The etiologies and prognosis associated with spinal cord infarction
Funding Information
Chang Gung Memorial Hospital, Linkou (Grant number: 201800769B0)
Abstract
Background
This study aims to investigate the etiology and prognosis of spinal cord infarction (SCI).
Methods
Over a period of 16 years, we retrospectively analyzed 31 patients with SCI. Demographic features and symptom presentations were carefully documented. Etiology-specific MRI features, such as the length and distribution of the lesions and owl’s eyes sign, were recorded and analyzed to determine their associations with the clinical signs/symptoms.
Results
In total, seven patients had aortic or vertebral artery dissections. We divided the patients with SCI into two groups: those with or without vessel dissection. Among SCI patients, the onset age was younger, and the proportion of patients with long-segment lesions and posterior pattern involvement on axial view was higher in the group with dissection than in the group without dissection (all P < 0.05). The lesions were frequently located in the upper cervical or lower thoracic-lumbar regions, and the lengths of the lesions were associated with 1-month outcomes, suggesting that artery dissection may contribute to the longitudinal and posterior extension of SCI. In contrast, among patients without dissection, the range of longitudinal extensions of in spans of vertebral bodies was broader (range, 1–8). A higher proportion of patients had focal pain adjacent to the lesion (P = 0.05) and a poorer 1-month outcome (P = 0.04) in the long-segment lesion group than in the short-segment lesion group.
Conclusions
A detailed history and the use of modern imaging tools may help clinicians search for vessel dissection and other etiologies, evaluate the spatial extension of lesions in SCI, and predict prognosis.
Introduction
Spinal cord infarction (SCI) is a rare disease that widely varies in its clinical presentations but may leave patients with devastating neurological sequelae, such as paraplegia, quadriplegia, and incontinence.1 Due to its rarity, there are few reliable estimates of its incidence; however, previous studies showed that SCI accounted for 1.2% of all strokes.2, 3 Recent studies have shown that myelopathy related to ischemic diseases accounts for 14–18% of patients with transverse myelitis, suggesting the underdiagnosis of SCI.4, 5 Clinically, three main characteristic symptoms could differentiate SCI from other etiologies of nontraumatic myelopathy (e.g., a hyperacute temporal course, new onset of back pain, and flaccid weakness).5 The MRI features of SCI include longitudinally extensive myelopathy more than three or more vertebral levels in length, gadolinium nonenhancement lesion, and the anterior involvement of the spinal cord on axial view, each of which is significantly different between this and other etiologies.5 Other specific findings of SCI found on MRI, such as the adjacent vertebral body infarction sign (defined by a geographic marrow hyperintensity on sagittal T2-weighted images) and the “owl’s eyes sign” (defined as “bilateral hyperintensities of the anterior horns on axial T2-weighted images”), have also been reported.6, 7
The vascular anatomy of the spinal cord is highly complex and has great individual variability. From the rostral to caudal axis, the spinal cord is mainly supplied by one anterior spinal artery (ASA) and two posterior spinal arteries (PSAs). The ASA supplies the anterior two-thirds of the cord, while the PSA pair supplies the rest.8 The ASA and PSAs are connected by the arterial vasocorona, which encircles the whole spinal cord and has many longitudinal and transverse interconnections.9 Many penetrating arteries from the arterial vasocorona supply the underlying spinal cord. These vascular networks construct numerous collateral systems of the spinal cord that account for rare cases of SCI. In SCI, most of the infarctions are located in the ASA territory (approximately 96.4%), while isolated PSA infarctions are relatively rare.10 The most common etiologies of SCI are vascular risk factors, aortic pathologies, and vessel dissection.10, 11 However, under such numerous collateral systems, factors associated with the longitudinal and posterior extensions of SCI remain unknown. Our hypothesis is that vessel dissection may be associated with the longitudinal and posterior involvement of SCI. To resolve this issue, we performed a retrospective study to evaluate the detailed clinical history, spinal cord MRI features, and cerebrospinal fluid (CSF) characteristics of a group of patients with SCI at our institution (the largest tertiary medical center in Taiwan).
Materials and Methods
Study design and patient population
We retrospectively analyzed the clinical presentations, initial neurological examinations, MRI features, and CSF profiles of a total of 38 patients with SCI enrolled from 2002 to 2018. The study protocol was approved by the institutional review board of the Chang Gung Memorial Hospital (IRB number: 201800769B0). All methods were performed in accordance with the relevant guidelines and regulations. Seven patients were excluded from this study due to a lack of complete clinical information or imaging data, leaving a final total of 31 patients in the analyses. We recorded demographic characteristics, medical histories, and information on clinical presentations, including temporal profiles, initial symptoms, the presence of acute focal pain adjacent to the spinal cord lesion, and neurologic examinations. CSF and MRI features, including the topography of the lesions at the initial assessment, were analyzed. The diagnosis of SCI was rendered based on the diagnostic criteria proposed by Zalewski et al.12 Briefly, acute nontraumatic myelopathy defined by onset to the nadir of clinical presentations within 12 h or less was mandatory and was combined with the corresponding MRI and/or CSF findings. Finally, three levels of diagnostic confidence in SCI were made: definite, probable, and possible SCI.
The temporal profiles covering the time from the initial symptom onset to the nadir of neurologic dysfunction were classified as sudden (<30 min), acute (30 min – 4 h), and subacute (>4 h) patterns. The nadir was defined as the point of the worst neurologic function before improvement or plateau was achieved and was based on the patient’s history and neurologic examinations. For each limb, a Medical Research Council (MRC) score (range, 0 to 5) was assigned to represent the muscle power in that limb. The muscle power values in the upper and lower limbs were measured by averaging the MRC scores of both sides. The total muscle power in all four limbs was calculated by summing the four-limb MRC scores. Associated medial history information, such as vascular risk factors (hypertension, diabetes mellitus, and dyslipidemia), previous trauma, and prior fever or upper respiratory tract infections, was also recorded. CSF profiles, including pleocytosis, sugar and protein levels, immunoglobulin G index, and oligoclonal band (OCB) measurements, were documented. Serum anti-aquaporin-4 (AQP4) antibody testing was performed by enzyme-linked immunosorbent assay (ELISA).13 We used modified Rankin scale (mRS) scores to compare the 1-month outcomes of patients with SCI.14 We classified outcomes with grades 0–3 as good prognoses and those with grades of 4–5 as a bad prognosis.
Evaluation of MRI parameters
MRIs of the spine obtained within 2 weeks of admission were reviewed by a board-certified neuroradiologist who was blinded to the clinical diagnosis. All studies included axial and sagittal T1- and T2-weighted sequences of spine images. The length of each lesion was measured by the sagittal extension of T2-hyperintense areas using the number of vertebral body spans. We defined long-segment lesions as longitudinal extension of lesions covering ≥ 3vertebral body spans and short-segment lesions as longitudinal extension of lesions covering < 3 vertebral body spans. The location of each lesion was recorded according to the vertebral body level (e.g., cervical, thoracic, or lumbar). Owl’s eyes sign and adjacent vertebral body infarction were also recorded.6, 15 Gadolinium enhancement [Gd+] on T1-weighted images was recorded as present or absent. The midpoint of each lesion was characterized using axial T2-weighted images. The distribution of the lesions in cross-sections of the spinal cord was classified as anterior, posterior, lateral, and central. In addition, the vascular territory of the spinal cord was used as a fit for the artery infarction observed in patients with SCI (e.g., ASA, PSA, spinal sulcal artery (SSA), or both ASA and PSA).10
Statistical analyses
All statistical analyses were performed using SPSS (version 21.0; IBM). Continuous variables are expressed as the means ± standard deviations. Categorical variables are presented as numbers and ratios. Independent t-tests were performed to compare mean ages between groups. Chi-squared tests, Fisher’s exact tests, and Mann–Whitney U tests were used to compare patients with and without vessel dissection in terms of sex, clinical presentations, 1-month outcomes, and imaging characteristics. Logistic regression was used to study the associations between 1-month outcomes and MRI features. Statistical significance was defined as P < 0.05.
Results
During the study period, 31 patients were confirmed to have SCI. In total, there were 16 male and 15 female patients, and the mean onset age of SCI was 55.3 ± 18.2 years old (range = 27–83 years old). There were 11 patients with definite SCI and 13 and 7 patients diagnosed with probable SCI and possible SCI, respectively. Table 1 presents a summary of the diagnostic groups in SCI and the involved vascular territories. Based on the classification of the vascular territory, 64.5% of the patients had ASA infarction, and 22.5% had both ASA and PSA infarctions. Only one case had PSA infarction with a definite SCI diagnosis. Figure 1 demonstrates the topographical distribution of spinal cord involvement in the 31 patients who received MRIs. The black lines represent the involved levels related to the vertebral bodies in each patient on T2-weighted images. Most of the lesions were located in the cervical or thoraco-lumbar regions. No lesion extended from the middle cervical to the middle thoracic region. Regarding the image features, only two patients had [Gd+] enhancement related to a long duration between symptom onset and MRI scanning time (more than 1 week). Eleven patients had a positive owl’s eyes sign (35%), and six patients had a positive adjacent vertebral body infarction sign (19%). With regard to 1-month outcomes, nine patients had good outcomes based on mRS scores. There was no significant association between the lengths of vertebral body spans and 1-month outcomes according to the results of the regression analysis (P = 0.45). Among the vascular risk factors, a history of hypertension was most frequently reported in patients with SCI (N = 14). Four patients with SCI had a history of aortic dissections, and three of these had aortic aneurysms with thrombosis. In the aortic dissection group, two patients had Marfan’s syndrome. Another three patients had vertebral artery dissection, including one with dissection in the V1–V2 segment of the vertebral artery and two with V3 segment dissection. To study the clinical characteristics and imaging features of patients with SCI who had a history of aorta or vertebral artery dissection, we divided these patients into two groups (with or without vessel dissection).
| Diagnostic group | Definite SCI | Probable SCI | Possible SCI | Total |
|---|---|---|---|---|
| Combined ASA and PSA infarct | 5 | 2 | 0 | 7 |
| ASA infarct | 5 | 10 | 5 | 20 |
| PSA infarct | 1 | 0 | 0 | 1 |
| SSA infarct | 0 | 1 | 2 | 3 |
- ASA, anterior spinal artery; PSA, posterior spinal artery; SSA, spinal sulcal artery.

Clinical and imaging features in SCI patients with vessel dissection
Table 2 shows the detailed comparisons between these two groups. The age of onset was significantly younger in SCI patients with vessel dissection than in those without (P < 0.05). The timing of performing MRI from the onset was longer in SCI patients with vessel dissection group (P = 0.03). There were no significant differences in sex, vascular risk factors, temporal profiles, focal pain adjacent to the lesion, muscle power in all limbs, and clinical outcomes after 1 month. A borderline difference in sphincter incontinence was noted between the groups (P = 0.05). Table 3 shows the differences in MRI features between SCI patients with and without vessel dissection. The mean value of the longitudinal extension of lesions in SCI patients with vessel dissection was 4.3 (range 3–10) vertebral body spans, while that in those without vessel dissection was 3.3 (range 1–8) vertebral body spans. Although the Mann–Whitney U test showed no significant group difference in the lengths of lesions based on the number of vertebral body spans (P = 0.06), we found that the proportion of patients with long-segment lesions was significantly higher in the vessel dissection group than in the nondissection group (Fisher’s exact test P = 0.03). In addition, the proportion of patients with an axial posterior pattern was significantly higher in the vessel dissection group (Fisher’s exact test P < 0.05), indicating the involvement of the PSA territory. Figure 2 demonstrates the number of cases and the ratio of lesions at the different vertebral body levels in SCI patients with a history of vessel dissection. In the vessel dissection group, patients frequently had lesions involving the upper cervical (C1–C4) and lower thoracic (T10–T12) vertebral body levels. In contrast, patients without vessel dissection more frequently had lesions distributed in the cervical regions (C5–T7) than in the thoraco-lumbar regions (Fig. 2B).
| SCI with vessel dissection (N = 7) | SCI without vessel dissection (N = 24) | P value | |
|---|---|---|---|
| Onset age | 39.8 ± 18.3 | 59.8 ± 16.8 | <0.05 |
| Sex (M:F) | 5:2 | 11:13 | 0.23 |
| Hypertension (Y:N) | 3:4 | 11:13 | 0.89 |
| Diabetes mellitus (Y:N) | 2:5 | 6:18 | 0.85 |
| Dyslipidemia (Y:N) | 2:5 | 8:16 | 0.81 |
| Fever (Y:N) | 1:6 | 1:23 | 0.34 |
| Temporal profile of onset | |||
| Sudden (<30 min) | 6 | 19 | 1.00 |
| Acute (30 min – 4 h) | 1 | 4 | |
| Subacute (>4 h) | 0 | 1 | |
| Onset to nadir time (minutes) | 12.8 ± 20.8 | 22.3 ± 48.6 | 0.51 |
| Focal pain adjacent to lesion (Y:N) | 4:3 | 12:12 | 0.74 |
| Timing of MRI from onset | 9.1 ± 8.6 | 4.5 ± 6.4 | 0.03 |
| All limbs muscle power (MRC score) | 13.1 ± 3.4 | 11.2 ± 4.4 | 0.35 |
| Upper limbs muscle power (MRC score) | 4.4 ± 1.1 | 4.3 ± 1.4 | 0.80 |
| Lower limbs muscle power (MRC score) | 2.3 ± 2.2 | 1.5 ± 1.8 | 0.53 |
| Hyporeflexia in affected limbs (Y:N) | 4:3 | 11:13 | 0.59 |
| Sphincter incontinence (Y:N) | 3:4 | 20:4 | 0.05 |
| mRS score (1 month later) | 3.1 ± 1.8 | 3.9 ± 1.0 | 0.47 |
- SCI, spinal cord infarction; MRC, Medical Research Council; mRS, modified Rankin scale; Y, yes; N, no.
| SCI with vessel dissection (N = 7) | SCI without vessel dissection (N = 24) | P value | |
|---|---|---|---|
| Lesion length (vertebral body span) | 4.3 ± 2.6 | 3.3 ± 1.8 | 0.30 |
| Longitudinal extension of lesions (Y:N) | 7:0 | 13:11 | 0.03 |
| Vertebral body infarction (Y:N) | 2:5 | 4:20 | 0.50 |
| Owl’s eyes sign (Y:N) | 1:6 | 10:14 | 0.18 |
| Axial anterior pattern (Y:N) | 4:3 | 18:4 | 0.20 |
| Axial central pattern (Y:N) | 5:2 | 13:9 | 0.55 |
| Axial lateral pattern (Y:N) | 6:1 | 16:6 | 0.46 |
| Axial posterior pattern (Y:N) | 5:2 | 4:18 | <0.05 |
- SCI, spinal cord infarction; long segments of lesion (≥3 vertebral body spans); Y, yes; N, no.
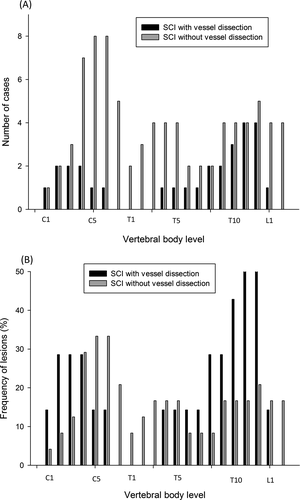
Clinical and imaging features of SCI without vessel dissection
Compared with SCI patients with vessel dissection, in those without, the longitudinal extension of lesions covered a broader range of vertebral body spans (range, 1–8). We divided these patients into short- and long-segment lesion groups and compared their clinical and imaging presentations. In the whole group, there was a significant association between the lengths of lesions and 1-month outcomes (a binary measure) in a logistic regression (P = 0.03). There was a trend toward a higher proportion of patients reporting focal pain adjacent to the lesion (Fisher’s exact test P = 0.05) and significantly poorer 1-month outcomes (Mann–Whitney U tests P = 0.04) in the long-segment group than in the short-segment group. The etiologies of the SCI observed in the long-segment group were very heterogeneous. Two cases had postoperative abdominal aortic aneurysms, two had coagulopathy related to bladder cancer or liver cirrhosis, four had diabetes mellitus, one had systemic lupus erythematosus, and one had pulmonary emboli. Only 12 patients with SCI underwent CSF studies, and all of these were in the nondissection group. The median cell count was one lymphocyte without red blood cells. The median values for CSF glucose levels and protein were 76 mg/dL and 49 mg/dL, respectively. There was no significant difference in CSF parameters between the two groups. Two cases received serum anti-AQP4 antibody testing, and the results were within a normal range.
Discussion
In our study, we found that compared to SCI patients without a history of dissection, those with a history of vessel dissection in either the aorta or vertebral artery had a younger age of onset and contained a significantly higher proportion of patients with long-segment lesions showing posterior-aspect involvement of the spinal cord. The frequently involved spine levels were located in the upper cervical or lower thoracic vertebral body levels. However, vessel dissection was not associated with muscle power in all limbs or 1-month outcomes. In contrast, SCI patients without a history of vessel dissection had an older age of onset and showed involvement of the lower cervical regions. Among these patients, those with long-segment lesions contain a significantly higher proportion of patients with focal pain adjacent to the lesion and had poorer 1-month outcomes.
Vessel dissection disrupts the longitudinal and axial blood supply
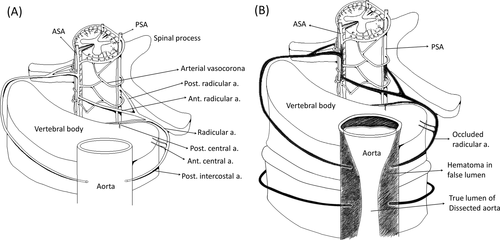
Our findings suggest that vessel dissection can directly disrupt the blood supply in both the horizontal and longitudinal axes. Figure 3A illustrates the anatomy of the cord, vertebrae, and major vessels under normal conditions.9 The radicular arteries originate from the vertebral artery or posterior intercostal arteries of the aorta and enter the spinal foramen before branching off to form the anterior and posterior radicular arteries, which anastomose to the ASA and PSAs, respectively. After parent vessel dissection, the orifices of the radicular arteries can become blocked, interrupting the blood supply to the spinal cord from the anterior to posterior aspects. In cases with longitudinal extension of the vessel dissection, both the upper and lower segments of the radicular arteries could be involved, leading to the breakdown of the longitudinal blood supply in the spinal cord (Fig. 3B).
The timing of performing an MRI may be associated with the lesion length. Early imaging may reveal a normal MRI or a short lesion before evolving to a long lesion. Our results showed a longer time between performing an MRI and the onset of SCI in the dissection group, which might be due to the fact that four patients with aortic dissection immediately received surgery, thus delaying the time of the MRI scan. After excluding these four patients, there was no significant group difference in the time between performing an MRI and onset (SCI with vessel dissection: SCI without vessel dissection = 4.5 ± 6.4: 3.0 ± 1.0 days, P = 0.43).
PSA infarction in SCI
Pure PSA infarction in SCI is relatively rare based on previous case studies.16-18 Multiple etiologies of PSA infarction have been previously reported; these include atherosclerosis,17, 19 fibrocartilaginous embolization,20 head injury,21 or mitral valve strands22. In a recent study that comprised the largest series of PSA infarctions (133 patients with SCI), 15 (11%), patients had a spontaneous PSA infarction.23 This figure suggests that the diagnosis of PSA infarction might be underrecognized in SCI.17 In the current study, acute onset large-fiber sensory impairment was found in all cases and was associated with weakness in 73% of the patients; this finding may have been related to the heterogeneous spinal arterial territories or initial coexisting ASA ischemia. Fourteen patients (93%) had vascular risk factors, such as dyslipidemia, hypertension, smoking, and diabetes mellitus. Approximately, 60% of the patients had long segments of vertebral body spans in the cervical or thoracic regions. The prognoses of these patient were good, and 93% of them were ambulatory. In our study, the only pure PSA infarction case (3.2%) in SCI was a 37-year-old male with left-side vertebral artery dissection demonstrated by angiography. The lesion extended from the occipital to the C2 vertebral body level. The 1-month outcome of this patient was good (grade 3 mRS score). Other patients with PSA involvement were associated with ASA infarction.
Possible etiologies are related to longitudinal extension of SCI
Numerous etiologies have been implicated in SCI; these include aortic disease,24 vertebral dissection,25 arterial or cardiac embolism,26 fibrocartilaginous embolism,20, 27 hypercoagulopathy,28 decompression sickness, vasculitis,29 systemic hypotension or global hypoperfusion from cardiac arrest,30 and radicular artery compression from a disc.31 Local or global hypoperfusion in the border zone area between the intrinsic and extrinsic spinal artery systems is often manifested in the anterior horns because of the gray matter and because motoneurons are notably more vulnerable to anoxia. Thus, the typical MRI features of SCI show bilateral hyperintense lesions on the axial T2-weighted sequence in the region of the anterior horns, illustrating the owl’s eyes configuration.10 Findings from previous studies indicated that the average length of SCI was approximately three vertebral body spans and that long cord lesions were associated with poorer outcomes.11, 25, 32 Approximately, 50–60% of SCI cases had lesions longer than three vertebral body spans.5, 11 In our study, we also found a similar proportion of long-segment lesions in patients with SCI without vessel dissection (58%). Worse 1-month outcomes were associated with long-segment lesions in patients without vessel dissection. Although vessel dissection may explain the longitudinal extension of SCI, other factors may also be present in SCI. We explored factors potentially associated with the longitudinal extension of SCI in our group. Thromboembolism (atheromatous plaque in the aorta or pulmonary emboli), coagulopathy (malignancy, liver cirrhosis, and hemodialysis), and diabetes mellitus contributed to 61% of our SCI patients in the no-dissection group with long-segment lesions. Interestingly, all of these patients were ASA infarction patients. In the short-segment group, the most common etiology of SCI was hypertension.
Vessel dissection can be recognized by modern imaging tools
Dissection has often been considered a single entity in previous reports, and the diagnosis is typically made by various imaging findings, including the absence of vertebral flow; stenosis of the vertebral artery; or the presence of a dissection flap, an intraluminal thrombus, a pseudoaneurysm, or an intramural hematoma.33 Using conventional MRI and MR angiography to study vertebral artery dissection, it was shown that the sensitivity ranged from 95% to 100%, and the specificity ranged from 67% to 99%. The CT angiography also showed a sensitivity ranging from 64% to 100%, and the specificity ranged from 82% to 95%.34 Both CT angiography and MR angiography can effectively visualize vertebral artery dissection; however, CT angiography can be performed rapidly, and it allows simple reconstruction of the dissection artery in an acute setting.35 Recently, it was shown that using the heavily T1-weighted sequence on MRI could increase the reliability of detection of intramural hematoma compared with using the T1-fat saturation method, which could improve the accuracy of vertebral artery dissection diagnosis.36 In aortic dissection, multi-detector row CT is the modality of choice for imaging acute aortic syndrome with a sensitivity and specificity approaching 100%.37
Limitations
Several limitations of the current study should be addressed. First, in this retrospective study, we collected patient data over a 16-year period. As such, it must be acknowledged that MRI protocols and quality can evolve over time. Some MRI sequences, such as diffusion-weighted imaging of the spine, are technically challenging to obtain and have only recently become available in our clinical setting. In the current study, we used only T1- and T2-weighted images reviewed by a single neuroradiologist to minimize discrepancies in the imaging data. Second, there are several principle vessels (e.g., one ASA and a pair of PSAs) involved in spinal cord vascularity. However, their connections with radicular arteries are quite capricious, and it remains difficult to visualize the detailed blood supply with noninvasive MRI methods.38, 39 Thus, we classified spinal artery involvement based on the territory covered by a typical vessel supply, which may have led to the misclassification of vessel involvement. An invasive superselective spinal angiographic study may identify spinal vessel involvement in equivocal cases. We performed a conventional angiographic study in half of our cases, but no superselective spinal angiography was performed in our study, although this procedure can be used to clearly visualize individual radicular artery occlusion. Third, SCI is a rare clinical condition, and the sample size in our single-center study was therefore small. This small sample size likely contributed to the failure to identify any meaningful relationships between MRI parameters and clinical features. A larger, multicenter study is warranted to validate our findings.
Conclusion
In our study of SCI, we found that patients in the aortic or vertebral artery dissection groups were associated with long-segment lesions and combinations of ASA and PSA infarctions. The disruption of the connection between the radicular arteries and their parent arteries may represent an underlying mechanism for these associations. In patients with SCI without dissection, thromboembolism (atheromatous plaques in the aorta or pulmonary emboli), coagulopathy (malignancy, liver cirrhosis, and hemodialysis), and diabetes mellitus were associated with long-segment lesions. The lengths of the lesions in spans of vertebral bodies were also associated with 1-month outcomes. These findings should advance the ability of clinicians to understand the probable underlying factors related to the longitudinal and posterior extensions of lesions in SCI.
Acknowledgments
This study was supported by the Chang Gung Memorial Hospital, Linkou, Taiwan (Grant number: 201800769B0). The funder had no role in the study design, data collection, and analysis, the decision to publish or the preparation of the manuscript.
Author Contributions
Study concept and design: JLH, MYC, MFL, and LSR. Collection and interpretation of data: all authors. Drafting or revision of the manuscript: LSR and JLH. Approval of the final version of the manuscript: all authors.
Conflict of Interest
The authors declare no conflicts of interest.




