intellectual content. Alberto J. Espay: Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; drafting the work or revising it critically for important intellectual content. Jamie Hamilton: Drafting the work or revising it critically for important intellectual content. Keith A. Hawkins: Substantial contributions to the conception or design of the work; the acquisition, analysis, or interpretation of data for the work; drafting the work or revising it critically for important intellectual content. James Leverenz: Drafting the work or revising it critically for important intellectual content. Irene Litvan: Drafting the work or revising it critically for important intellectual content. Irene Richard: Drafting the work or revising it critically for important intellectual content. Liana Rosenthal: Drafting the work or revising it critically for important intellectual content. Andrew Siderowf: Substantial contributions to the conception or design of the work; the acquisition, analysis, or interpretation of data for the work; drafting the work or revising it critically for important intellectual content. Michele York: Drafting the work or revising it critically for important intellectual content.




