Relationship between left atrial volume and ischemic stroke subtype
Funding Information
Kamel is supported by NIH/NINDS grant R01NS097443 and by the Michael Goldberg Research Fund. Merkler is supported by AHA grant 18CDA34110419 and by the Leon Levy Fellowship in Neuroscience. Navi is supported by NIH/NINDS grant K23NS091395 and by the Florence Gould Endowment for Discovery in Stroke. Weinsaft is supported by NIH/NHLBI grant R01HL128278. Kim is supported by NIH/NHLBI grant K23HL140092. This study received support from New York-Presbyterian Hospital and Weill Cornell Medicine, including the Clinical and Translational Science Center (UL1TR000457) and Joint Clinical Trials Office.
Abstract
Objective
Atrial cardiopathy without atrial fibrillation (AF) may be a potential cardiac source of embolic strokes of undetermined source (ESUS). Atrial volume is a feature of atrial cardiopathy, but the relationship between atrial volume and ESUS remains unclear.
Methods
We compared left atrial volume among ischemic stroke subtypes in the Cornell Acute Stroke Academic Registry (CAESAR), which includes all patients with acute ischemic stroke at our hospital since 2011. Stroke subtype was determined by neurologists per the TOAST classification and consensus ESUS definition. Left atrial volume index (LAVI) was obtained directly from our echocardiography image system (Xcelera, Philips Healthcare). We used t-tests and analysis of variance for unadjusted comparisons and targeted minimum loss-based estimation for comparisons adjusted for demographics and comorbidities.
Results
Among 2116 patients in CAESAR from 2011 to 2016, 1293 had LAVI measurements. LAVI varied across subtypes (P < 0.001) from 48.8 (±30.0) mL/m2 in cardioembolic strokes to 30.3 (±10.5) mL/m2 in small-vessel strokes. LAVI was larger in ESUS (33.3 ± 13.6 mL/m2) than in small- or large-vessel stroke (30.9 ± 10.7 mL/m2) (P = 0.01). The association between LAVI and ESUS persisted after the adjustment for demographics and comorbidities: a 10 mL/m2 increase in LAVI was associated with a 4.4% increase in ESUS probability (95% CI, 2.3%–6.4%). Results were similar after excluding patients with AF during post-discharge heart-rhythm monitoring.
Interpretation
We found larger left atria among patients with ESUS versus non-cardioembolic stroke. There was significant overlap in left atrial size between ESUS and non-cardioembolic stroke, highlighting that many ESUS cases are not cardioembolic.
Introduction
Approximately 20% of ischemic strokes do not have a known mechanism.1 Most of these cryptogenic strokes appear embolic and are sometimes referred to as embolic strokes of undetermined source (ESUS).2 It is hypothesized that most cases of ESUS result from either occult cardiac or large-artery lesions,2 but the exact nature of these mechanisms remain unclear. Recent evidence suggests that some cases of ESUS may be the result of left atrial thromboembolism that goes unrecognized because it occurs in the absence of atrial fibrillation/flutter (AF).3 Several markers of abnormal atrial anatomy and function have been associated with ischemic stroke independently of AF.3 Left atrial volume is a core feature of atrial cardiopathy but the relationship between left atrial volume and ESUS remains unclear given conflicting results from prior studies.4-8 If atrial cardiopathy is an under-recognized risk factor for ESUS, it should be more prevalent among ESUS cases than strokes of known non-cardioembolic etiology. We therefore tested the hypothesis that ESUS patients have larger left atrial size than patients with non-cardioembolic stroke.
Methods
Design
We conducted a cross-sectional study among ischemic stroke patients enrolled in the Cornell AcutE Stroke Academic Registry (CAESAR). The institutional review board at Weill Cornell Medical College approved this study and waived the requirement for informed consent. De-identified data and the analytic methods that produced the results of this study are available upon reasonable request from the corresponding author.
Patient population
All patients hospitalized at NewYork-Presbyterian Hospital/Weill Cornell Medical Center for acute stroke are prospectively enrolled in the American Heart Association’s Get With The Guidelines (GWTG)—Stroke registry. Trained hospital analysts prospectively collect data on demographics, vascular risk factors and comorbidities, stroke severity, and in-hospital treatments and outcomes. CAESAR combines the GWTG data plus additional retrospectively collected clinical, laboratory, and radiographic data.9 All cases of ischemic stroke are reviewed by a panel of at least three neurologists who adjudicate the etiology per the Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification10 and per the consensus definition for embolic stroke of undetermined source (ESUS).2 For this analysis, we included all ischemic stroke patients who were registered in CAESAR from 2011 through 2016 except those with a subtype of other known cause, who represented a small and heterogeneous fraction of our sample.
Measurements
The exposure variable was the left atrial volume index (LAVI) measured during transthoracic echocardiography (TTE). LAVI values were imported directly from our hospital’s echocardiographic image management system (Xcelera, Philips Healthcare) into a Microsoft SQL server and merged into the main CAESAR registry. We excluded TTEs without a recorded LAVI measurement. For patients with multiple TTEs with LAVI measurements, we included the qualifying study performed closest in time to the index stroke. A board-certified cardiologist with specific training in echocardiography verified LAVI measurements <10 or >125 mL/m2, thresholds that were chosen based on clinical expertise and prior to beginning analyses. The outcome variable was ischemic stroke subtype. Covariates were age, sex, race, insurance status, chronic kidney disease, heart failure, coronary artery disease, diabetes, alcohol or drug abuse, hypertension, prior stroke, peripheral vascular disease, tobacco use, and valvular heart disease.
Statistical analysis
We compared LAVI among three categories of ischemic stroke subtype: cardioembolic, non-cardioembolic (defined as small-vessel occlusion or large-artery atherosclerosis), and ESUS. In sensitivity analyses, we used the TOAST cryptogenic stroke definition instead of the ESUS definition. We used t-tests and analysis of variance (ANOVA) for unadjusted comparisons and targeted minimum loss-based estimation for comparisons adjusted for demographics and comorbidities.11, 12 We performed a post hoc analysis in which we additionally adjusted for body mass index, ejection fraction, and the E/e′ ratio. We performed subgroup analyses stratified by sex. For descriptive purposes, left atrial enlargement was defined as LAVI ≥ 35 mL/m2.13 In a sensitivity analysis, we excluded cryptogenic stroke patients found to have AF during poststroke heart-rhythm monitoring; the standard protocol at our institution is to perform heart-rhythm monitoring with a 30-day external loop recorder, starting within a few weeks after hospital discharge. Additionally, we performed sensitivity analyses limited to echocardiograms performed as part of clinical care prior to the index stroke and analyses using the cryptogenic stroke definition instead of the ESUS definition.
Results
Among 2116 patients in CAESAR from 2011 to 2016, 1293 (72.5%) had a recorded LAVI measurement. The likelihood of a missing LAVI measurement did not differ between ESUS and non-ESUS cases. Among the 1293 patients with a recorded LAVI measurement, there were 448 cases of cardioembolic stroke, 314 cases of non-cardioembolic stroke (small- or large-vessel stroke), and 531 cases of ESUS (Table 1). Among the cardioembolic stroke cases, 85% had AF. The mean LAVI was 38.7 (±21.9) mL/m2. The 638 patients with any left atrial enlargement were older; had more severe strokes; and were more likely to have hypertension, chronic kidney disease, coronary heart disease, heart failure, and prior stroke; rates of other vascular comorbidities such as peripheral vascular disease did not differ between groups (Table 2).
| Characteristica | Cardioembolic (N = 448) | Embolic stroke of undetermined source (N = 531) | Non-Cardioembolic (N = 314) |
|---|---|---|---|
| Age, mean (SD), y | 74.0 (14.0) | 69.9 (15.9) | 69.8 (13.1) |
| Female | 239 (53.4) | 285 (53.7) | 132 (42.0) |
| White race | 389 (86.8) | 463 (87.2) | 257 (81.9) |
| Insurance status | |||
| Medicare | 215 (48.0) | 253 (47.7) | 141 (44.9) |
| Medicaid | 175 (39.1) | 197 (37.1) | 117 (37.3) |
| Private | 51 (11.4) | 68 (12.8) | 51 (16.2) |
| Self-pay | 7 (1.6) | 13 (2.5) | 5 (1.6) |
| Hypertension | 1209 (68.7) | 316 (70.5) | 351 (66.1) |
| Diabetes | 452 (25.7) | 100 (22.3) | 137 (25.8) |
| Coronary heart disease | 360 (20.5) | 115 (25.7) | 94 (17.7) |
| Heart failure | 91 (5.2) | 46 (10.3) | 24 (4.5) |
| Peripheral vascular disease | 90 (5.1) | 22 (4.9) | 31 (5.8) |
| Chronic kidney disease | 55 (3.1) | 18 (4.0) | 20 (3.8) |
| Prior stroke | 379 (21.6) | 88 (19.6) | 114 (21.5) |
| Active tobacco use | 142 (8.1) | 24 (5.4) | 29 (5.5) |
| Valvular disease | 33 (1.9) | 15 (3.4) | 6 (1.1) |
| NIHSS score, mean (SD) | 7.5 (8.3) | 9.9 (9.2) | 6.2 (7.4) |
| Systolic BP, mean (SD), mm Hg | 150.8 (29.2) | 144.6 (27.1) | 151.8 (28.1) |
| Body mass index, mean (SD) | 26.9 (9.4) | 26.9 (15.0) | 26.5 (5.8) |
| LDL, mean (SD), mg/dL | 98.4 (40.4) | 92.0 (38.0) | 96.8 (37.8) |
| Hemoglobin A1c, mean (SD), % | 7.0 (1.9) | 6.7 (1.5) | 7.0 (1.9) |
| Ejection fraction, mean (SD), % | 60.1 (17.5) | 69.2 (10.4) | |
| E/e′, mean (SD) | 15.5 (8.0) | 13.8 (7.9) | |
- Abbreviations: BP, blood pressure; LDL, low-density lipoprotein; NIHSS, National Institutes of Health Stroke Scale; SD, standard deviation.
- a Data are presented as number (%) unless otherwise specified.
| Characteristica | Left atrial enlargementb (N = 638) | No left atrial enlargement (N = 655) | P value |
|---|---|---|---|
| Age, mean (SD), y | 75.4 (13.4) | 67.2 (14.9) | <0.001 |
| Female | 336 (52.7) | 320 (48.9) | 0.17 |
| White race | 553 (86.7) | 556 (84.9) | 0.36 |
| Insurance status | 0.06 | ||
| Medicare | 291 (45.6) | 318 (48.6) | |
| Medicaid | 261 (40.9) | 228 (34.8) | |
| Private | 78 (12.2) | 92 (14.1) | |
| Self-pay | 8 (1.3) | 17 (2.6) | |
| Hypertension | 38 (6.0) | 60 (9.2) | <0.001 |
| Diabetes | 166 (26.0) | 185 (28.2) | 0.37 |
| Coronary heart disease | 164 (25.7) | 104 (15.9) | <0.001 |
| Heart failure | 57 (8.9) | 18 (2.8) | <0.001 |
| Peripheral vascular disease | 37 (5.8) | 27 (4.1) | 0.16 |
| Chronic kidney disease | 34 (5.3) | 15 (2.3) | 0.004 |
| Prior stroke | 227 (35.6) | 44 (6.7) | <0.001 |
| Active tobacco use | 145 (22.7) | 131 (20.0) | 0.23 |
| Valvular disease | 38 (6.0) | 60 (9.2) | 0.03 |
| NIHSS score, mean (SD) | 16 (2.5) | 9 (1.4) | 0.14 |
| Systolic BP, mean (SD), mm Hg | 8.4 (8.6) | 6.0 (7.4) | <0.001 |
| Body mass index, mean (SD) | 151.8 (28.2) | 150.9 (29.6) | 0.58 |
| LDL, mean (SD), mg/dL | 27.0 (12.4) | 26.9 (7.2) | 0.87 |
| Hemoglobin A1c, mean (SD), % | 91.9 (36.3) | 103.9 (43.0) | <0.001 |
| Ejection fraction, mean (SD), % | 6.7 (1.5) | 7.3 (2.1) | <0.001 |
| E/e′, mean (SD) | 64.4 (15.7) | 68.7 (11.3) | <0.001 |
- Abbreviations: BP, blood pressure; LDL, low-density lipoprotein; NIHSS, National Institutes of Health Stroke Scale; SD, standard deviation.
- a Data are presented as number (%) unless otherwise specified.
- b Left atrial enlargement was defined as a left atrial volume index ≥ 29 mL/m2.
LAVI varied significantly across subtypes (P < 0.001), from 48.8 (±30.0) mL/m2 in cardioembolic strokes to 30.3 (±10.5) mL/m2 in small-vessel strokes. Patients with ESUS had significantly larger LAVI (33.3 ± 13.6 mL/m2) than those with small- or large-vessel strokes (30.9 ± 10.7 mL/m2) (P = 0.01). Although there was substantial overlap in LAVI between these two groups (Fig. 1), we found a curvilinear relationship between LAVI and the likelihood of ESUS (vs. small- or large-vessel stroke) (Fig. 2).
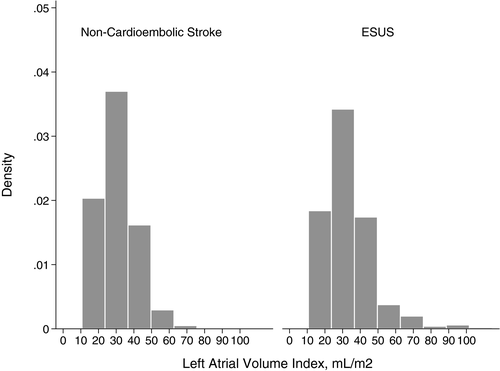
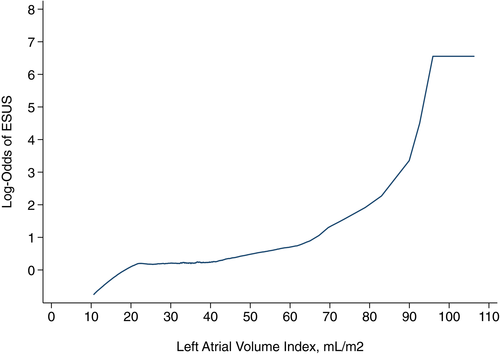
The association between LAVI and ESUS (vs. small- or large-vessel stroke) persisted after adjustment for demographics and comorbidities: a 10 mL/m2 increase in LAVI was associated with a 4.4% increase in ESUS probability (95% confidence interval, 2.3%–6.4%). These results were unchanged after additional adjustment for body mass index, ejection fraction, and the E/e′ ratio. In subgroup analyses, LAVI was associated with ESUS in both men and women and we found no interaction between sex and LAVI in relation to ESUS (P = 0.32).
Our findings were the same in sensitivity analyses using the TOAST cryptogenic stroke definition, limiting our sample to the 297 patients with echocardiograms performed prior to the index stroke, and excluding patients found to have AF during poststroke heart-rhythm monitoring.
Discussion
In a single-center registry of patients with acute ischemic stroke, we found that patients with ESUS had larger left atria than patients with non-cardioembolic stroke. This association was found in both men and women and persisted after adjustment for demographics and comorbidities, after excluding ESUS patients who were later found to have AF, and when restricting the sample only to those patients with available prestroke echocardiograms. These findings support the hypothesis that atrial dilatation may be a risk factor for ischemic stroke even in the absence of clinically apparent AF. At the same time, there was significant overlap in left atrial size between cryptogenic and non-cardioembolic stroke patients, highlighting that many cryptogenic strokes may not be cardioembolic.
Based on prior studies, it has been unclear if left atrial size is associated with ischemic stroke independently of AF. Left atrial size has been associated with incident6, 14 and recurrent stroke15 after adjustment for AF in some cohorts, but other cohort studies have found no relationship7 or an association only in men.4, 5 These prior studies did not use the ESUS definition, which ensures uniform evaluation of potential etiologies, and did not include continuous heart-rhythm monitoring to rule out underlying AF, which may be involved in the relationship between left atrial size and stroke. A recent study found no significant difference in left atrial enlargement in ESUS patients versus non-cardioembolic stroke patients, but this study was likely underpowered based on its reported confidence intervals.8 In this context, our study provides novel information about the relationship between left atrial size and ischemic stroke risk, suggesting that left atrial structural derangements may play a pathogenic role in strokes that are currently labeled as being of undetermined source. Based on emerging evidence, we and others have argued for the possibility of a thrombogenic atrial substrate that may lead to embolic stroke even in the absence of AF.16-20 Such an atrial cardiomyopathy would explain the temporal dissociation between AF and stroke21, 22 and the link between markers of atrial cardiomyopathy and stroke independently of AF.6, 16, 23 If atrial cardiomyopathy can cause stroke in the absence of AF but is currently not recognized as a cause of stroke, then we would expect it to be over-represented among cases of ESUS. In that context, our finding of larger left atrial size among ESUS cases supports the concept of a thrombogenic atrial cardiomyopathy. However, the mean LAVI in our ESUS cases (approximately 33 mL/m2) was within the normal range, indicating that LAVI may not be a very useful discriminator, in which case imaging biomarkers of atrial function such as left atrial strain may help distinguish between myopathic and non-myopathic atria.24
The difference in LAVI between cardioembolic stroke and ESUS cases was much larger than the difference between ESUS and non-cardioembolic stroke cases. This may partly represent a ceiling of LAVI above which patients very often develop AF and become classified as cardioembolic stroke, thereby limiting the upper threshold of LAVI in the ESUS group. At the same time, the substantial overlap between ESUS cases and patients with known non-cardioembolic etiologies is consistent with the hypothesis that many ESUS cases are not the result of cardiac embolism. It is likely that a substantial proportion of ESUS cases results from large-artery atherosclerotic plaques that are currently unrecognized because they do not cause >50% stenosis of the arterial lumen.25, 26 This heterogeneity in the ESUS population may explain the lack of benefit of anticoagulant therapy in the overall ESUS population,27, 28 since atherosclerotic lesions may respond more favorably to strong antiplatelet therapy.29
Our study should be interpreted in light of its limitations. First, we studied a single-center cohort and our findings may not be generalizable to other populations. On the other hand, with the exception of a higher proportion of white patients and somewhat more severe strokes, the baseline characteristics of our patients were broadly similar to more representative samples such as the overall population of the national GWTG registry.30 Second, our ESUS patients generally did not undergo more than 30 days of heart-rhythm monitoring, and prior studies have indicated a substantial yield of AF detection with continuous monitoring beyond this point. Third, LAVI measurements were not available in all patients in our registry. An echocardiogram was not performed in 17% of our population, usually due to early death, patients’ goals of care, or an obvious stroke etiology. Among those who did have an echocardiogram, about one-quarter did not have LAVI documented, usually due to technically limited images or practice variation on the part of the echocardiographer. This is unlikely to have affected our results since the likelihood of a missing LAVI value did not differ between ESUS and non-ESUS cases.
In conclusion, we found significantly larger left atria among patients with ESUS versus non-cardioembolic stroke. However, there was significant overlap in left atrial size between ESUS and non-cardioembolic stroke patients, highlighting that many ESUS cases are not cardioembolic.
Acknowledgments
None.
Conflict of Interest
Kamel serves on the Steering Committee for Medtronic’s Stroke AF trial (uncompensated), has served on an advisory board for Roivant Sciences, and receives in-kind study support for the ARCADIA trial from the BMS-Pfizer Alliance and Roche Diagnostics. The remaining authors have no conflict of interest. All authors abide by the ICMJE guidelines regarding the declaration of conflict of interest.




