Citrus
Abstract
Citrus is the most important fruit tree crop in the world, with a production of more than 100 million tons annually. The area of origin of Citrus is believed to be southeastern Asia, where its domestication started. It has become clear that only citron, mandarin, and pummelo are true species within genus Citrus, being other important Citrus types, as sweet orange, sour orange, lemon, lime, grapefruit and other mandarins originated from hybridization between these ancestral species. In spite of the many efforts put in classical breeding programs in the last 100 years, current citrus industry relies on various groups of varieties that are grafted onto rootstocks adapted to different abiotic and biotic stresses. Most of these genotypes have been generated by chance, mostly as budsports but also as natural hybrids or seedlings selected by men in the wild or in orchards. Citrus breeding is complicated due to its complex reproductive biology. In this context, genetic transformation offers an important alternative for the genetic improvement of citrus. Moreover, it is probably the most efficient approach to make reverse genetics in citrus to investigate gene function and thus to gain better understanding in metabolic processes and plant-pathogen-environment interactions.
1 Introduction
1.1 History, Origin, and Distribution
The area of origin of Citrus is believed to be Southeastern Asia, including South China, the Indo-Chinese peninsula, Northeastern India, and Burma. This is a wide area, but attempts to localize more precisely the centers of origin of the most important Citrus types are still now controversial. It has become clear in recent times that only citron (Citrus medica), mandarin (C. reticulata), and pummelo (C. grandis) are true species within genus Citrus, being other important Citrus types, as sweet orange, sour orange, lemon, lime, grapefruit, and other mandarins originated from hybridization between these ancestral species. This view was convincingly supported by the phylogenetic study of Barrett and Rhodes 1976 who evaluated 146 morphological and biochemical tree, leaf, flower, and fruit characteristics, and it was later confirmed also using molecular markers (reviewed by Nicolosi, 2007). Therefore, Southeastern Asia would not only be the site of origin of most important Citrus types but also its major center of diversity. Domestication could have started in this area and expanded progressively in all directions. The genus Citrus is by far the most important among the Rutaceae family, but there are two other genera that have played a relevant role in citriculture, which are Fortunella, producing edible fruits and commonly called kumquat, and Poncirus, sometimes vulgarized as trifoliate orange. All authors coincide in ascribing their origin to central China, since both genera are most cold-hardy than Citrus and are reported as growing wild in the Yellow river area in ancient Chinese literature.
The first historical documents mentioning the use of citrus come from China and India. Confucious describes prehistoric China in its classic “Book of History”, written ca. 500 BC, and in the “Tribute to Yu” states that the Chu and Yu were sent as annual tributes to the emperor Ta Yu (2205–2197 BC) from An Yang (north of the Yellow river) by the people of central and southern provinces, Chu and Yu being referred to Citrus types, most likely to mandarin and mandarin/pummelo natural hybrids, respectively. The Chu is mentioned in several Chinese classic books and also in the Bretschneider's “Notes on Chinese Botany from Native and Western Sources” as cultivated along Yellow river central regions since 12th century BC. During the 2nd century BC, the Han emperor Wu Ti conquered and annexed the barbarian provinces of the south, and soon after, new Citrus types appeared in Chinese references. Kan possibly referred to sweet orange early types, Yu or Yau was then a pummelo type, and Chang was first used to describe sour orange types and later as a generic term for oranges, mainly sweet oranges. The first written reference to citrus fruits appeared in India in the “Vajasaneyi Samhita”, a collection of sacred Brahma texts written in Sanskrit prior to 800 BC, where ancient lemon and citron types are called “jambila”.
The citron was the first Citrus type noticed by the Europeans and the only one known for centuries. Theophrastus describes the tree in his “Historia Plantarum” (around 300 BC) and its fruit is called “Persian citron” or “Median apple”, assuming that it was indigenous from that region. Although there are conflicting opinions on how citron arrived to Europe, most authorities agree in supporting that Alexander the Great brought it to Greece when returning from India (about 300 BC). According to a very famous Greek myth, one of the labors of Heracles was the theft of the golden apples of Hesperides, in which golden apples would be translated as citrons. Citrus were called Hesperides by Roman writers. Linnaeus gave the name Hesperideae to an order containing the genus Citrus. Nowadays, in botany the term hesperidium names a berry whose fleshy parts are divided into segments surrounded by a rind or hard shell, also in allusion to the golden apples of the Hesperides garden. Hebrews were also attracted by this “Persian tree” and it was adopted for worship during the feast of the Tabernacles, playing an important role in Jewish religious rituals. Latin writers as Vergil and Pliny later cited the citron and it was probably introduced to southern regions of Italy during the 1st century AD. Greeks and Romans held the citron in high esteem because of its delicate and penetrating fragrance. It was used both as a perfumant and moth repellent. It was not extended further in the continent until many years later (15th century) likely due to its cold sensitivity.
Although old mosaics indicate that orange and lemon were known by Romans, spread of sour orange and lemon in Europe through India, western Asia, and North Africa is due to the expansion of the Arab empire. The Crusaders further extended these and other Citrus types as limes and pummelos into Europe. It is not clear for historians when sweet orange first appeared in this continent, but it became widely spread only after the Portuguese established the commercial route with India and China in early 16th century. Before then, orange types were bitter and used mainly as condiments. Therefore, Citrus might be introduced into Europe many times at various periods of history by successive invaders and traders, each being new introduction of increasing quality in terms of edibility and fragrance. There were attempts to establish Citrus trees in northern areas of Europe since the 1st century AD, but frost injury caused limiting problems. Because of this, Citrus types were cultured in special protected houses, first known as “stanzone per i cidri” and later as orangeries (14th century), which were the predecessors of greenhouses. Mandarin types, which were widely known and cultivated in Southeast Asia from ancient times, were not introduced to Europe from China until the 19th century.
There are many written references of Citrus cultivation in Japan since the 1st century AD, citrus being mostly referred to mandarin types. Poncirus was brought from China around the 8th century, but pummelo and sweet orange were introduced in Japan by Spanish and Portuguese travelers just in the 15th century. The origin of most popular satsuma mandarin from Japan is uncertain but it was not until the end of 19th century that it became expanded nationwide and commercialized (Mizutani, 2006).
Columbus took seeds of oranges, lemons, and citron to America on his second voyage, which arrived at Hispaniola (Dominican Republic and Haiti) in 1483. It was soon brought to other islands and continental America where citrus trees were fully adapted, spread widely, and become very abundant and even feral in some places of tropical/subtropical climate. Citrus were brought to Florida by the early Spanish explorers sometime between 1513 and 1565. About the same time, Citrus fruits were introduced into Brazil by the Portuguese. Portuguese travellers also introduced Citrus in West Africa while sweet oranges were the first Citrus introduced in South Africa by the Dutch colony in 1654. Citrus was first planted in Australia by the colonists of the First Fleet who brought oranges, limes, and lemons from Brazil. Oranges and lemons were first planted in California around 1769, after the settlement of Franciscan missions at San Diego area.
Due to their apomictic character, most Citrus varieties were propagated as seedlings during many centuries. In the case of monoembryonic genotypes, propagation by seeds led to generation of a lot of genetic variation and horticultural diversity, as it is exemplified by the high number of different mandarin types that have been grown in China and Japan during many years. Although there are ancient Chinese references reporting the graft of mandarins onto Poncirus trifoliata, grafting only became a common practice in citriculture from the mid-19th century, after sweet orange seedlings grown in Europe were seriously affected by Phytopththora epidemics. Nowadays, the citrus industry relies on trees composed of two different genotypes: a mature fruit-producing Citrus scion grafted onto a highly apomictic juvenile rootstock.
Most of the information compiled in this section comes from Webber 1967 and Cooper 1982 where more detailed information on the origin and history of Citrus can be found.
1.2 Botanical Description
1.2.1 Taxonomy
The genus Citrus is one of the 33 genera in the subfamily Aurantoideae of the family Rutaceae. Within this subfamily, most taxonomists recognize that “true citrus fruit trees” belong to the tribe Citreae, subtribe Citrinae, with three genera of economic importance: Poncirus, Fortunella, and Citrus. The taxonomy of the genus Citrus is controversial. The system most commonly used comes from the classification of Swingle with modifications provided by the much more complex Tanaka's classification. While Swingle recognizes 10 and 6 species, respectively, in the two subgenera Citrus and Papeda (Swingle and Reece, 1967), Tanaka identifies up to 157 species in different groups and subgroups (Tanaka, 1954).
From the 10 Citrus species designated by Swingle, 8 are of commercial importance: C. sinensis (L.) Osb. (sweet oranges), C. reticulata Blanco (mandarins), C. paradisi Macf. (grapefruits), C. grandis (L.) Osb. (pummelos), C. limon (L.) Burm. f. (lemons), C. aurantifolia (Christm.) Swing. (limes), C. aurantium L. (sour oranges), and C. medica L. (citrons).
Tanaka's system is better adapted to horticultural traits paying also special consideration to cultivated species. This concerns to Citrus genotypes that are widely cultivated and of high economic importance, such as clementine mandarins (C. clementina Hort. ex Tan.), satsuma mandarins (C. unshiu (Mak.) Marc.), or Rangpur lime (C. limonia (L.) Osb.) among others, for which most citrus researchers use the Tanaka's classification.
Based on biochemical and molecular marker data, there are only three true Citrus species: citron, mandarin, and pummelo. Since the three ancestral species reproduce only sexually and are original from the same geographical area, several generations of hybridization among these species would generate the highest levels of genetic diversity within the genus Citrus and sexually compatible relatives. The appearance of facultative apomixis together with the selection of specific genotypes propagated as seedlings by men gave rise to most of the Citrus types currently cultivated. Obviously, this has led to a narrow genetic base within Citrus, in spite of the wide diversity of horticultural traits that can be found in the cultivated varieties.
Isozymes, organelle genomes, microsatellites, restriction fragment length polymorphism (RFLP), random amplified polymorphic DNA (RAPD), and sequence-characterized amplified region (SCAR) analyses have provided important clues on the genetic relationships among Citrus types. Concerning economically important Citrus “species”, sweet oranges are thought to be hybrids between mandarin and pummelo, sour oranges would come from a mandarin genotype introgressed with genes from pummelo, which is the maternal donor, lemon would originate from citron and sour orange (female parent), and lime would also have citron as one of the parents and a Papeda (Citrus micrantha Webster) species is proposed to be the other parent. Grapefruit is a very recent species, first described in Barbados in 1750, and originated from a natural hybridization between pummelo and sweet orange probably followed by introgression with pummelo (reviewed in Nicolosi, 2007).
1.2.2 Citrus biology: some clues on growth and development
All Citrus types are evergreen and do not show winter dormancy but just a bud-resting period. However, the Poncirus relative is deciduous, showing winter leaf abscission and bud dormancy. Citrus species show a sympodial pattern of growth, based on flushes of growth in which the shoot apical meristem senesces after the production of several leaves and axillary buds. The closest bud to the senescent shoot apical meristem drives the following growth flush. Whereas in subtropical conditions, three to five flushes occur per year, in tropical areas shoot growth occurs almost uninterruptedly. These growth pattern and number of flushes per year is maintained in plants grown under temperature-controlled greenhouses. However, it can be forced up by increasing day length artificially. Moreover, the elongation of the shoot can be manipulated by controlling light intensity.
Citrus shoots develop several axillary buds in the axil of each leaf, together with an axillary thorn. Shoot length is related with the genotype and the vigor/age of the shoot. Presence of thorns is commonly considered as a juvenile character, but there are genotypes showing thorns being fully mature, as for example some lemon varieties. There are also juvenile genotypes without thorns, such as Cleopatra mandarin. Leaves are unifoliate and in most species the petioles are winged. Poncirus shows trifoliate leaves, reminiscent of other Aurantoideae genera with composite leaves. Elongated leaf shape and larger petiole wings are considered juvenile characters.
Flower bud differentiation is induced photoperiodically in subtropical areas when the day becomes shorter during winter months. Cold temperatures are also important in floral induction. In the deciduous Poncirus, flower bud induction is initiated during late summer. In tropical areas without photoperiod changes, water stress is the major flower-inducing signal. In Citrus, blooming usually occurs in spring, following flower development. As evergreen, reproductive and vegetative developments are intimately related, and four main shoot types can be distinguished: vegetative shoots, leafy inflorescences, leafless inflorescences, and solitary flowers. Poncirus and Fortunella also flower in spring but usually sooner and later than Citrus, respectively.
The citrus fruit is a hesperidium, namely a berry arising from growth and development of the ovary, consisting of fleshy parts divided by segments, the whole being surrounded by a separable skin. It is composed of two major regions: the pericarp, commonly known as the peel, and the endocarp, often called the pulp. The pericarp is composed of external colored peel known as flavedo, and the internal usually white layer known as albedo. Citrus fruits are nonclimacteric, ethylene changes being extremely low during fruit development. During maturation the dark green, photosynthetically active flavedo transforms its chloroplasts into carotenoid-rich chromoplasts. On the other hand, maturation of the pulp is characterized by a decline in acidity and an increase in sugars, the ratio of both components being used to define the “maturity index”. A good summary on citrus biology can be found in Spiegel-Roy and Goldschmidt 1996.
1.3 Economic Importance
Citrus is the most important fruit tree crop in the world, with a production of more than 105 million tons in 2005 (FAO, 2006). It is grown in more than 130 countries all over the world, mainly in tropical and subtropical areas (approximately 40° latitude in each side of the equator) where favorable soil and climatic conditions occur, extending over 7.6 million hectares. Major producing countries include Brazil, the United States, China, Spain, Mexico, India, Iran, Italy, Egypt, Argentina, Turkey, Japan, Pakistan, South Africa, Greece, Thailand, Morocco, Israel, Indonesia, Korea, and Australia, from major to minor. The first five countries account for about 55% of the world production.
Sweet orange represents more than 55% of total citrus production, being marketed as fresh fruit or as processed juice. Traditionally, oranges were consumed as fresh fruits but in the last 30 years consumption of processed oranges (mainly as concentrated fruit juice) has increased extraordinarily all over the world, and especially in Europe and the United States. It represents the primary force supporting expanded world consumption and is the basis of Brazilian and Florida citrus industries. Brazil and the United States produce almost 45% of sweet orange in the world and about 60% is processed.
Mandarins, including clementines, tangerines and satsumas, represent about 20% of total citrus fruit production. The first producer is China, followed by Spain and Japan. Most mandarins are intended for the fresh market and are generally consumed in the country of production, with the important exception of Spain that is the first producer of clementines and exports more than half of its production. Japanese mandarins are nearly all satsumas. It is difficult to track the production of processed mandarin since most juice is blended with orange juice. There is an increasingly important industry of production of canned fruit segments (mainly from satsuma) developed in Japan and Spain, and more recently in China that has become the first producer.
Lemons and limes differ from other citrus types in that they are typically consumed processed or mixed with other foods. Whereas lemons are widely adapted to different climates, limes are highly sensitive to cold, being grown exclusively in tropical climates. Mexico is the first producer and exporter of limes with a production of almost 2 million tons in 2005, followed by Brazil. India is a major producer of both lemons and limes. Argentina is the first world producer and exporter of lemons. The United States, Spain, and Italy are also major producers of lemon. There is an important industry of processed lemon and lime fruits with the juice and essential oils used as flavorings in beverages and foods.
Production of other citrus types is much smaller compared to the four major groups, no more than few hundred tons in the case of pummelos, citrons, and kumquats, mostly are commercialized in local Asian markets. Processed Citrus fruits have many uses apart from those mentioned above. Sour orange fruits are used to produce marmalade and flowers are used to extract neroli oil, which is highly appreciated in perfumery. Peel essential oils, mainly monoterpenes, have a wide industrial use as food additives to provide a citrus flavor, as a fragrance in perfumes, air fresheners and personal care products, and as a natural replacement for petroleum-based solvents in paints and cleaning products. By-products of concentrated citrus fruit juice are used as molasses for animal feed. There is an increasing utilization of citrus trees as ornamental plants in the United States and Europe.
Citrus fruits have been shown to possess many constituents, which have important effects on the human health: vitamin C, carotenoids (as provitamin A), folic acid, flavonoids, monoterpenes/essential oils, limonoids, and others.
1.4 Citrus Scions and Rootstocks: Needs for Genetic Improvement
1.4.1 Origin of currently cultivated citrus scions and rootstocks
Citrus are diploid species having a haploid chromosome number of 9 and an approximate genome size of 0.9pg (picogram) (∼385Mb). Most Citrus rootstocks and varieties grown commercially nowadays have been originated by budsport mutations and chance seedlings and have been selected directly by growers due to their excellent fruit quality, performance, and stress resistance.
In case of sweet oranges, there are two major types: blond oranges, mainly used for juice production, as the most representative Valencia orange, and navel oranges, mainly used for fresh consumption. Valencia orange was probably originated in Azores or Portugal as a mutant from the superior class of oranges brought by the Portuguese from China in the early 16th century. Many nucellar clones and likely budsport mutations of outstanding interest have been generated from the original Valencia, which are currently the basis of the Brazilian and Florida citriculture. The origin of navel orange is uncertain but it has been claimed that it was generated as a limb sport from the blond variety Selecta at Salvador de Bahia (Brazil). Worldwide expansion of this type started after it was brought to the United States and renamed as Washington navel at the end of 19th century. Many early and late-ripening bud mutants have been found covering all seasons with one of the most excellent fresh fruit types achievable in markets. A third group of minor importance is that of blood oranges, characterized by the accumulation of anthocianins in the flesh and juice, red pigment synthesis being usually dependent on low night temperatures. They probably originated in the Mediterranean area as mutants from blond oranges. Nowadays, blood oranges are only important in Italy, Tarocco being their most popular selection.
Natural and man-made mandarin hybrids have been cultivated in China and Southeast Asia during many centuries. Two of the most commercially successful mandarin types in current times are satsumas in China and Japan, and clementines in Spain and Morocco. The original satsuma was most probably generated as a chance seedling in Kyushu Island (Japan) around 15–16th century. Many early and late maturing, small and larger fruited types are commercially relevant and all of them have been generated as limb sports, bud sports, or nucellar seedlings from pre-existing scions. Clementine first appeared in the garden of an orphanate near Oran (Algeria) and it was discovered by Father Clement Rodier as a seedling from a cross between a Mediterranean mandarin and an ornamental sour orange known as Granito, according to historical records. However, molecular markers data support that it was originated from a mandarin × sweet orange cross (reviewed by Nicolosi, 2007). It is not strange that the first describers were confused about the actual pollen donor. Clementine was introduced into Spain from Algeria in 1925, and since then many excellent bud mutants of different fruit size, shape, color and maturing season have been found and propagated, constituting the main basis of the Spanish citrus industry.
Another natural hybrid mandarin is the Ponkan, widely cultivated in Asian citrus countries and in Brazil. Some other mandarin cultivars of relative importance that were originated as chance seedlings are Dancy, from Florida, and Ellendale and Imperial, found in Australia, all in the 19th century. “Murcott” is most probably a tangor (mandarin × sweet orange) of unknown parentage cultivated in Florida, Brazil, Argentina, and Japan. Another important tangor of natural hybrid origin is “Ortanique”, discovered in Jamaica around 1920. A more recent natural hybrid is “Afourer” or “Nadorcott”, found recently in Morocco (most probably a “Murcott” × clementine hybrid), which is considered the most significant new mandarin variety currently available.
All important common varieties of lemon and lime are natural hybrids, chance seedlings or budsport mutations selected by men either in ancient time in Southeast Asia or since the 19th century in other major citriculture areas.
Regarding rootstocks, Rangpur lime is the predominant one in Brazil, due to its combined tolerance to several important biotic and abiotic stresses. It is an ancient Asian natural hybrid. P. trifoliata, a true species, is widely used in several parts of the world, mainly in Asia, especially due to its cold-hardiness and semi-dwarfing abilities. Importantly, both are resistant or highly tolerant to the major pathogens Phytophthora spp. and Citrus tristeza virus (CTV). Other relevant citrus rootstocks, as sour orange, Cleopatra and Sunki mandarins, rough and Volkamer lemons, and alemow or Citrus macrophylla are ancient natural hybrids of Southeast Asian origin.
In spite that present-day citriculture is based on cultivars that grower directly selected from the wild or from the orchards, they usually have outstanding quality. This makes very difficult the obtention of new improved cultivars, especially in the case of scion varieties for the fresh fruit market. Citrus rootstocks and varieties of the world are extensively detailed by Saunt 2000.
1.4.2 Needs for genetic improvement
Many different citrus genotypes are commercially grown in a wide diversity of soil and climatic conditions, implicating that trees are subjected to important abiotic and biotic stresses that limit the production and, in some instances, the use of certain rootstocks and varieties. The main abiotic stresses are acid, alkaline, and salty soils, flooding and drought, freezing, and high temperatures.
Citrus trees are also affected by many pests, diseases caused by nematodes, fungi, oomycetes, bacteria, spiroplasmas, phytoplasmas, viruses and viroids, and diseases of unknown etiology. Some diseases are spread throughout the world, as those produced by the oomycete Phytophthora spp., or by the CTV, which preclude the use of certain excellent rootstocks, and severely restrict fruit production and quality of important varieties in some countries. Other diseases are restricted to specific geographic areas, as those caused by the bacteria Xylella fastidiosa or by citrus sudden death associated virus in Sao Paulo state (Brazil). There are also diseases spread in most citrus areas, as citrus canker, caused by the bacteria Xanthomonas axonopodis pv. citri. Whereas Brazil has been able to implement a quite successful eradication program, the bacterium is currently expanding without control in Florida. Finally, there are diseases that were locally important but in more recent times have become widely spread and are seriously threatening important citricultures, as it is the case of the Huanglongbing caused by the bacterium Candidatus Liberobacter asiaticum, which affects all citrus varieties. It has impeded the development of citriculture in certain Southeast Asian countries and at present day is devastating millions of trees in Florida and Brazil. In the cases of these three bacteria there are no means for efficient control. At the same time that citrus industry is threatened by important biotic and abiotic stresses, markets demand fresh fruit and juice of increasing quality. In this situation, genetic improvement of citrus has a very high priority.
Major current goals of rootstock breeding are resistance to CTV and Phytophthora spp., cold-hardiness in citrus areas as Japan, Florida, or New Zealand, scion size-controlling abilities, higher tolerance to calcareous, and saline soils in areas with poor-quality water, and resistance to the citrus and the burrowing nematodes, particularly in Florida. Scion breeding is mainly focused in resistance against major pests and diseases, and in fruit quality aspects. For the fresh fruit market, major goals include adequate size for each citrus type, easy peeling, seedlessness, attractive color and aroma, compensated acid/sugar content in the fruit, extension of the maturity season for all year round supply, and good storage and shipment. When the fruit is going to be used for juice production, prime goals are juice content of the fruit, good color, and lack of bitterness.
1.5 Limitations of Conventional Breeding, Achievements, and Rationale for Transgenic Breeding
1.5.1 Biological limitations of citrus breeding
Conventional breeding by hybridization has important limitations. Citrus species have a complex reproductive biology. Most genotypes are facultative apomictic, which means that adventitious embryos initiate directly from maternal nucellar cells, limitating or precluding the development of less vigorous zygotic embryos. Although this is the basis for propagation of citrus rootstocks, apomixis seriously limits the recovery of sexual progeny populations in breeding programs. Some important genotypes have total or partial pollen and/or ovule sterility and cannot be used as parents in breeding programs; for example, most navel oranges are male sterile while satsuma mandarins and most navel and Valencia oranges are female sterile. There are many cases of cross- and self-incompatibility. Clementines, grapefruits, and certain important lemons are self-incompatible, and many hybrids between self-incompatible cultivars are also cross-incompatible. They have a long juvenile period and most species need at least 5 years to start flowering in subtropical areas, and usually several years more to achieve fully mature characteristics. Citrus have high heterozigosity, there is a lack of basic knowledge about how the most important horticultural traits are inherited, and they show quantitative inheritance of important characters, many of them related to fruit quality and maturity time. All these features together with their large plant size have greatly impeded genetic improvement of citrus through conventional breeding methods. Moreover, sources of efficient resistance against important pathogens as Candidatus L. asiaticum and X. fastidiosa have not been found in the citrus germplasm.
1.5.2 Breeding goals and new tools for citrus improvement
In principle, breeding objectives in citrus are different depending on whether improved rootstocks or scions would like to be generated. Citrus trees, mainly sweet orange, were mostly produced from seedlings until mid-19th century, when Phytophthora spp. was recognized as a major disease of trees first in Azores (1840) and later in France and Spain. There are records indicating that sweet orange trees were grafted onto different rootstocks as citron, lemon, sour orange and other sweet orange genotypes in certain citrus areas of Spain. After spreading of Phytophthora spp. in Spain, only scions grafted onto sour orange remained alive (Wolffenstein, 1880). Since then, all citrus trees were budded onto this rootstock, which is not only resistant to the oomycete pathogen but also provides excellent agronomic attributes, particularly fruit yield, quality, rusticity, and tolerance to calcareous and saline soils. Thus bud grafting of scion varieties onto sour orange became a universal practice. Between 1910 and 1930, trees grafted onto sour orange started to decline with a disease later identified as caused by CTV. Since then, different citrus genotypes and relatives have been used as rootstocks depending on the requirements of each citriculture area, but very few of them were originated in breeding programs. The first recorded artificial hybridization of citrus was carried out by Swingle and Webber in Florida in 1893 with the aim of incorporating resistance to diseases, but a severe freeze destroyed most of the seedlings. Then, they decided to use the cold-hardy P. trifoliata as a parent in crosses to try to incorporate higher cold tolerance to citrus scions. None of the progeny trees combined hardiness with good fruit quality. However, the excellent Carrizo and Troyer citrange hybrids (sweet orange × P. trifoliata) rootstocks resulted undeliberately from these crosses. Both hybrids are highly tolerant to CTV and Phytophthora spp., and are widely used as rootstocks in countries as Spain and the United States. Another rootstock hybrid obtained in the same program, though released in 1974, was the Swingle citrumelo, coming from a grapefruit × P. trifoliata cross performed in 1907. It is widely used in Florida and South Africa. All other important rootstocks used now-a-days are true species or ancient natural hybrids.
Very active variety hybridization programs were performed along 20th century in most citrus-growing countries, but none of the important scion varieties cultivated at present day came from such programs though some hybrid scions of relative importance were generated. In Florida, grapefruit × mandarin hybrids gave rise to the “Orlando” and “Minneola” tangelos. Clementine × these tangelos yielded a number of hybrids of some importance as “Nova”. In California, Frost performed interesting crosses among different mandarin types between 1914 and 1916, and obtained the “Kinnow” mandarin hybrid (King mandarin × Mediterranean mandarin), widely cultivated in Pakistan and India. “Fortune”, a hybrid of clementine × Dancy mandarin made by Furr in California in 1964, has been the most important late mandarin hybrid in Spain in the last two decades of the 20th century. “Kiyomi” is a tangor (satsuma × sweet orange) obtained in Japan and released in 1979. Ponkan × “Kiyomi” recently gave rise to “Shiranui” that is probably the best success of hybridization breeding programs in Japan.
Artificial induction of genetic changes was initiated in 1935 in the United States by treating seeds with x-rays. Then the most important results of irradiation programs came from Henz, who obtained several thousand plants from irradiated seeds and budwood from grapefruit and Valencia orange in Texas, resulting in two of the most widely grown pigmented grapefruit varieties of present times. “Star Ruby” was produced by irradiating seeds from the Hudson variety in 1959, and “Rio Red” was selected as a bud mutation of a tree obtained after irradiation of budwood from a “Ruby Red” seedling (see also Grapefruit). More recently, irradiation programs have been carried out for the generation of seedless mandarin hybrids. Examples of recent highly promising seedless releases are “Orri”, generated from the Israeli hybrid “Orah” (“Temple” × “Dancy”), and “Tango”, originated from irradiated “Afourer”.
Although diploidy is the prevalent state in citrus, tetraploids spontaneously arise due to chromosomal duplication in nucellar cells, which are able to generate embryos and plants, their frequency being highly dependent on genotype and environmental conditions. Tetraploidy has been also induced by treating nucellar callus, and caulinar apices (for monoembryonic cultivars) with colchicine. Some authors have proposed the use of tetraploid citrus as semi-dwarfing rootstocks. Nevertheless, tetraploid genotypes have been mostly used to generate triploids in crosses with diploid parents. Most triploid citrus trees are sterile, producing seedless fruit. A natural triploid variety is “Tahiti” lime (Citrus latifolia Tan.), increasingly important for Mexican citrus industry. Seedlessness is very difficult to obtain by conventional hybridization. Production of triploid hybrids is currently the most promising approach to obtain seedless cultivars that do not produce seeds. Recovery of citrus sexual triploid hybrids (3n = 27) has been reported since the early 1960s after 2n × 4n, 4n × 2n, and 2n × 2n crosses (Ollitrault et al., 1998). In the last case, the triploid embryos are originated by the fertilization of an unreduced diploid female gamete with a normal reduced haploid male gamete. Seeds with triploid embryos are generally underdeveloped or aborted due to uneven embryo/endosperm chromosome balance, and it is very difficult to regenerate plants regularly. In addition, analysis of ploidy level of large populations of citrus plants by cytological methods is very difficult. The development of methodologies for in vitro culture of embryos and small seeds and for ploidy analysis by flow cytometry is allowing a much more efficient production of citrus triploid sexual hybrids (Navarro et al., 2003). Several promising triploid mandarin hybrids have been recently released in the United States and Italy.
Another promising technology to generate tetraploid breeding parents is somatic hybridization. It allows producing somatic hybrids that incorporate genomes of the two parents without recombination, thus avoiding the problem of the high heterozygosity in citrus. Somatic hybrids are generally produced from the fusion of protoplasts isolated from embryogenic callus or suspension cultures of one parent with leaf-derived protoplasts of the second parent. Protoplast fusion is induced either by polyethylene glycol (PEG), electrically, or by a combination of both methods. After fusion, the embryogenic parent provides to the hybrid most of the capacity of regeneration through callus formation and somatic embryogenesis. In citrus this technology has been extensively used and has many important applications, including the generation of new tetraploid hybrids, utilizable as parents for triploid breeding or directly as new rootstocks, the production of triploids from haploid + diploid somatic hybridization, and the generation of cybrids, namely new diploid hybrids with the nuclear genome from one parent and either the cytoplasmic genome from the other parent or a combination of both parents (Grosser et al., 2000). There is a large somatic hybridization program in Florida, with many hybrids being tested in the field as potential rootstocks and several flowering ones are being used as pollen donors in a breeding program aimed to produce triploid mandarin hybrids (Grosser and Gmitter, 2005).
Grosser et al. 2002 are investigating the phenomenon of somaclonal variation as an alternative method for improving sweet orange. Somaclonal variation is defined as genetic variation that is either induced or uncovered by plant tissue culture in vitro. This generally slight variation could modify plant horticultural performance and give rise to new improved germplasm. They are currently field evaluating several Valencia sweet orange selections obtained through somatic embryogenesis from nucellar callus or from protoplasts, which possess the superior fruit color and quality of Valencia orange, but mature earlier or later than the standard variety.
The development of genetic markers is also providing a potential tool for citrus breeding. Linkage maps have been constructed using isozymes, RFLP, RAPD, SCARs, amplified fragment length polymorphism (AFLP), microsatellites (single sequence repeat; SSR), and cleaved amplified polymorphic sequences (CAPs). These studies have served to determine the mode of inheritance of some traits and they could be useful for early selection of the progeny and genotype identification in breeding programs. More recently, other markers as resistance gene candidates (RGCs) and single nucleotide polymorphisms (SNPs) have been developed and are currently being used in citrus breeding.
1.5.3 Rationale for transgenic breeding
In spite of the many efforts put in classical breeding programs in the last 100 years, current citrus industry relies on various groups of varieties of outstanding quality that are grafted onto a narrow diversity of rootstocks adapted to different abiotic and biotic stresses. Most of these excellent genotypes have been generated by chance, mostly as budsports but also as natural hybrids or seedlings selected by men in the wild or in orchards. In addition citrus breeding is complicated due to its complex reproductive biology. In this context, genetic transformation offers an excellent alternative for genetic improvement of citrus because it is based on the introduction of specific traits into known genotypes without altering their elite genetic background. Theoretically, it would be possible to incorporate the CTV-resistance trait into the otherwise primary sour orange rootstock, or seedlessness independent of cross-pollination into clementine mandarins. The transgene of interest could come from another Citrus species or relative, from another plant species, or from another organism such as a bacterium, an insect or a virus, widening the possibilities for genetic improvement. Moreover, it allows overcoming the heterozygosity, inbreeding depression, and genetic incompatibility barriers associated with hybridization. Facultative apomixis is in principle an advantage because it could be possible to use vigorous juvenile material genetically identical to the elite mature germplasm as a source of plant tissue for transformation. However, this cannot be applied to important monoembryonic citrus types as Clementine mandarins. In addition, female sterility is extended in citrus cultivars making it difficult to obtain seeds (e.g., in navel sweet oranges). More important, the juvenile period of citrus is extremely long compared to other fruit trees, taking about 5 years for first flowering and fruit setting, and at least 3 years more to loose completely juvenile growth and developmental characteristics. For the purpose of genetic improvement, transformation of mature tissue then becomes necessary. Sweet orange was the first fruit tree from which adult material was transformed (Cervera et al., 1998a) providing the only biotechnology-based system able to overcome the juvenility constraint of citrus breeding.
2 Development of Transgenic Citrus
2.1 Plant Regeneration: Organogenesis and Somatic Embryogenesis
2.1.1 Source plant material
Compared to other fruit trees, Citrus, Poncirus, and their hybrids are more amenable to tissue culture. The ability to regenerate whole plants from protoplasts, cell suspensions, callus, tissues and organs has been fully established (Figure 1), and regeneration studies have been successful for different applications including recovery of pathogen-free plants (Navarro et al., 1975; Navarro, 1992), ploidy manipulation (Ollitrault et al., 1998), generation of new hybrids and cybrids (Grosser and Gmitter, 2005), and genetic transformation (Table 1).
References |
Citrus genotypes |
Plant material |
Vector/method |
Results/remarks |
|---|---|---|---|---|
A |
||||
Kobayashi and Uchimiya, 1989 |
Citrus sinensis cv. Trovita |
Protoplasts |
PEG |
nptII No plant regeneration |
Vardi et al., 1990 |
Citrus jambhiri |
Protoplasts |
PEG |
cat, nptII Scarce plant regeneration |
Hidaka et al., 1990 |
Citrus sinensis cv. Washington navel and cv. Trovita |
Cell suspensions |
Agrobacterium tumefaciens |
hpt, nptII No plant regeneration |
Hidaka and Omura, 1993 |
Citrus reticulata cv. Ohta ponkan |
Protoplasts |
Electroporation |
uidA No plant regeneration |
Niedz et al., 1995 |
Citrus sinensis cv. Hamlin |
Protoplasts |
Electroporation |
gfp Efficient plant regeneration |
Yao et al., 1996 |
Tangelo (Citrus reticulata × Citrus paradisi) |
Cell suspensions |
Particle bombardment |
nptII, uidA No plant regeneration |
Fleming et al., 2000 |
Citrus sinensis cv. Itaborai |
Protoplasts |
PEG |
gfp No plant regeneration |
Li et al., 2002 |
Citrus reticulata cv. Ponkan |
Embryogenic callus |
Agrobacterium tumefaciens |
bar, pAT29-barnase Efficient plant regeneration |
Olivares-Fuster et al., 2003 |
Citrus sinensis cv. Itaborai |
Protoplasts |
PEG |
gfp, CTV-derived sequences No plant regeneration |
Li et al., 2003 |
Citrus sinensis cv. Valencia |
Embryogenic callus |
Agrobacterium tumefaciens |
bar, pAT29-barnase Efficient plant regeneration |
Niedz et al., 2003 |
Citrus sinensis cv. Hamlin |
Protoplasts |
Electroporation |
egfp Efficient plant regeneration |
Guo et al., 2005 |
Citrus sinensis cv. Valencia |
Protoplasts |
PEG |
gfp, TSPME Efficient plant regeneration |
B |
||||
Moore et al., 1992 |
Carrizo citrange (Citrus sinensis × Poncirus trifoliata) |
In vitro internodal stem segments |
Agrobacterium tumefaciens |
nptII, uidA Scarce transgenic plant regeneration |
Kaneyoshi et al., 1994 |
Poncirus trifoliata |
In vitro epicotyl segments |
Agrobacterium tumefaciens |
nptII, uidA Efficient transgenic plant regeneration |
Peña et al., 1995b |
Carrizo citrange (Citrus sinensis × Poncirus trifoliata) |
In vitro epicotyl segments |
Agrobacterium tumefaciens |
nptII, uidA Efficient transgenic plant regeneration |
Peña et al., 1995a |
Citrus sinensis cv. Pineapple |
Greenhouse internodal stem segments |
Agrobacterium tumefaciens |
nptII, uidA Efficient transgenic plant regeneration |
Kobayashi et al., 1996 |
Poncirus trifoliata |
In vitro epicotyl segments |
Agrobacterium tumefaciens |
hEGF gene Transgenic plants |
Peña et al., 1997 |
Citrus aurantifolia cv. Mexican |
Greenhouse internodal stem segments |
Agrobacterium tumefaciens |
nptII, uidA Efficient transgenic plant regeneration |
Gutiérrez et al., 1997 |
Carrizo citrange Citrus aurantium Citrus aurantifolia |
In vitro internodal stem segments |
Agrobacterium tumefaciens |
CTV-CP gene Transgenic plants |
Cervera et al., 1998a |
Citrus sinensis cv. Pineapple |
Greenhouse internodal stem segments |
Agrobacterium tumefaciens |
nptII, uidA Mature transgenic plants |
Cervera et al., 1998c |
Carrizo citrange (Citrus sinensis × Poncirus trifoliata) |
In vitro epicotyl segments |
Agrobacterium tumefaciens |
nptII, uidA Efficient transgenic plant regeneration |
Bond and Roose, 1998 |
Citrus sinensis cv. Washington navel |
In vitro epicotyl segments |
Agrobacterium tumefaciens |
nptII, uidA Efficient transgenic plant regeneration |
Gentile et al., 1998 |
Troyer citrange Citrus sinensis cv. Tarocco |
In vitro epicotyl segments |
Agrobacterium tumefaciens |
nptII, rolA, rolB, rolC genes Aberrant transgenic plants |
Pérez-Molphe and Ochoa-Alejo, 1998 |
Citrus aurantifolia cv. Mexican |
In vitro internodal stem segments |
Agrobacterium rhizogenes |
nptII, uidA Efficient transgenic plant regeneration |
Luth and Moore, 1999 |
Citrus paradisi cv. Duncan |
In vitro epicotyl segments |
Agrobacterium tumefaciens |
nptII, uidA Efficient transgenic plant regeneration |
Ghorbel et al., 1999 |
Carrizo citrange Citrus aurantium Citrus aurantifolia |
In vitro epicotyl or greenhouse internodal stem segments |
Agrobacterium tumefaciens |
nptII, gfp Efficient transgenic plant regeneration |
Kaneyoshi and Kobayashi, 1999 |
Poncirus trifoliata |
In vitro epicotyl segments |
Agrobacterium tumefaciens |
RolC Better rooting ability of transgenic plants, dwarfism |
Cervera et al., 2000b |
Carrizo citrange |
In vitro epicotyl segments |
Agrobacterium tumefaciens |
nptII, uidA Stability of transgene integration and expression over years |
Cervera et al., 2000a |
Carrizo citrange |
In vitro epicotyl segments |
Agrobacterium tumefaciens |
HAL2 gene Transgenic plants |
Domínguez et al., 2000 |
Citrus aurantifolia cv. Mexican |
Greenhouse internodal stem segments |
Agrobacterium tumefaciens |
CTV-CP gene Transgenic plants, transgenic protein accumulation |
Ghorbel et al., 2000 |
Citrus aurantium |
Greenhouse internodal stem segments |
Agrobacterium tumefaciens |
CTV-CP gene Transgenic plants |
LaMalfa et al., 2000 |
Troyer citrange |
In vitro epicotyl segments |
Agrobacterium tumefaciens |
nptII, gfp Efficient transgenic plant regeneration |
Yang et al., 2000 |
Citrus paradisi cv. Rio Red |
In vitro epicotyl segments |
Agrobacterium tumefaciens |
nptII, uidA, unCTV-CP, gna genes Transgenic protein accumulation |
Koltunow et al., 2000 |
Citrus aurantifolia cv. Mexican |
In vitro hypocotyl and epicotyl segments |
Agrobacterium tumefaciens |
Genes for decreased seed set Transgenic plants |
Wong et al., 2001 |
Carrizo citrange Citrus sinensis Poncirus trifoliata |
In vitro epicotyl segments |
Agrobacterium tumefaciens |
CS-ACS1 gene Repression of ACC content increase following chilling treatment |
Ghorbel et al., 2001b |
Different genotypes |
Greenhouse internodal stem segments |
Agrobacterium tumefaciens |
Enhancement of A. tumefaciens strain virulence by adding virG genes |
Ghorbel et al., 2001a |
Citrus aurantifolia cv. Mexican |
Greenhouse internodal stem segments |
Agrobacterium tumefaciens |
CTV-p23 gene Development of CTV symptoms in transgenic plants |
Fagoaga et al., 2001 |
Citrus sinensis cv. Pineapple |
Greenhouse internodal stem segments |
Agrobacterium tumefaciens |
PR-5 gene Resistance to Phytophthora citrophthora in one transgenic line |
Peña et al., 2001 |
Carrizo citrange |
In vitro epicotyl segments |
Agrobacterium tumefaciens |
AP1, LFY genes Precocious flowering of transgenic plants |
Domínguez et al., 2002c |
Citrus aurantifolia cv. Mexican |
Greenhouse internodal stem segments |
Agrobacterium tumefaciens |
CTV-CP gene Protection against CTV in several transgenic lines |
Domínguez et al., 2002a |
Citrus aurantifolia cv. Mexican |
Greenhouse internodal stem segments |
Agrobacterium tumefaciens |
Transgenic plants under nptII-selective and nonselective conditions. Silencing. |
Domínguez et al., 2002b |
Citrus aurantifolia cv. Mexican |
Greenhouse internodal stem segments |
Agrobacterium tumefaciens |
unCTV-CP gene versions Some delay in virus infection |
Costa et al., 2002 |
Citrus paradisi cv. Duncan |
In vitro internodal stem segments |
Agrobacterium tumefaciens |
Phytoene synthase, phytoene desaturase, lycopene-β-cyclase genes Transgenic plant regeneration |
Yu et al., 2002 |
Carrizo citrange Citrus sinensis |
In vitro internodal stem segments |
Agrobacterium tumefaciens |
nptII, uidA Efficient transgenic plant regeneration |
Mendes et al., 2002 |
Citrus sinensis cv. Hamlin |
In vitro epicotyl segments |
Agrobacterium tumefaciens |
nptII, gfp Efficient transgenic plant regeneration |
Febres et al., 2003 |
Citrus paradisi cv. Duncan |
In vitro internodal stem segments |
Agrobacterium tumefaciens |
ntCTV-CP, RdRp, 3′ end genes Great variability in virus titer in control and transgenic plants in CTV challenge |
Almeida et al., 2003a |
Citrus sinensis cv. Natal, cv. Valencia Citrus limonia cv. Rangpur |
In vitro epicotyl segments |
Agrobacterium tumefaciens |
nptII, uidA Efficient transgenic plant regeneration |
Almeida et al., 2003b |
Citrus sinensis |
Greenhouse internodal stem segments |
Agrobacterium tumefaciens |
nptII, uidA Mature transgenic plants |
Boscariol et al., 2003 |
Citrus sinensis cv. Valencia, cv. Hamlin, cv. Natal, cv. Pera |
In vitro epicotyl segments |
Agrobacterium tumefaciens |
PMI gene (positive selection) Efficient transgenic plant regeneration |
Molinari et al., 2004a |
Swingle citrumelo (Citrus paradisi × Poncirus trifoliata) |
In vitro epicotyl thin sections |
Agrobacterium tumefaciens |
nptII, uidA Efficient transgenic plant regeneration |
Molinari et al., 2004b |
Carrizo citrange |
In vitro epicotyl segments |
Agrobacterium tumefaciens |
p5cs gene Proline accumulation, superior behavior of transgenic plants under drought stress |
Kayim et al., 1994 |
Carrizo citrange |
In vitro epicotyl segments |
Agrobacterium tumefaciens |
p12 gene (sense and antisense) Transgenic plant regeneration |
Domínguez et al., 2004 |
Different genotypes |
In vitro epicotyl or greenhouse internodal segments |
Agrobacterium tumefaciens |
nptII, uidA, gfp Study of phenomena as chimeras, escapes or silencing |
Peña et al., 2004a |
Different genotypes |
In vitro epicotyl or greenhouse internodal segments |
Agrobacterium tumefaciens |
nptII, uidA, gfp Study of citrus cell transformation process |
Iwanami et al., 2004 |
Poncirus trifoliata |
In vitro epicotyl segments |
Agrobacterium tumefaciens |
CiMV-CP gene One immune and several tolerant transgenic lines |
Trainin et al., 2005 |
Citrus paradisi cv. Duncan |
In vitro internodal stem segments |
Agrobacterium tumefaciens |
Transposable element activator Ac Ac activity maintenance in transgenic plants after 4 years of growing |
Endo et al., 2005 |
Poncirus trifoliata |
In vitro epicotyl segments |
Agrobacterium tumefaciens |
Ci-FT gene Precocious flowering of transgenic plants |
Fagoaga et al., 2005 |
Citrus aurantifolia cv. Mexican Citrus aurantium Poncirus trifoliata |
Greenhouse internodal stem segments |
Agrobacterium tumefaciens |
CTV-p23 gene Development of CTV symptoms in transgenic plants |
Fagoaga et al., 2006 |
Citrus aurantifolia cv. Mexican |
Greenhouse internodal stem segments |
Agrobacterium tumefaciens |
CTV-p23 gene Some CTV-immune transgenic plants |
Boscariol et al., 2006 |
Citrus sinensis cv. Hamlin |
In vitro epicotyl segments |
Agrobacterium tumefaciens |
attA gene Significant reduction in susceptibility to citrus canker in some transgenic lines |
Rai, 2006 |
Citrus paradisi |
In vitro epicotyl segments |
Agrobacterium tumefaciens |
Ten candidate CTV resistance genes Evaluation of gene expression in transgenic lines and of CTV accumulation in infected plants |
Ballester et al., 2007 |
Carrizo citrange Citrus sinensis cv. Pineapple |
In vitro epicotyl or greenhouse internodal segments |
Agrobacterium tumefaciens |
ipt gene (positive selection), R/RS recombinase system |
Cervera et al., 2006 |
Carrizo citrange |
In vitro epicotyl segments |
Agrobacterium tumefaciens |
hpt, bar, gfp Retransformation of early flowering AP1 transgenic plants |
Batuman et al., 2006 |
Citrus macrophylla |
In vitro epicotyl segments |
Agrobacterium tumefaciens |
ds(p23 + 3'UTR) gene construct Delayed appearance of symptoms but no durable resistance |
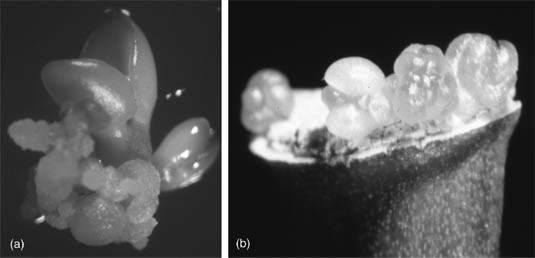
Regeneration of whole citrus plants through somatic embryogenesis and organogenesis. (a) Somatic embryo at the cotyledonary stage developing from nucellar-derived callus tissue. (b) Direct organogenesis from the cut end of an internodal stem segment
Sweet orange was the first tree crop in which plant regeneration from protoplasts was achieved (Vardi et al., 1982; Kobayashi et al., 1983). Since then, protoplast totipotency has been demonstrated and exploited for most citrus types of interest (reviewed in Grosser and Gmitter, 2005). Embryogenic suspension cultures initiated from ovule-derived nucellar callus have been the most appropriate source of protoplasts able to regenerate plants through somatic embryogenesis.
Protoplasts were the first source of plant material used to attempt genetic transformation in citrus. Kobayashi and Uchimiya 1989 obtained transgenic callus from Trovita sweet orange by PEG treatment of protoplasts with a plasmid containing the neomycin phosphotransferase II (nptII) marker gene, but regeneration of transgenic plants from that callus was unsuccessful. Cell suspension cultures were originated from nucellar callus and maintained in MT (Murashige and Tucker, 1969) liquid medium with 10mgl−1 6-benzylaminopurine (BAP). Two-week-old cells were transferred to hormone-free MT medium, and 2 weeks later were collected and subjected to protoplast isolation by incubation with an enzyme solution containing macerozyme, cellulase Onozuka, and driselase in ½ MT salts plus mannitol as osmoprotectant. Incubation was carried out at 25°C in a rotary shaker (25rpm) for 16h in dark. Protoplasts were filtered through a nylon mesh and washed with mannitol and MT plus mannitol through low centrifugation cycles to remove the enzymatic solution. Protoplasts were purified from a sucrose-mannitol gradient (Kobayashi et al., 1985), suspended in a mannitol solution and mixed with a plasmid vector solution. After 5min, a PEG solution was added to the mixture. Dilutions were made with a glucose-containing solution to adjust the osmotic level of the mixture. Protoplasts were then cultured in MT plus mannitol medium in small Petri dishes. The plates were sealed and maintained under 16-h/day illumination (500lux) at 26°C. After 2 weeks, the medium was diluted with an equal volume of MT plus mannitol. Two weeks later, the medium was solidified by an equal MT plus mannitol medium supplemented with 50mgl−1 kanamycin and 1.2% agarose. After 2 months, cell colonies were transferred to MT plus 0.8% agar and 25mgl−1 kanamycin for a second round of selection. Only eight colonies larger than 1mm were able to survive the two rounds of selection. They were transferred to kanamycin-free MT medium supplemented with 5mgl−1 BAP, 0.8% agar, but apparently they did not progress further. Integration of the nptII transgene was demonstrated in 4–5 of the callus lines by Southern blot analysis.
Vardi et al. 1990 produced transgenic callus from rough lemon (Citrus jambhiri Lush.) by PEG treatment of protoplasts with a plasmid containing the marker genes chloramphenicol acetyltransferase (cat) and nptII, and obtained several stably transgenic embryogenic lines, and at least two of them regenerated whole plants. Nucellar callus subcultured at least twice on MT was macerated in an enzyme solution containing macerozyme, cellulase Onozuka, and driselase in ½ MT salts plus mannitol and sucrose. Protoplasts were isolated by sequential filtering through nylon screens, and washed several times by centrifugation at 100 × g in MT plus sucrose/mannitol solutions. Washed protoplasts were resuspended and centrifuged in a Ficoll/mannitol gradient. Intact protoplasts formed a prominent band at the upper interface while the debris remained at the bottom. Then, protoplasts were collected and rewashed. For transformation, protoplasts were cocultivated with the linearized plasmid vector in a buffer medium containing PEG. Protoplasts were layered on top of a feeder layer consisting of γ-irradiated Citrus × P. trifoliata protoplasts plated in small Petri dishes. According to Vardi and Galun 1989, the feeder layer would promote protoplast division. However, the PEG treatment delayed the initiation of cell division from protoplast-derived cell colonies, and only about 8 weeks after transformation protoplast-derived microcallus attained about 0.5mm. Then, it was exposed to paromomycin selection (20–40mgl−1) in MT plus 4% sucrose, since the more common aminoglycoside antibiotic kanamycin did not provide a reproducible inhibition curve. Green embryoids were formed from 21 callus colonies, which were picked up and plated on a medium devoid of paromomycin to promote further growth and development. Individual embryos were isolated and regenerated to plants. Integration of the nptII gene in several plants and embryos was demonstrated by Southern blot analysis, as well as cat and nptII expression in putative transgenic embryos.
Hidaka et al. 1990 produced transformed callus of Washington navel and Trovita sweet oranges by co-cultivation of embryogenic cell suspension lines with Agrobacterium tumefaciens, but only one transgenic plantlet of Washington navel was regenerated. Six- to eight-year-old nucellar callus from Washington navel orange, Ohta ponkan, and Kara mandarin, and pollen-derived somatic callus from Trovita sweet orange, all of them maintaining their embryogenic potential, were transferred to liquid MS (Murashige and Skoog, 1962) medium supplemented with 0.2M sucrose and 50μM kinetin. Cultures were incubated at 25°C, 16-h light period, in an orbital shaker at 130rpm, and refreshed at least three times at 2-week intervals. Seven days after subculture, cell colonies were suspended in MS-sucrose liquid medium and inoculated with A. tumefaciens at a ratio of 100–200 bacteria per cell colony. Two bacterial strains were used, each carrying the nptII or the hygromycin phosphotransferase (hpt) marker genes within the transfer-DNA (T-DNA) of their disarmed Ti plasmids. After 3, 5, or 7 days of co-cultivation, cell colonies were washed several times with MS plus 0.2M sucrose supplemented with 100mgl−1 kanamycin or 20mgl−1 hygromycin, and then they were spread on the same medium but gelified with 0.8% agar. A second round of selection was performed with double antibiotic concentration. Aliquots from both the first and the second round of selection were spread on MS plus 0.2M sucrose for callus proliferation, or on MS plus 0.1M galactose and 0.1M sorbitol for embryoid differentiation. Co-cultivation of 3 days provided the best results. Antibiotic pressure, even at low concentration, inhibited callus proliferation. However, green embryoids were formed from Washington navel and Trovita orange callus after 6–10 weeks of culture in the first selection medium. Transfer of embryoids and callus to the second selection medium precluded further progress in most cases but 15 embryoids in total were able to survive. At least one Washington navel orange embryoid was able to regenerate a whole transgenic plant, which was confirmed as hpt-positive by Southern blot.
Hidaka and Omura 1993 obtained transformed ponkan mandarin callus by electroporation of protoplasts, but no plants were regenerated. Ohta ponkan nucellar callus was transferred from liquid MS and subcultured at least three times at 2-week intervals. Then cell suspensions were transferred to liquid MS plus 0.1M sorbitol and 0.1M galactose for other three subcultures. One week after the last subculture, protoplasts were isolated according to Vardi et al. 1990. The maceration medium consisted of ½ MS with 0.3% macerozyme Onozuka, 0.3% cellulase Onozuka, 0.35M mannitol, and 0.35M sorbitol. Protoplasts were resuspended in electroporation buffer containing 0.6M mannitol, and plasmid DNA was added at a concentration of 20mgl−1. The plasmid used carried the β-glucuronidase (uidA) gene as reporter marker. Samples were subjected to electroporation with an exponential decay pulse provided by a pulse generator. After electroporation, the suspension was transferred to a buffer containing 0.25% gelrite, 0.15M sucrose, and 0.45M glucose. Callus colonies formed after 2 months of culture were stained with 5-bromo-4-chloro-3-indolyl-β-d-glucuronic acid (X-Gluc) for testing histochemical GUS (β-glucuronidase; uidA) expression. Apparently, regeneration of transgenic plants was not attempted in this study.
Yao et al. 1996 reported transformation of Page tangelo embryogenic cells using particle bombardment, and produced 15 transgenic embryo lines, but they did not progress further. Highly embryogenic nucellar callus was used also here as source of tissue for transformation. Cell suspensions were prepared in liquid MS without growth regulators basically as described before. They were collected on filter paper containing sorbitol + mannitol, and were bombarded with tungsten particles coated with plasmid DNA using a Biolistic PDS-1000/HE Particle Delivery System. The plasmid vector used carried the uidA reporter and the nptII selectable marker transgenes. Stably transformed cells were detected by GUS staining at 8 weeks post bombardment. Transgenic cells and callus passed through rounds of 100 and 200mgl−1 kanamycin selection. Between 10 and 100 embryos were produced and transferred to germination medium, but conversion of embryos to plantlets was generally unsuccessful. Integration of the nptII transgene in the callus lines was demonstrated by Southern blot analysis.
J. Grosser's laboratory has used extensively protoplasts for citrus genetic transformation (Fleming et al., 2000; Olivares-Fuster et al., 2003; Guo et al., 2005). The protoplast transformation protocol was adapted from the PEG protoplast fusion method developed by Grosser and Gmitter 1990 for citrus somatic hybridization. Cell suspensions are prepared from ovule-derived nucellar callus with high embryogenic potential in a basal medium free of growth regulators. Protoplast isolation medium must be fine-tuned for every genotype but basically consists of the three enzymes mixture, mannitol, and buffer solutions. The suspension culture is digested overnight in the isolation medium, protoplasts are purified by centrifugation in a sucrose-mannitol gradient, and resuspended in 0.6M modified MT medium (BH3; Grosser and Gmitter, 1990). Protoplast suspension is aliquoted and plasmid DNA is added followed by a 40% PEG solution. After several cycles of incubation, centrifugation, and washings with different culture media, protoplasts are plated in small petridishes on BH3 medium, and incubated at 25°C under low light for 4 weeks. Although transgenic callus and embryoid production is generally very efficient, whole transgenic plant regeneration is difficult, and transgenic plants usually show atypical morphology. This has been attributed to the use of long-term cultures as starting materials.
Niedz et al. 2003 used a 2-year-old embryogenic callus line from Hamlin sweet orange for protoplast transformation by an improved electroporation method. In this case, many normal transgenic plants were regenerated as it was demonstrated by Southern blot analysis of 18 lines. In a previous report, Niedz et al. 1995 electroporated embryogenic protoplasts of sweet orange with a plasmid vector, but no transgenic plants were regenerated, probably due at least in part to the toxicity of the reporter marker gene used.
Li et al. (2002, 2003) exploited the embryogenic potential of different citrus (Ponkan, Valencia sweet orange) callus lines to attempt the Agrobacterium-mediated transformation system. The use of proper vector, co-cultivation, and selection conditions allowed producing whole transgenic plants at high frequencies. All relevant reports on genetic transformation of citrus through somatic embryogenesis are summarized in Table 1(A).
2.1.2 Source plant material: citrus seedling explants
Plant regeneration through organogenesis has been reported for many Citrus types and relatives, and from different tissues and explants, including leaf pieces, epicotyl segments, stem internodes, root segments, thin layers, and other tissues, being epicotyl and stem segments the most preferred ones. First works on citrus organogenesis reported the regeneration of whole plants from callus tissue formed from the primary explants (Grinblat, 1972; Chatuverdi and Mitra, 1974; Barlass and Skene, 1982; Edriss and Burger, 1984), likely reflecting how responsive they are to the addition of growth regulators in vitro. The cytokinin BAP has been essential for secondary organogenesis from disorganized callus, with auxins having only a marginal effect (García-Luis et al., 1999). A promotive effect in shoot regeneration is shown at low BAP concentration range, but raising the concentration over 5mgl−1 usually inhibits bud formation and shoot regeneration while enhances callusing (Moreira-Dias et al., 2000). In the absence of BAP, direct shoot regeneration occurs from epicotyl and stem segments (García-Luis et al., 1999; Bordón et al., 2000).
In early transformation works, Moore et al. 1992 compared the organogenic ability of 0.7cm leaf disks and stem segments of different lengths from in vitro-grown 2–4-month-old seedlings of several citrus genotypes. Stem segments of about 1cm in length were the most effective in shoot production. Consequently, they decided to use this material as source explant for A. tumefaciens-mediated genetic transformation. A disarmed A. tumefaciens strain that contained two different transformation vectors carrying nptII and uidA marker transgenes was used in the experiments. Internodal stem segments were inserted vertically with either the basal or the apical end protruding from a medium consisting of MT with 5% sucrose, 5mgl−1 BAP, and 0.8% agar, pH 5.7. The protruding ends were inoculated with an overnight culture of A. tumefaciens by placing a small drop of the culture on the end of the segment with a syringe. After 2–3 days of co-cultivation, explants were transferred to the same medium supplemented with the antibiotic kanamycin (100mgl−1), as selective agent, and mefoxin (200mgl−1), to control bacterial growth. At 4 weeks, shoots started to arise from the protruding cut ends of the explants with little or no callus production. They were excised and rooted in cups containing sterile potting soil moistened with ½ MT medium. More than 95% of the regenerated shoots were GUS-negative, suggesting that kanamycin was not a reliable indicator of transformation in this system. In addition, rooting of GUS-positive shoots was highly inefficient, so only two whole transgenic Carrizo citrange plants could be produced.
Kaneyoshi et al. 1994 established the first efficient protocol for transformation of seedling plant material and applied it to the generation of transgenic P. trifoliata plants. Importantly, they used 1cm long etiolated epicotyl segments from 20-day-old in vitro-grown seedlings as starting material for transformation, because they had previously shown that epicotyl segments were highly responsive to shoot regeneration. Seeds were sterilized with 1% sodium hypochlorite solution containing 0.1% Tween-20 for 20min, and rinsed three times with sterile distilled water. Seeds, with their seed coats peeled off, were placed on MS plus 5% sucrose and 0.8% agar, and then incubated at 27°C in darkness. A disarmed A. tumefaciens strain was used as transformation vector, and nptII and uidA were used as marker transgenes. Explants were immersed in a bacterial suspension at 5 × 108 cells/ml for 15min, blotted on sterile filter paper, and transferred to co-cultivation medium consisting of hormone-free MS plus acetosyringone at 100μM for 3 days. Then, explants were subcultured to MS plus 5mgl−1 BAP, 0.1mgl−1 α-naphthalene acetic acid (NAA), supplemented with kanamycin at 100mgl−1 for transgenic selection, and cefotaxime at 500mgl−1 to prevent bacterial growth. Explants regenerating shoots were transferred to a new medium with much reduced BAP concentration (0.5mgl−1) and increased kanamycin level (200mgl−1) to favor transgenic shoot development. GUS assays revealed that more than 50% of the regenerants were transgenic, suggesting that kanamycin was a reliable selectable marker in this system. Attempts to avoid escape regeneration by growing the explants in 200mgl−1 kanamycin were unsuccessful. Elongated shoots were rooted in MS plus 0.5mgl−1 NAA without problems. Efficient stable integration was demonstrated by Southern blot analysis of uidA gene in several transformants. Authors claimed that this procedure permitted them to generate more than 100 transgenic plants within 2–3 months with an average transformation efficiency (transgenic shoots × 100 per total number of explants) higher than 60%. Moreover, it has been successfully used by this and other groups to incorporate transgenes of potential interest into P. trifoliata (Kobayashi et al., 1996; Kaneyoshi and Kobayashi, 1999; Wong et al., 2001; Iwanami et al., 2004; Endo et al., 2005).
Peña et al. 1995b used a similar protocol to transform Carrizo citrange, but it had to be modified because this genotype responded much worse than Poncirus to Agrobacterium-mediated transformation and shoot rooting. Stored seeds coming from the same tree stock were peeled, removing both seed coats, disinfected for 10min in a 0.5% (v/v) sodium hypochlorite solution containing 0.1% (v/v) Tween-20, and rinsed three times with sterile distilled water. Five-week-old germinating seedlings were used as the starting material for genetic transformation. These seedlings were grown in MS salt solution plus 10gl−1 agar, pH 5.7, at 26°C in darkness for the first 2 weeks, and under a 16-h photoperiod and illumination of 45μEm−2s−1 for three additional weeks. An A. tumefaciens strain carrying a transformation plasmid with nptII and uidA marker transgenes was used as vector system for transformation. Bacteria were cultured overnight in an orbital shaker at 28°C and 200rpm in Luria Broth (LB) medium containing the proper antibiotics to grow the binary system. Bacterial cells were pelleted at 3500rpm for 10min, resuspended and diluted to 4 × 107 or 4 × 108 cells/ml in liquid inoculation medium, which consisted of MS salt solution, 0.2mgl−1 thiamine hydrochloride, 1mgl−1 pyridoxine hydrochloride, 1mgl−1 nicotinic acid, and 3% (w/v) sucrose, pH 5.7.
Either epicotyl or internodal stem segments (about 1cm long) were cut transversely and incubated for 15min in 10-cm-diameter plates containing 15ml of the bacterial suspension in inoculation medium by gentle shaking. The infected explants were blotted dry on sterile filter paper and placed horizontally on plates with the same medium but gelified with 0.8% agar for a 2-day co-cultivation period. In parallel, co-cultivation was tested as in Moore et al. 1992, by placing a drop of the bacterial culture on the cut end of the segments inserted vertically in the co-cultivation medium.
After co-cultivation, the explants were blotted dry with sterile filter paper and transferred to shoot regeneration medium (SRM), which consisted of MS salts, 0.2mgl−1 thiamine hydrochloride, 1mgl−1 pyridoxine hydrochloride, 1mgl−1 nicotinic acid, 3% (w/v) sucrose, 1% (w/v) agar, pH 5.7, plus 100mgl−1 kanamycin for the selection of transgenic shoots, and 250mgl−1 vancomycin and 500mgl−1 cefotaxime to control bacterial growth. This medium was supplemented with 3mgl−1 BAP. Cultures were maintained in the dark for 4 weeks at 26°C and then were transferred to 16-h photoperiod, 45μEm−2s−1 illumination, and 26°C. A high frequency of GUS-positive shoots (55.1%) was obtained when explants were disposed horizontally in co-cultivation and regeneration medium, and when the bacterial culture was used at 4 × 107. However, escapes (21%) and GUS-chimeric shoots (23.9%) were also produced. Attempts to root the transgenic regenerants were unsuccessful. Alternatively, shoots were excised from the explants and cut in two pieces. The basal portion was GUS-assayed and, if the reaction was positive, the apical part was grafted in vitro onto a nontransgenic decapitated in vitro-grown seedling (Navarro, 1992). This resulted in 100% recovery of transgenic shoots. About 3–4 weeks after shoot-tip grafting, plantlets were again grafted on a vigorous seedling rootstock in a greenhouse at 18–27°C. The system resulted in transformation efficiency higher than 20% and allowed to generate Carrizo citrange transgenic plants routinely.
Gloria Moore's group also compared co-cultivation and regeneration/selection of the explants in vertical and horizontal orientations and concluded that horizontal disposition permitted to perform a better kanamycin selection. However, the use of older (3–4-month-old) seedlings, inefficient selection, and poor rooting frequency allowed them to only produce two sour orange, nine lime, and nine Carrizo citrange transgenic plants (Gutiérrez et al., 1997). The same group later found that the transformation system previously established for P. trifoliata by Kaneyoshi et al. 1994 also worked for transformation of Duncan grapefruit, though at much lower efficiency (Luth and Moore, 1999; Costa et al., 2002; Febres et al., 2003).
Leandro Peña's group improved the transformation system of Carrizo citrange epicotyl segments by studying critically several factors affecting transformation and regeneration (Cervera et al., 1998c). It was determined that co-cultivation of 3 days with A. tumefaciens in a medium rich in auxins and postcultivation in regeneration/selection medium for 4 weeks in darkness increased transformation efficiency to 41.3%, making possible to produce so many transgenic plants as needed in 3–6 months. The same basic procedure with more or less similar modifications has been used by other laboratories to transform Carrizo citrange (LaMalfa et al., 2000; Wong et al., 2001; Yu et al., 2002; Kayim et al., 2004), Washington navel orange (Bond and Roose, 1998), Tarocco sweet orange (Gentile et al., 1998), Rio Red grapefruit (Yang et al., 2000; Rai, 2006), Mexican lime (Koltunow et al., 2000), Xuegan sweet orange (Yu et al., 2002), Rangpur lime, Valencia, Natal, Pera, and Hamlin sweet oranges (Mendes et al., 2002; Almeida et al., 2003a; Boscariol et al., 2003, 2006), Duncan grapefruit (Trainin et al., 2005; Rai, 2006), and Ruby Red grapefruit (Rai, 2006) (Figure 2).
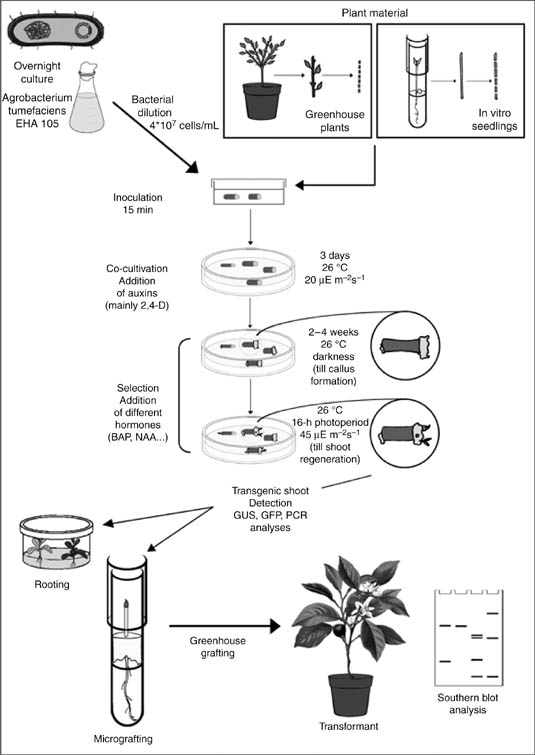
Procedure for the genetic transformation of citrus plants. Aseptically germinated 4–5-week-old seedlings are used as the starting material for genetic transformation of P. trifoliata and Carrizo citrange. Older tissues, such as shoots from 4- to 12-month-old glasshouse-grown vigorous seedlings, are also used as the source of tissue for transformation of sweet orange, sour orange, lime, alemow, lemon, and mandarins. For transformation of mature tissues, new shoots elongated from buds, collected from trees maintained in a screenhouse (pathogen-free Germplasm Bank Collection of the IVIA), grafted onto seedlings of a vigorous rootstock grown under glasshouse conditions, are used as starting material. Agrobacterium tumefaciens strain EHA 105 carrying a binary plasmid is used for transformation. Bacteria are cultured overnight in an orbital shaker at 28°C and 200rpm in LB medium containing the appropriate antibiotics. After bacterial cells are pelleted at 3500rpm for 10min, they are resuspended and diluted to 4 × 107 cells/ml in liquid inoculation medium. Epicotyl or internodal stem segments (1cm long) are cut transversely and incubated for 15min in 15ml of bacterial suspension by gentle shaking. The infected explants are blotted dry on sterile filter paper and co-cultivated for 3 days on co-cultivation medium. After co-cultivation, explants are blotted dry with sterile filter paper and transferred to selection/regeneration medium. Cultures are grown in the dark for 2–4 weeks at 26°C and then maintained under a 16-h photoperiod with 45μEm−2s−1 of light intensity. Shoots usually develop from the cut ends 3–8 weeks after co-cultivation. Their transgenic nature is evaluated by histochemical assays for GUS activity or by visualizing GFP expression. The apical portions of transgenic shoots are then grafted in vitro onto decapitated seedlings of a citrange rootstock. Rooting is generally not easy in many citrus genotypes. New grafting of the in vitro-grown plants on vigorous rootstocks in the greenhouse allows the rapid acclimatization and development of putative transgenic plants. Genetic transformation is then confirmed by Southern blot analysis
Pérez-Molphe and Ochoa-Alejo 1998 used Agrobacterium rhizogenes as transformation vector and in vitro-grown internodal stem segments of Mexican lime as explants. In this case, the bacteria carried the root inducing plasmid (pRi) plasmid, which is necessary for transformation and confers the characteristic hairy root phenotype, and the disarmed Ti plasmid with nptII and uidA transgenes within the T-DNA. Explants were inoculated by immersion in the bacterial culture diluted at 1 × 108 cells/ml in MS liquid medium plus B5 vitamins and 3% sucrose during 45min, and then were placed horizontally on the same medium but with 5% sucrose and gelified with 0.8% agar for a 3-day co-cultivation period at 28°C in darkness. Infected explants were washed for 45min with liquid MS medium containing 750mgl−1 cefotaxime to eliminate A. rhizogenes. Finally, the explants were transferred to the medium used for co-cultivation but supplemented with 80mgl−1 kanamycin and 300mgl−1 cefotaxime, and with two different growth regulators treatments: without regulators or with 7.5mgl−1 BAP and 1mgl−1 NAA. In the first case, transformed roots appeared directly from the cut ends of the explants 30 days after infection. Some explants produced shoots and roots simultaneously, but in general the frequency of regenerated shoots was low. When growth regulators were added to the regeneration medium, a pale green callus was formed at the cut ends and about 60 days later shoots were formed without hairy root phase. Transformed roots transferred to a medium with growth regulators also produced shoots at high frequency. Shoots were excised from explant and root segments and easily rooted in a medium without growth regulators. This profuse organogenic response was due to the integration and expression of the root loci (rol) genes from the Ri plasmid of A. rhizogenes in the transformed cells. The survival rate upon transfer to soil was 79%. Stable transformation was confirmed by Southern blot analysis of the nptII transgene. This is a rather efficient transformation system for citrus but it is not being widely used due to the integration of the T-DNA genes from the Ri plasmid in the transgenic cells. Their expression caused not only active regeneration but also important phenotypical alterations, including short internodes, reduced apical dominance, and wrinkled leaves in the transgenic plants, seriously limiting the biotechnological possibilities of the procedure.
Several groups have modified the original epicotyl transformation system with the aim of increasing the wounded area of the explants and the number of explants generated per seedling. Yu et al. 2002 proposed cutting longitudinally the epicotyl segments in two halves to enhance both regeneration and transformation frequency. This was used for Carrizo citrange transformation (Yu et al., 2002; Kayim et al., 2004), but attempts to adapt it to other citrus genotypes have been generally unsuccessful mainly due to problems associated to Agrobacterium overgrowth (Cervera and Peña, unpublished results; W. Guo, personal communication). Another alternative has been using thin layers of about 1–2mm cut transversally from etiolated epicotyls. This explant type was highly organogenic, as demonstrated by Le et al. 1999 in P. trifoliata, and reduced the occurrence of escapes in Swingle citrumelo and Carrizo citrange transformation (Molinari et al., 2004a, 2004b). However, transformation efficiency is much lower than using 1cm long explants, probably due to the toxicity of A. tumefaciens inoculation and co-cultivation of so small explants (Molinari et al., 2004b; Cervera and Peña, unpublished results).
Although using either embryogenic nucellar callus or epicotyl segments from in vitro-grown seedlings as source material for transformation has led to workable transformation efficiencies for some citrus genotypes and relatives, transgenic plants were juvenile. In addition, regeneration from these materials could result in some cases in production of plants that are not true-to-type (Cervera et al., 2000b; Grosser et al., 2002; Cervera and Peña, unpublished results). Given the long juvenile period of most citrus genotypes, several years of cultivation would be needed before horticultural and commercial traits of the transgenic plants could be evaluated, if juvenile tissues were used for transformation. Therefore, development of transformation systems from mature plants was very important in citrus to be able to overcome juvenility.
The first approach in such direction was the use of source material coming from the greenhouse. Peña et al. 1995a used 6–12-month-old greenhouse-grown (18–27°C) seedlings of Pineapple sweet orange as the source of tissue for transformation. Internodal stem segments (0.5–1cm in length) from stem pieces (20cm in length) were inserted vertically in SRM (Peña et al., 1995b) without antibiotics and inoculated with A. tumefaciens carrying nptII and uidA marker transgenes by placing a drop of the bacterial culture on the cut end of the segment protruding from the medium. The explants were co-cultivated for 2 days with the bacteria, blotted dry with sterile filter paper and transferred to SRM supplemented with kanamycin, as selectable agent, and cefotaxime and vancomycin to prevent further bacterial growth. The pots were maintained in dark at 27°C during 8 weeks and then at 25°C, 16-h photoperiod, illumination of 10μEm−2s−1 and 60% relative humidity during 4 weeks. Regenerated shoots of 0.2–0.3cm height were harvested from the stem segments. Portions of 0.1–0.2cm were excised from the shoot basal ends and assayed for GUS activity, and the remaining portions were shoot-tip grafted on Troyer citrange seedlings, as in Peña et al. 1995b. As transformation efficiency was low using this system (7.9%), test inoculation was done by immersion in the bacterial culture diluted at 107 cell/ml in SRM with BAP at 1mgl−1, and co-cultivation by disposing the explants horizontally under two different conditions: 2 days in SRM with BAP 1mgl−1, agar 0.8%, or 3 days on tomato feeder layers. Feeder plates were prepared by pippeting 2ml of 6–7-day-old tomato cell suspensions on the surface of 25ml of tomato cell suspension (TCS) solid medium with sterile Whatman 5 filter paper on the top in 10 × 1.5cm (diameter × height) plates. TCS medium consisted of MS salts, 1mgl−1 thiamine hydrochloride, 1mgl−1 pyridoxine hydrochloride, 1mgl−1 nicotinic acid, 3% sucrose, 2mgl−1 indole-3-acetic acid (IAA), 1mgl−1 2-isopentenyl-adenine (2-iP), 2mgl−1 2,4-dichlorophenoxyacetic acid (2,4-D), 0.8% agar, pH 5.7. TCSs were maintained in TCS liquid medium in a shaker at 100rpm and 25°C and were subcultured in fresh medium every 15 days. After co-cultivation, the explants were blotted dry with sterile filter paper and transferred to SRM (BAP 1mgl−1) plates containing 100mgl−1 kanamycin, for selection and 500mgl−1 cefotaxime, 250mgl−1 vancomycin to control further bacterial growth. The cultures were maintained in the dark for 15 days at 26°C and then transferred to 16-h photoperiod, 45μEm−2s−1 illumination, 26°C, and 60% relative humidity. For Mexican lime, co-cultivation in feeder plates allowed to increase transformation efficiency twice (Peña et al., 1997).
2.1.3 Source plant material: citrus mature material
Once Leandro Peña's were able to achieve 15–20% transformation efficiency working with greenhouse-grown juvenile material, they attempted transformation of mature tissues (Cervera et al., 1998a). However, in the first preliminary experiments, internodal stem segments from aged mature citrus trees showed very limited regenerative potential. Then, buds from adult trees were grafted on vigorous seedlings. Buds were collected from Pineapple sweet orange trees maintained in a screenhouse (pathogen-free Germplasm Bank Collection of the IVIA) and were grafted onto seedlings of a vigorous rootstock under greenhouse conditions (18–27°C), and newly elongated shoots were used as starting material. Stem pieces (20cm in length) were stripped of their leaves and thorns disinfected for 10min in a 2% (v/v) sodium hypochlorite solution and rinsed three times with sterile distilled water. Regeneration from stem segments from the first, second, and third flushes of newly grafted invigorated mature sweet orange plants was evaluated in comparison to regeneration from stem segments from juvenile plants. Results indicated that explants from the first and second flushes produced similar regeneration frequencies, significantly higher than that of the explants from the third flush. As expected, stem segments from juvenile plants produced the highest regeneration frequency. The first flush of the adult plants was selected as the source of tissue for genetic transformation experiments. Different A. tumefaciens concentrations and co-cultivation time and media for transformation were tested. A bacterial concentration of 107 cells/ml and 3-day co-cultivation in tomato feeder plates provided the best results and were used in further experiments. Regeneration/selection medium and conditions were identical to those used for transformation of juvenile material. Shoot regeneration was observed after 2–5 months on selective medium, and transformation efficiency achieved was of about 6%, approximately threefold lower than that obtained for juvenile material. It is probable that aging decreased the susceptibility of plant cells to Agrobacterium-infection as well as their organogenic potential. Southern blot analysis confirmed the stable integration of uidA and nptII gene cassettes into the plants' genome. The putative mature transgenic sweet orange plants showed morphology and growth habits of an adult plant, as compared to control mature plants. In fact, whereas juvenile plants showed a pronounced thorniness, transgenic mature plants were almost thornless, similar to the mature plants from which the explants were taken for transformation. After 14 months in the greenhouse, the transgenic and control plants started to flower, confirming their mature nature (Figure 3a). Both flowers and immature fruits from the transgenic plants showed a dark blue color after overnight incubation in X-Gluc, a substrate of the β-glucuronidase enzyme (Figure 3b). These results confirmed the maintenance of the ontogenic mature stage of the invigorated mature plants as well as the transgenic plants. Interestingly, transgenic events kept their epigenetic mature state even after a process of de-differentiation, induction and redifferentiation, necessary to shift the cells to a competent state for transformation. Therefore, Cervera et al. 1998a directly transformed and regenerated mature tissues of citrus, bypassing the juvenile stage. This process greatly shortens the period of time until flowering and bearing fruits and decreases the time to achieve horticulturally acceptable characteristics by years. Pineapple sweet orange transgenic juvenile plants generated in the same series of experiments needed at least 5 years to start flowering, and 4 years later still keep juvenile characteristics. This transformation procedure for mature citrus tissues has been patented in Spain (no 9700491/X), Europe (no EP 0870838 B1), and United States (no 6.103.955). Moreover, it could be worth trying to apply this transformation and regeneration strategy to transgenic plant production of other woody fruit species with long juvenile periods.

Genetic transformation of mature citrus plants. (a) About 1 year after transformation, regenerated plants started to flower and set fruit, confirming their mature nature. (b) GUS expression in a longitudinal cut from a developing fruit of a transgenic mature sweet orange plant (right) compared to the corresponding nontransgenic control (left)
In subsequent experiments, Leandro Peña's group investigated the influence of the components of the feeder plates, namely the TCSs, the filter paper layer, and the TCS culture medium rich in auxins, separately and in combination, in the genetic transformation of Mexican lime explants. TCS basal medium was responsible for the increased lime transformation frequency previously attributed to the feeder plates as a whole. The role of the TCSs and the filter paper was even detrimental, as they drastically decreased transformation frequency in comparison with co-cultivation of explants in TCS. Not only was the transformation frequency much higher in explants co-cultivated in TCS, but also the percentage of explants with GUS-positive spots and the maximum number of GUS-positive spots per explant. The liquid layer provided by the cell suspension affected negatively the explants, favoring excessive formation of abnormal callus. On the contrary, the filter paper layer between the basal medium and the explants precluded callus formation at their cut ends (Domínguez et al., 2000). Therefore, TCS was used for co-cultivation in further experiments with Mexican lime and other citrus genotypes. As it was previously shown for Carrizo citrange epicotyl segments (Cervera et al., 1998c), phytohormones (mainly auxins) in TCS seemed to play a crucial role in inducing de-differentiated cells competent for transformation.
The use of vigorous shoots from plants growing in the greenhouse as source of material for transformation in the case of citrus varieties provides with the possibility of using the same procedure first established to transform juvenile tissues of a given species and also to transform adult materials from the same species (Figure 2). Thus, Leandro Peña's group extended the genetic transformation system for juvenile material to mature citrus tissues of many citrus types of interest, including several sweet orange varieties, sour orange, Mexican lime, Fino lemon, rough lemon, Cleopatra mandarin, and C. macrophylla (reviewed in Peña et al., 2004a), and more recently also clementine (Cervera et al., 2008). Alternative to invigoration by grafting, Leandro Peña's group directly used new shoots from the Pineapple sweet orange tree maintained in a screenhouse (at the Germplasm Bank Collection) as starting material for mature transformation (Peña et al., 2004a). Although transgenic plants were generated, the efficiency of the system was much lower (Cervera and Peña, unpublished results). Almeida et al. 2003b used this system with minor modifications to transform mature Hamlin sweet orange with low efficiency. They also used leaf disks from mature plants as explants but poor bud induction and Agrobacterium overgrowth even at 1-day co-cultivation impeded transgenic plant recovery. A summary of reports on regeneration of transgenic citrus plants by organogenesis is shown in Table 1(B).
2.2 Methods Employed for Transformation
2.2.1 Electroporation and PEG-mediated transformation of citrus protoplasts
First reports on genetic transformation of citrus used PEG, electroporation or A. tumefaciens as systems for protoplast transformation. Electroporation or addition of PEG to a protoplast suspension induces pore formation in the membranes, so plasmid DNA is able to pass through them and in some cases the DNA becomes integrated in the nuclear genome. Kobayashi and Uchimiya 1989 mixed 10μg of the circular form of the bacterial plasmid vector carrying the nptII selectable marker gene with a protoplast suspension (2.2 × 106) in 0.6M mannitol. Vardi et al. 1990 also used PEG 6000 for protoplast transformation but in this case the plasmid was linearized and calf thymus DNA was used as a carrier. Preliminarly, these authors compared transient transformation mediated either by PEG or by electroporation and found that both the systems worked well, but since the former procedure was simpler and resulted in more consistent transgene transient activity, further stable transformation experiments were PEG-mediated. Electroporation was also attempted by Hidaka and Omura 1993 and conditions were fixed to get transgenic callus colonies. Whereas maximal transgene transient expression was shown in protoplasts electroporated at 1200V/cm, callus colonies were only formed using voltages between 200 and 600V/cm. A heat shock treatment at 49°C before electroporation greatly enhanced transient transformation, as well as using 0.1mM CaCl2 in the electroporation buffer. Plasmid DNA concentration of 100μg/ml and a capacitance of 5.5μF were also determined as optimal for transient transgene expression.
More recently, PEG-mediated citrus protoplast transformation is being routinely used in J. Grosser's laboratory (Fleming et al., 2000; Olivares-Fuster et al., 2003; Guo et al., 2005), as an extension of their PEG protoplast somatic hybridization method (Grosser and Gmitter, 1990). The protoplast suspension at 2 × 106 cells/ml is aliquoted into 15ml round-bottomed tubes at 0.5ml per tube and 20μg of plasmid DNA in buffer or water is added to each tube, followed by 0.5ml of a 40% PEG solution. Niedz et al. 2003 optimized electroporation conditions for protoplast transformation. They found that an electric field strength of 375–450V/cm, a vector DNA concentration of 100μg/ml, a carrier DNA (salmon sperm DNA) concentration of 100μg/ml, the use of electroporation buffer at pH 8.0, and preelectroporation heat shock of protoplasts for 5min at 45°C, were optimum for DNA uptake into protoplasts as determined by assaying GUS activity 24h after electroporation. These improvements allowed producing transgenic plants quite efficiently.
2.2.2 Agrobacterium-mediated transformation of citrus epicotyl and internodal stem segments
Moore et al. 1992 tested Carrizo citrange, Swingle citrumelo, Mexican lime, and Hamlin sweet orange for susceptibility to various A. tumefaciens wild-type strains by injecting seedling hypocotyls with bacterial cultures. Although the detailed results of these experiments were not shown, it was clear that all tested citrus types were susceptible to Agrobacterium as evidenced by the production of tumors at the wound site following inoculation, and that the succinamopine strain A281 gave rise to very large, rapidly growing tumors. Because of the apparent hypervirulence of its Ti plasmid, pTiBo542, a nononcogenic derivative of this strain, EHA 101, was used in subsequent transformation experiments. Due to the low transformation efficiency achieved, in a second work the same group attempted using the oncogenic strain A518 harboring a binary plasmid with the aim of enhancing transgenic shoot regeneration. GUS-positive shoots were obtained, sometimes at higher frequencies than those obtained with EHA 101, but all of them also contained the oncogenic T-DNA, appearing morphologically abnormal. Therefore, EHA 101 was used in further experiments.
Peña et al. (1995a, 1995b, 1997) used A. tumefaciens strain EHA 105 for transformation of epicotyl segments from in vitro-grown seedlings and of internodal stem segments of greenhouse-grown juvenile plants. EHA 105 is a derivative of EHA 101 in which the bacterial kanamycin resistance gene from the disarmed Ti plasmid has been removed. Thus, this Agrobacterium strain can be used with most binary plasmid systems, as pBin or pCambia vectors, which harbor bacterial resistance to kanamycin. In order to choose a proper A. tumefaciens strain for transformation of mature material, we investigated the virulence of three different wild-type strains by inoculating stems of greenhouse-grown sweet orange seedlings. These three strains were A281 (succinamopine type), Ach5 (octopine type), and C58 (nopaline type). A. tumefaciens A281 produced the earliest and the highest frequency of tumor formation. Therefore, Leandro Peña's group followed using A. tumefaciens EHA 105, a nononcogenic derivative of this strain, as favorite vector for citrus transformation (Cervera et al., 1998b).
Tumors incited by Agrobacterium tumefaciens strains A281, Ach5, and C58 on: (a) Carrizo citrange and (b) Mexican lime. On each photograph, stem marked with CONT corresponds to a mock-inoculated plant. Photographs were taken 28 weeks after inoculation. Three inoculations were performed per stem segment
To gain more insight on the relationships between A. tumefaciens strains and citrus, and to aid in the choice of proper Agrobacterium vectors for genetic transformation, Cervera et al. 1998b investigated the virulence of A. tumefaciens strain A281, compared to that of Ach5 and C58 on a wide range of agronomically important woody fruit crops. This study included Pineapple sweet orange, Mexican lime, Clemenules clementine, and Carrizo citrange as citrus types. Plants (4–6-month-old) were grown in individual 2.5l pots and were fertilized weekly. They were kept inside temperature-controlled greenhouses at 24–26/15–16°C day/night temperatures, with relative humidity of 60–80%. For each Agrobacterium strain and host pair, three inoculations per plant were performed at the same time in all the tested species, at intervals of 5–7cm and practicing the first wound at 5cm from the soil. Five microliters of A. tumefaciens suspensions in water (OD595 nm ≈ 0.3), from 48-h-old cultures, were added to the wounds with a micropipette. The wounds were then covered with plastic wraps for 2 weeks to prevent bacterial desiccation. All the inoculations were performed in April, when plants were actively growing up, and tumor development was allowed to progress for 8–28 weeks. Strain A281 was the most virulent in all citrus types, since it produced earlier appearance and higher tumor formation frequency. On sweet orange and lime, tumor weight was comparable in plants inoculated with strains A281 and C58. However, strain C58 was incapable of inciting tumors in clementine. On citrange, A281 produced earlier-appearing tumors but C58 was as efficient as A281 in tumor formation and incited larger tumors than A281 (Figure 4). Interestingly, tumors induced by strain A281 on the four citrus hosts showed a progressive blackening after 3 months from inoculation. When tumors incited by A281 were excised, 7 months postinoculation, necrosis was severely affecting their morphology and final weight, thus explaining the larger size of tumors produced by strain C58 on citrange, and equivalent sizes of tumors produced by A281 and C58 on sweet orange and Mexican lime. Since tumor necrosis seems to be strongly correlated with the supervirulent phenotype induced by A281, and this strain incited earlier-appearing tumors and at higher frequencies than the other Agrobacterium strains, A281 can be considered supervirulent on the four citrus hosts tested. Strain Ach5 was not effective on sweet orange and clementine, and only produced small tumors at low frequencies on Mexican lime and citrange.
Later, Leandro Peña's group extended this study to other five agronomically important citrus types, Fino lemon, Cleopatra mandarin, C. macrophylla, sour orange, and Mediterranean mandarin. Tumors were induced by the three strains in all the genotypes tested, but time of appearance of tumors, tumor frequency, and final tumor weight were clearly different depending on the bacterial strain. A281 was again the most virulent strain, because it induced earlier appearance of tumor and higher tumor formation frequency in most of the hosts, at least until 2 months postinoculation. However, in the cases of Mediterranean and Cleopatra mandarins, C58 was as virulent as A281. Tumors induced by A281 in all the citrus hosts started to become necrotic 2–3 months postinoculation. Such necrosis possibly had a slight effect on final tumor formation frequency, but clearly affected tumor development. Whereas tumors induced by strains Ach5 and, over all, C58 continuously grew until 4 months postinoculation, tumors produced by A281 grew more slowly when necrosis started to affect them. As a consequence, final tumor weight was higher for C58 than for A281 in Cleopatra mandarin and C. macrophylla, and similar for C58 and A281 in Mediterranean mandarin. Ach5 incited in general less tumor frequency and weight than the other two strains (Ghorbel and Peña, unpublished results).
Interestingly, the octopine strain LBA 4404, which is a disarmed derivative of Ach5, has been widely used to transform P. trifoliata (Kaneyoshi et al., 1994; Kobayashi et al. 1996; Kaneyoshi and Kobayashi, 1999; Iwanami et al., 2004; Endo et al., 2005). However, this citrus relative has also been successfully transformed with strain EHA 105 (Wong et al., 2001; Fagoaga et al., 2005), suggesting that it is highly receptive to Agrobacterium-mediated gene transfer and that several strains could be used to obtain efficient transformation.
Experiments from Leandro Peña's group clearly indicated that C58 and A281 were appropriate strains for citrus transformation, A281 being generally much more virulent. A281 is a transconjugant of C58 with pTiBo542 instead of pTiC58, meaning that both strains should interact identically with citrus cells. Therefore, differences in virulence would probably reflect differences in transformation and/or expression of T-DNA and/or vir genes. To investigate this, Ghorbel et al. 2001b tested stable transformation in internodal stems segments from Fino lemon, Cleopatra mandarin, C. macrophylla, sour orange, and Mediterranean mandarin co-cultivated with the disarmed Agrobacterium strains C58 (pMP90) and EHA 105, carrying a binary plasmid (p35SGUSINT) containing uidA and nptII gene cassettes in the T-DNA. After co-cultivation in the medium rich in auxins that favors transformation (Cervera et al., 1998c), the explants were transferred to a regeneration medium containing kanamycin to allow growth of transformed tissues only. GUS assays were performed 6 weeks after bacterial infections, when T-DNA gene expression was fully stable. For all the citrus genotypes tested, transformation frequency produced by both strains was very different. C58 (pMP90)/ p35SGUSINT produced very low transformation in lime, sour orange, lemon, and C. macrophylla, and no transformation in Cleopatra mandarin, while EHA 105/p35SGUSINT produced much higher transformation in all cases, from sixfold in sour orange to 22-fold in lime. Although recovery of transgenic citrus plants was not the main objective of these experiments, transformation frequency provided by EHA 105/p35SGUSINT was so high that we were able to regenerate transgenic shoots from lime, lemon, C. macrophylla, and Cleopatra mandarin, just 6 weeks postinoculation. Since the chromosomal background from C58 as well as the T-DNA from p35SGUSINT are identical for the bacterial strains tested, the supertransformation ability of EHA 105/p35SGUSINT in citrus may be attributed to the vir region of its Ti plasmid pTiBo542 (Ghorbel et al., 2001b). Consequently, EHA 105 has been the most commonly used Agrobacterium strain in citrus transformation.
Bond and Roose 1998 published that C58 was more efficient than EHA 101/105 for transformation of Washington navel sweet orange epicotyl segments. However, EHA 101 and EHA 105 were indistinguishable in their analysis, being probably very different strains in terms of genetic transformation efficiency in this case, since kanamycin was used as selection marker of transgenic cells being EHA 101 also resistant to kanamycin. Yang et al. 2000 also reported that C58 was more efficient than EHA 105 for Rio Red grapefruit transformation, but their experiments were based in transient expression analyses (3 days after co-cultivation) with few explants. More recently, the same group has been using only EHA 105 for transformation of three different grapefruit genotypes including Rio Red (Rai, 2006).
As the supertransformation ability of EHA 105 was attributed to the vir region of pTiBo542, Ghorbel et al. 2001b investigated whether supplementary copies of vir genes could increase transformation frequency in citrus. The helper plasmid pCH30 (Hamilton et al., 1996) was introduced into C58 (pMP90)/p35SGUSINT and EHA 105/p35SGUSINT. C58 (pMP90) is a disarmed derivative of C58. p35SGUSINT is a binary plasmid carrying nptII an uidA with an intron, as selectable and reporter marker transgenes, respectively. pCH30 provides additional copies of virG from pTiBo542, so this allowed also to investigate the role of this virG in the supertransformation ability provided by this Ti plasmid in citrus. Transformation of citrus explants in vitro was carried out as described before, and stable transformation was tested 6 weeks after inoculation by performing GUS assays. Two different citrus species were chosen for carrying out these experiments: lime, which was efficiently transformed by EHA 105/p35SGUSINT, and lemon, in which transformation mediated by EHA 105/p35SGUSINT was rather low. C58 (pMP90)/p35SGUSINT produced extremely low transformation frequency in both citrus species. In lime, additional copies of virG from pTiBo542 increased transformation frequency induced by C58 (pMP90)/p35SGUSINT about 80-fold, reaching similar transformation frequency than that provided by EHA 105/p35SGUSINT. However, additional copies of virG from pTiBo542 in EHA 105/p35SGUSINT did not allow increasing transformation frequency. No further increase in transformation frequency was shown by introducing pCH32 (carrying virG and virE) instead of pCH30 in any of the two bacterial strains. In lemon, when pCH30 was introduced into C58 (pMP90)/p35SGUSINT, an increase in transformation frequency of sixfold was obtained, resulting in a transformation frequency similar to that provided by EHA 105/p35SGUSINT, and affording the possibility of regenerating transgenic plants with the use of this strain. Strikingly, pCH30 also enhanced the transformation frequency provided by EHA 105/p35SGUSINT in 1.7-fold. pCH32 provided a similar increase in transformation frequency as pCH30 for both the bacterial strains (Ghorbel et al., 2001b).
VirG is a transcriptional activator that specifically recognizes a 14bp DNA sequence, called the vir box, which is found in all vir gene promoter regions. The supervirulent phenotype of A281 in its infection to certain hosts has been correlated with higher expression of vir genes after induction. Ghorbel et al. 2001b have shown that extra supply of only virG from pTiBo542 greatly increased Agrobacterium-mediated stable transformation of several citrus genotypes, demonstrating the importance of vir induction, and mainly virG activation, in the supertransformation ability of this Ti plasmid in citrus. In addition, Ghorbel et al. 2001b have shown that extra copies of this virG could complement vir induction of pTiC58, providing also to C58 (pMP90)/p35SGUSINT the capacity to supertransform citrus. Looking back to our results on comparison of virulence induced by oncogenic strains in different citrus genotypes, there are genotypes for which C58 is as efficient as A281 for tumor induction, and cases in which A281 incites a too severe reaction at the wounds. In current experiments, Leandro Peña's group is finding citrus types that are severely affected by inoculation and co-cultivation with EHA 105, making very difficult further progress to induce shoot regeneration. For these citrus types, alternative A. tumefaciens strains become an almost indispensable need. Using a disarmed derivative of C58 carrying additional copies of virG is representing an excellent option in our hands (Peña, unpublished results).
Vir gene inducers, such as acetosyringone, wounded cell extracts, feeder cells, sugars, hormones, wounding, etc. have been extensively used to enhance genetic transformation frequency in many plants. The aim of these treatments was to stimulate virG activation and virA sensing. Acetosyringone is a phenolic compound produced during the wounding of plant cells that induces the transcription of the virulence genes of A. tumefaciens. Its beneficial role has been demonstrated in the genetic transformation of many plants, including some woody fruit species, as apple (James et al., 1993) and kiwifruit (Janssen and Gardner, 1993). It has being also widely used in citrus transformation, during Agrobacterium culture and/or during co-cultivation (Kaneyoshi et al., 1994; Bond and Roose, 1998; Luth and Moore, 1999; Yang et al., 2000; Molinari et al., 2004a), normally at 100–200μM concentration. However, its role as transformation enhancer was not investigated. Cervera et al. 1998c showed that its addition during co-cultivation increased transformation frequency twofold in Carrizo citrange explants, but when the medium rich in auxins was used for co-cultivation, its effect was not so clear (Cervera et al., unpublished results). Because of this, acetosyringone was not further used in Leandro Peña's lab. More recently, Almeida et al. 2003a used a co-cultivation medium supplemented with acetosyringone at 0, 100, or 200μM for transformation of epicotyl segments from Rangpur lime and Valencia and Natal sweet oranges. Transgenic shoots were only generated in absence of acetosyringone. It is possible that the effect of acetosyringone in citrus transformation is dependent on the genotype, explant type, and co-cultivation conditions.
2.2.3 Agrobacterium-mediated transformation of citrus embryogenic cells and callus
There are a few reports on the use of A. tumefaciens as vector for embryogenic callus transformation. Hidaka et al. 1990 tested two disarmed A. tumefaciens strains and co-cultivation of 3, 5, or 7 days for transformation of cell suspensions coming from embryogenic callus cultures. The bacterial strains were octopine LBA 4404 carrying a binary vector with the nptII marker gene, and the nopaline GV3031 harboring a cointegrate vector system with the hpt transgene as selectable marker. In general, transformation was very low but 3-day co-cultivation provided a higher number of cell colonies formed with both bacterial vectors. A major problem in the system was that citrus cells were very sensitive to both kanamycin and hygromycin selective antibiotics, since even concentrations of 10 and 20mgl−1, inhibited callus proliferation.
Li et al. 2002 successfully used the strain EHA 105 for transformation of embryogenic callus from Ponkan by immersion for 25min in the bacterial culture and 3-day co-cultivation in MT plus 100μM acetosyringone, in darkness. Transformation conditions were optimized for adapting this procedure to Valencia sweet orange, by preculturing the callus 4 days in MT liquid medium with 0.5gl−1 malt extract, 1.5gl−1 glutamine, and 50gl−1 sucrose. Almost no resistant callus was obtained if the co-cultivation medium lacked acetosyringone.
2.2.4 Less-used transformation systems: A. rhizogenes and particle bombardment systems
There is only one report on the use of each of A. rhizogenes and particle bombardment systems for citrus transformation. Yao et al. 1996 bombarded embryogenic callus cultures of Page tangelo with M-10 tungsten particles coated with plasmid DNA using the Biolistic PDS-1000/HE particle delivery system. Transgenic embryos were produced but most of them were abnormal and conversion to plantlets was generally unsuccessful. Although these results were not satisfactory, Leandro Peña and his co-workers still think that gene bombardment could be a promising transformation system, especially for those explant types that are highly sensitive to Agrobacterium inoculation/co-cultivation but have high organogenic/embryogenic potential.
The A. rhizogenes A4 agropine-type strain was used for transformation of internodal stem segments of in vitro-grown Mexican lime seedlings (Pérez-Molphe and Ochoa-Alejo, 1998). Transformation efficiency achieved was very high, but the aberrant phenotype, the integration of the T-DNA from the Ri plasmid provided to the transformants, has probably precluded its subsequent use for incorporation of transgenes of agricultural interest into citrus types. In spite of this, several authors have proposed the use of rol genes from the Ri plasmid as transgenes to produce dwarf P. trifoliata and citrange rootstocks (Gentile et al., 1998; Kaneyoshi and Kobayashi, 1999).
2.2.5 Optimizing A. tumefaciens and cell/tissue co-cultivation conditions: cell competence for transformation in citrus
As A. tumefaciens has been the most commonly used transformation vector for citrus, different research groups have attempted to establish proper infection and co-cultivation conditions with the aim of enhancing transformation frequency. Thus, there are as many protocols on citrus epicotyl segment transformation as laboratories working on this issue. For instance, bacterial inoculation time fluctuates between 5min (Molinari et al., 2004a) and 20min (Yang et al., 2000; Almeida et al., 2003a), bacterial concentration for inoculation varies between 4 × 107 (Peña et al., 1995b; Yu et al., 2002) and 5 × 108 (Kaneyoshi et al., 1994; Bond and Roose, 1998; Luth and Moore, 1999), co-cultivation time is usually of 2 or 3 days, and co-cultivation temperature varies between 19°C (Li et al., 2002, 2003) and 28°C (Luth and Moore, 1999). Very few papers compare different treatments and then recommend the best conditions for transformation of each citrus genotype, and even less reports investigate why a given treatment is better than others to enhance transformation.
Histochemical GUS-positive spots in cut ends from transformed epicotyl segments. (a) Computing the average number of GUS-positive events at the cut end of inoculated explants is very useful to determine optimal transformation culture media and conditions. (b) Upper view of the cut end of an explant revealing localization of sites of transgenic GUS expression in callus derived from cambial cells
In spite of the high number of studies showing organogenesis and attempting genetic transformation in citrus, little is known about how these two events together contribute to the success of the entire transgenic plant production process. The use of the uidA reporter gene allowed to localize competent cells for transformation in callus presumably formed from the cambium tissue of citrus explants in early steps after co-cultivation with A. tumefaciens (Peña et al., 1997; Cervera et al., 1998c). Furthermore, treatments favoring the development of such callus tissue, as co-cultivation in a culture medium rich in auxins and exposure of the explants to darkness during the first 2–4 weeks after bacterial inoculation, greatly increased transformation frequencies and consequently regeneration of transgenic shoots (Cervera et al., 1998c) (Figure 5a). Then, we decided to investigate cell competence and the role of phytohormones for transformation and regeneration of shoots from citrus explants. For this purpose, we used a highly responsive citrus genotype as Carrizo citrange and well-defined tissue culture conditions to perform a histological examination of morphogenesis from citrus explants after co-cultivation with Agrobacterium in different culture media: without growth regulators, with BAP at 1mgl−1, and with 2mgl−1 2,4-D, 2mgl−1 IAA, and 1mgl−1 2-iP. Moreover, we used flow cytometry to investigate the role of auxins in the co-cultivation medium as possible enhancers of transformation. Although in all culture media tested regeneration proceeded through indirect organogenesis, co-cultivation in media without phytohormones or with BAP at 1mgl−1 and subsequent culture in medium with BAP at 3mgl−1 promoted a faster differentiation response and bud formation. A conspicuous callus was formed from the cambium cells in explants cocultivated in the medium rich in auxins and bud differentiation did not occur during the first weeks after Agrobacterium-inoculation. This is consistent with the totipotent state of cambial cells that were able to rapidly respond to external stimuli during in vitro culture. Thus competence for regeneration and cell division were strongly related in citrus epicotyl segments. Interestingly, GUS-assayed explants showed that transgenic sectors were only localized in callus cells coming from the cambium, clearly indicating that the development of the cambial callus was also essential to obtain transformation from citrus explants. Therefore, cells competent for transformation and for regeneration were localized in the same callus tissue.
Flow cytometry analysis revealed that co-cultivation in the medium rich in auxins rapidly favored cell entry into the cell cycle because a higher rate of cells at the cut ends were actively dividing and duplicating their DNA after 2 days of culture in the medium rich in auxins. Thus, addition of auxins to the culture medium promoted active cell division and de-differentiation. This coincided with a much higher transformation frequency in the cut ends of such explants. On the other hand, a lower ratio of S-phase cells was found in the explants during co-cultivation in other culture media, and much lower stable transformation was obtained. Taken together, our data suggest that de-differentiation is crucial for transformation of citrus cells.
Another remarkable observation was that callus from the cambium and therefore stable transformation was preferentially produced at the basal end of the explants, probably reflecting an increase in auxin concentration at the basal ends due to the basipetal auxin transport. Then, it could be hypothesized that citrus cells at the explants would be highly responsive not only to an exogenous auxin supplement but also to endogenous auxin accumulation. Leandro Peña's group results demonstrated that auxins play an essential role in cell competence for transformation in citrus. The promotive effect of phytohormones in shifting cells to a competent state for transformation, especially in the case of auxins is well known. Leandro Peña's group observed that addition of BAP promoted cell division and rapid differentiation of buds, as shown by García-Luis et al. 1999, but most of these buds were escapes, indicating that cell division and callus formation were not enough to ensure efficient transformation. Only buds regenerating later, after more prominent callus formation, were transgenic. Leandro Peña's group results demonstrated that de-differentiation was required to shift citrus cells to a competent state for stable transformation, and that de-differentiation was especially triggered by the addition of auxins (mainly 2,4-D) to the co-cultivation medium and possibly by their endogenous accumulation in specific explant sectors. At the same time, co-cultivation in the medium, rich in auxins, could prevent early differentiation of citrus cells into meristemoids that would result in regeneration of escapes. Once transformation at high frequency was achieved in the proper co-cultivation medium, addition of BAP to the regeneration medium could promote differentiation of the transgenic events into buds and shoots. All these results together indicate that the proper combination of phytohormones in the co-cultivation medium shift the cells at the cut ends of the explants to a competent state for integrative transformation (Peña et al., 2004b). These conclusions have been later extended and applied to the genetic transformation of juvenile and mature internodal stem segments from many different citrus types (Ghorbel et al., 1999; Peña et al., 2004a; Rai, 2006; Peña et al., unpublished results).
2.3 Selection of Transformed Tissue
The nptII transgene, conferring resistance to aminoglycoside antibiotics as kanamycin by inhibiting protein synthesis, has been the most widely used selectable marker in citrus transformation and regeneration. However, the first reports on citrus transformation already indicated that kanamycin selection was not working efficiently to regenerate transformants. Vardi et al. 1990 showed that kanamycin did not provide a reproducible inhibition curve in Rough lemon protoplast transformation experiments. Therefore, they preferred to use paromomycin, another aminoglycoside antibiotic, over kanamycin, as the selective agent, because it inbibited growth of microcalli at 20mgl−1. In any case, a few whole plants were generated. Kobayashi and Uchimiya 1989 got transgenic sweet orange microcalli from protoplasts, but these did not develop further by using kanamycin at 25mgl−1 as selective agent. Hidaka et al. 1990 found that embryogenic callus lines from different sweet orange genotypes were highly sensitive to kanamycin and hygromycin even used at low doses.
When internodal stem and epicotyl segments coming from either in vitro or greenhouse-grown seedlings were used, kanamycin at 100mgl−1 did not control the regeneration of escapes (Moore et al., 1992; Peña et al., 1995a, 1995b; Gutiérrez et al., 1997). The number of escapes was higher than 90% of the regenerated shoots when the explants were inserted vertically in the culture media (Moore et al., 1992; Peña et al., 1995a; Gutiérrez et al., 1997), but this frequency was strongly reduced (to 45% in Carrizo citrange) when epicotyl segments were disposed horizontally in the media (Peña et al., 1995b). Transformation frequency in etiolated epicotyl segments from P. trifoliata was rather high, but even with this species, escapes were produced (16–45%) with kanamycin at 100mgl−1. When kanamycin concentration was raised to 200mgl−1, transformation efficiency became lower because high kanamycin dose inhibited the proliferation of transformed as well as untransformed cells (Kaneyoshi et al., 1994). The same effect was shown by Peña et al. 1997 in internodal stem segments of Mexican lime and by Cervera et al. 1998c in Carrizo citrange epicotyl segments. The aminoglycoside antibiotic geneticin was also tested at 10mgl−1 as an alternative to kanamycin, but it was toxic for transgenic shoot regeneration in Mexican lime (Peña et al., 1997). Another phenomenon apparently associated to the regeneration of escapes was the production of chimeric shoots at high frequencies, which were likely regenerating from transformed and nontransformed cells (Peña et al., 1995a, 1995b, 1997).
As attempts to raise concentrations of kanamycin and use of alternative aminoglycoside antibiotics failed, utilization of the reporter marker gene uidA (or gus gene from Escherichia coli), present in most transformation vectors used in the 1990s, for shoot screening and selection became the best option to enhance citrus transformation efficiency, because screening revealed transformation more efficiently than lethal selection. In this way, part of all shoots regenerating under a 100mgl−1 kanamycin regime was necessarily screened for histochemical GUS expression, and only the few positive ones were selected for whole plant production. Therefore, the uidA reporter transgene was used as a second selectable marker in most citrus transformation protocols. In addition, uidA was instrumental for developing appropriate inoculation, co-cultivation, and regeneration media and conditions. Computing the average number of GUS-positive events at the end of inoculated explants was useful to determine in different genotypes the optimal values for factors affecting transformation (Figure 5a). Moreover, in Leandro Peña's laboratory, uidA was very important to localize the sites of transgene expression in citrus explants in order to favor the regeneration of whole plants from such competent cells (Figure 5b) (Peña et al., 1997; Cervera et al., 1998c).
Another major step for the improvement of citrus transformation systems was the employment of the green fluorescent protein gene (gfp, from the jellyfish Aequorea victoria) as a screenable marker. In contrast to uidA and other reporters, the fluorescence emission of GFP only requires excitation of living cells by ultraviolet (UV) or blue light, which results from an internal p-hydroxy-benzylideneimidazolinone chromophore generated by an autocatalytic cyclization and oxidation of a Ser-Tyr-Gly sequence at amino acid residues 65–67 (Cody et al., 1993; Heim et al., 1994). It does not require either exogenous substrates or cofactors, thus their applications are not limited by problems in substrates' penetration. Moreover, GFP assays are not destructive, allowing performing in vivo monitoring of genetic transformation. Earlier attempts to stably incorporate the wt-gfp into citrus protoplasts and plants failed, probably due to the toxicity of the GFP protein to citrus protoplasts (Niedz et al., 1995). In Leandro Peña's laboratory, several different gfp gene versions were tested, and the so-called sgfp gene provided the most easily identifiable GFP expression in citrus explants. The sgfp gene is a re-engineered gfp gene sequence with the favored codons of highly expressed human proteins, designed to encode a peptide sequence identical to wild-type protein, combined with the replacement of the serine at position 65 with a threonine. It had given a high GFP expression in maize, tobacco, onion, and Arabidopsis cells and in stably transformed tobacco plants (Chiu et al., 1996). Leandro Peña's group first used it for Agrobacterium-mediated transformation of epicotyl segments from Carrizo citrange and internodal stem segments from Mexican lime and sour orange (Ghorbel et al., 1999). After 1 month in darkness, explants developed callus at the cut ends, which was formed from the cambium tissue. When this callus was examined under a stereomicroscope with 480nm-excited blue light, it exhibited yellow autofluorescence probably emitted by the walls of de-differentiated cells. In some callus tissue, red autofluorescence cell clusters were also visualized. Since chlorophyll fluoresces red at the wavelength used for GFP excitation, these clusters could be considered nontransformed differentiated cells initiating shoot regeneration. Green fluorescent sectors were also observed that were clearly distinguishable from the red and yellow ones. Green fluorescent cell clusters corresponded to transgenic events (Figure 6a). As each GFP molecule represents one fluorophore, high-level expression was detected in these cells. Furthermore, continuous monitoring of transformation events led us to observe that they always occurred in callus tissue formed from the cambium in all the three citrus genotypes analyzed. Thus, in vivo monitoring of Agrobacterium-inoculated plant tissues allowed us to localize competent cells for transformation (Figure 6b).
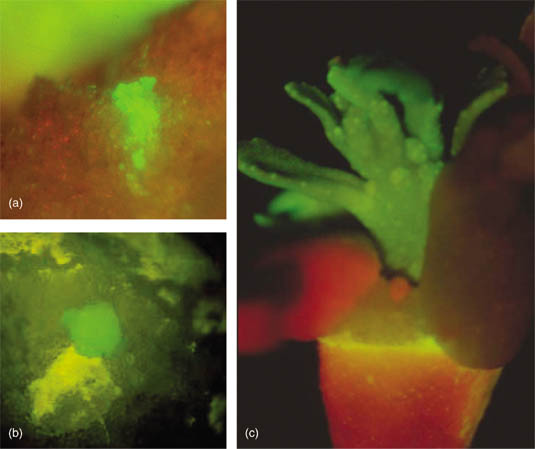
Use of gfp as reporter gene for the localization of competent cells and the recovery of transgenic citrus plants. (a) Green fluorescent cell cluster developed in callus tissue from the cut end of an explant. (b) Upper view of an explant showing a green fluorescent transgenic event at the callus formed from the cambium. (c) GFP expression permits a rapid and easy discrimination of transgenic (green) and escape (red) shoots
Mexican lime and citrange shoots started to regenerate from the explants after 1–4 weeks in the light. When they were excited at 480nm blue light, transgenic shoots showed a bright green fluorescence, whereas escapes and nontransformed control shoots appeared red, again because of the autofluorescence of the chlorophyll. GFP expression permitted a rapid and easy discrimination of transgenic and escape shoots of citrus (Figure 6c). Competition for growth between transformed and nontransformed shoots could be avoided eliminating the escapes soon after arising. In some cases, Mexican lime and citrange regenerated shoots showed sectorial expression of GFP. These plantlets were derived from transgenic and nontransgenic cells originating from one shoot. Transgenic chimeras could be recognized at a very early stage of development and tissue pattern expression could be followed during the whole life of the plant (Ghorbel et al., 1999).
GFP expression was usually detected in transgenic cells, buds, and shoots, without interfering with the transformation and regeneration of plants. All cells or tissues from leaves of the transgenic plants showed green fluorescence. However, fluorescence intensity varied among different parts of the plant, being higher in new growing leaves than in old ones. In old tissues, lower metabolic activity and chlorophyll accumulation partially masked the green fluorescence provided by GFP. Furthermore, differences in green fluorescence intensity could be observed associated to different GFP expression levels in apical leaves from independent transgenic lines. Therefore, GFP could be used as reporter of gene expression for early nondestructive identification and selection of transgenic buds or shoots expressing the highest levels of protein (Ghorbel et al., 1999).
Efficient monitoring by using GFP for early identification and rescue of transgenic buds could circumvent the use of antibiotic or herbicide marker genes to produce transgenic plants. Interestingly, green fluorescent shoots were regenerated from explants inoculated with Agrobacterium but cultured in a medium without kanamycin. These results opened up the possibility to produce transgenic plants without using selective agents. Nontransformed buds could be periodically eliminated to avoid competition with the transgenic ones to favor proper development of shoots only from the transgenic events and thus to increase the frequency of GFP-positive shoots regenerating in a medium without kanamycin selection (Ghorbel et al., 1999). This GFP system has also been used to monitor the process of somatic hybridization through protoplast fusion between a GFP-positive leaf parent and a nontransgenic callus donor, and to successfully localize and select hybrid colonies, callus, embryos, and plants (Olivares-Fuster et al., 2002). GFP-based screening/selection has been widely used for citrus protoplast transformation, since they seem to be highly sensitive to antibiotics even used at low concentrations. Following protoplast culture in liquid medium and transfer to solid medium, transformed calli could be identified through in vivo monitoring of GFP expression, physically separated from nontransformed tissue, and cultured on somatic embryogenesis induction medium (Fleming et al., 2000; Olivares-Fuster et al., 2003; Guo et al., 2005; Niedz et al., 2003).
Even with the use of reporter genes as uidA and gfp as efficient screenable markers, the number of nontransgenic regenerants and chimeras was high enough to become a problem in most citrus transformation systems. Because of this, we decided to investigate in detail the origin and frequency of escape regenerants in transformation of citrus explants, specifically in Carrizo citrange epicotyl and Mexican lime internodal stem segments. Several possibilities have been proposed to explain the regeneration of escapes: transient expression of the selectable marker transgene in many plant cells during the first transformation steps, selection of mutant plant cells resistant to the selective agent, endogenous nonspecific tolerance of plant cells to the selective agent, protection of the nontransformed cells from the selective agent by the surrounding transformed cells, and persistence of A. tumefaciens in infected tissues (Peña et al., 1995b). NPTII accumulation in leaves from 20 randomly selected GUS or GFP-positive plantlets of each genotype ranged from 1100 to 6000 and from 2900 to 9100ng NPTII/mg total protein in citrange and lime, respectively. No NPTII activity was detected in leaves from control plantlets regenerated from noninoculated explants cultured in regeneration medium without kanamycin. Taken together the lack of escapes from noninoculated explants cultured in selection medium, and the absence of endogenous NPTII activity in nontransformed regenerated control shoots, the hypothesis of endogenous nonspecific tolerance of citrus cells to kanamycin to explain the regeneration of escapes and chimeric shoots can be discarded (Domínguez et al., 2004).
To investigate the origin of GUS and GFP-negative/chimeric regenerants, citrange and lime explants were analyzed for GUS expression with the histochemical GUS assay, and for GFP expression under blue light, respectively, from a few weeks to 5 months after infection, when most shoots had already arose. In citrange, most GUS-negative/chimeric shoots regenerated close to or even from GUS-positive spots. In lime also, a very high rate of GFP-negative/chimeric regenerants was observed coming from GFP-positive transgenic events. This strongly suggested that protection of nontransformed cells from kanamycin by the surrounding transformed cells accounted for most of the escapes and chimeric shoots generated in both citrus genotypes. In fact, detailed observation of certain prominent transgenic events allowed us to observe that in many cases they were composed of mixtures of GUS or GFP-positive and GUS or GFP-negative cells. However, a considerable number of GUS and GFP-negative/chimeric regenerants arose from cut ends without any transgenic event indicating that factors other than protection of escapes by close transgenic cells should also be considered (Domínguez et al., 2004). It has been proposed that transient expression of the selectable marker transgene in many plant cells could play an important role in generation of this type of nontransgenic shoots during genetic transformation of tobacco leaf disks (Park et al., 1998). However, this is unlikely to occur in citrus because shoot regeneration is slow, starting from 1 month after co-cultivation.
Then Leandro Peña's group tested persistence of Agrobacterium in citrus explants. Nine months after co-cultivation, lime explants were subcultured to selection medium without cefotaxime and vancomycin for 3 months. During this time, 80–90% of the explants became contaminated by Agrobacterium overgrowth, as confirmed by polymerase chain reaction (PCR) analysis of the agrobacterial-like colonies. The remaining explants were analyzed for the presence of the bacteria at the cut ends by culture of basal sections in selective media and subsequent PCR for identification. In two experiments, still 49% and 12.5% of the explants cultured in selective medium contained persisting Agrobacterium cells. Interestingly, these frequencies reached 65% and 45% when the explants were cultured in enriched selective medium, indicating that a more sensitive analysis revealed a higher frequency of bacterial detection in the explants. Moreover, these frequencies could be even higher because nonculturable Agrobacterium cannot be detected using this method. After co-cultivation, citrus explants are transferred to a regeneration/selection medium containing the antibiotics cefotaxime and vancomycin to control Agrobacterium overgrowth. However, complete elimination of the bacteria seemed to be difficult mainly because the antibiotics used are bacteriostatic rather than bactericidal. It is also possible that resistance of the engineered Agrobacterium strains used to kanamycin could provide a selective advantage to bacterial cells over nontransformed plant cells and tissues in the in vitro culture medium. In any case, it is clear that the consistent presence of bacterial colonies resistant to kanamycin in certain tissues at the cut ends of the explants could detoxify the surrounding nontransgenic tissues and favor the regeneration of escapes. This could especially explain the regeneration of escapes in cut ends without any transformation event (Domínguez et al., 2004).
In addition, Southern blot characterization of individual propagations from GUS-positive shoots revealed that 12% were actually chimeras from either transgenic and nontransgenic events or from different transformation events, indicating that chimeric shoots resulted not only from the convergence of transgenic and nontransgenic cells as shown by reporter gene expression, but also from the union of different transgenic events (Domínguez et al., 2004).
These facts have important implications in the production of transgenic citrus plants. With the aim of preventing the generation of escapes and chimeras, strategies directed to avoid Agrobacterium persistence in plant tissues, and the use of alternative, preferably antibiotic-free positive selection marker genes that could not be expressed in the bacteria would be advisable. The use of alternative selective agents has been attempted. Li et al. 2002 used the phosphinothricin acetyl transferase (PAT) bar gene, providing resistance to glufosinate ammonium (active ingredient of the BASTA® herbicide), to transform Ponkan calli with A. tumefaciens and efficiently regenerate whole plants through somatic embryogenesis in culture media supplemented with Basta at 50mgl−1. Few escapes and no chimeras were generated with this transformation system. One year later, the same group (Li et al., 2003) also used the hpt gene, providing resistance to the antibiotic hygromycin, as selection marker gene for developing successful Agrobacterium-mediated embryogenic callus transformation of Valencia sweet orange. We have tested both bar and hpt as selectable markers for epicotyl and internodal stem segment transformation of several citrus genotypes. Initial regeneration experiments were performed in order to choose the appropriate hygromycin and basta concentrations to control regeneration from nontransformed explants. In the case of hygromycin, regeneration was impeded from a concentration of 10mgl−1 in Carrizo citrange epicotyl segments. A slight toxicity was, however, noticed at this and at lower concentrations, causing developmental anomalies in regenerated shoots. Results from tests using bialaphos as regeneration-controlling agent indicated that a concentration of 5mgl−1 was appropriate for citrus transformation. In transformation experiments, the use of any of both selective agents allowed to produce transgenic plants (Cervera et al., 2006; Cervera et al., unpublished results).
Most of the selectable markers genes used to date, including nptII, bar, and hpt, are based on the addition to the culture media of a substance that is toxic to untransformed cells. There is a concern that the transformation efficiencies are suboptimal with toxic substrates because dying untransformed cells may inhibit transformed cells from proliferating by secreting inhibitors or preventing transport of essential nutrients to the living transformed cells (Haldrup et al., 1998). Alternatively, the manA gene, which codes for phosphomannose isomerase (PMI), is an example of a conditional-positive selection system where the selection substrate is not toxic. In this system, the substrate mannose is unable to act as a carbon source for untransformed cells but it will promote the growth of cells transformed with manA. This system has been used for sweet orange transformation from epicotyl segments (Boscariol et al., 2003). Mannose used as the sole carbon source, at 73–112.3mM concentrations depending on the genotype, promoted transgenic shoot regeneration. Transformation efficiency achieved varied between 3% and almost 24%, but escape regeneration was not avoided and about 57% of the total regenerants were not transgenic.
Nonconditional positive selection systems do not require external substrates, yet promote the selective growth and differentiation of transformed material. An example is the isopentenyl transferase gene (ipt) from A. tumefaciens, which catalyzes the production of a precursor of several cytokinins that enhance shoot development by modifying the plant hormone levels endogenously. Leandro Peña's group have used this system in citrus transformation and compared it with nptII selection. An important characteristic of ipt used as positive selectable marker in genetic transformation of citrus was that ipt overexpression was sufficient to promote shoot organogenesis in citrus (Figure 7). Regeneration was comparable to that obtained by supplementing a cytokinin (BAP) to the regeneration medium. Moreover, ipt was a reliable positive selectable marker that permitted to select transgenic sweet orange shoots from internodal stem segments at the first stages of development, since they showed a clearly distinctive phenotype. In citrange epicotyl segments, ipt overexpression induced adverse pleiotropic effects in the explants and their regenerants, and transgenic selection was only possible after PCR analysis of the ipt transgene in the regenerated shoots or plantlets. These results suggest that citrange cells are highly sensitive to IPT accumulation, likely causing hormonal imbalances detrimental for development. Compared to kanamycin selection, the ipt system permitted to increase transformation efficiency in sweet orange about three times, but was much less efficient in Carrizo citrange (Ballester et al., 2007). Due to the induction of significant alterations in the morphology, development, and physiology of the transgenic plants, this marker had to be combined with a site-specific recombination and DNA removal system to generate ipt-free plants (Ebinuma et al., 1997; Sugita et al., 1999).

Isopentenyl transferase gene (ipt) as positive selectable marker in genetic transformation of citrus. ipt overexpression is sufficient to promote shoot organogenesis in citrus. Different phenotypes are exhibited by ipt-positive (a) and ipt-negative (b) Pineapple sweet orange shoots
2.4 Regeneration of Whole Plants
For regeneration of whole transgenic citrus plants through organogenesis and somatic embryogenesis, tissue culture media and conditions are similar to those fully established for several other biotechnological applications. However, these media are usually supplemented with antibiotics, which are toxic to most cells/tissues in culture, since only a few cells of those put in contact with the vector become transformed. In addition, gene transfer itself provokes important alterations in transformed cells and regenerated shoots or embryos. Therefore, the ideal regeneration medium should facilitate the development of buds or microcalli from transgenic cells, and at the same time avoid the generation of escapes. As escape regeneration is a major obstacle in all citrus transformation systems and with all marker genes tested to date, regeneration media and conditions have been adapted to favor the growth and development of transgenic events. Peña et al. (1995a, 1995b, 1997) proposed the cultivation of explant in darkness for 2–4 weeks in regeneration/selection medium after co-cultivation with A. tumefaciens. This enhanced not only transformation frequency, but also promoted the generation of large transgenic events able to regenerate whole transformed buds (Domínguez et al., 2004; Peña et al., 2004b). Its beneficial role for citrus explant transformation has been demonstrated in several laboratories (Cervera et al., 1998c; Yu et al., 2002; Almeida et al., 2003b; Boscariol et al., 2006). For some genotypes and in general for mature tissue transformation, the addition of NAA at different concentrations to the regeneration medium during cultivation in darkness strongly promoted the development of the transgenic events (Ghorbel et al., 2000; Almeida et al., 2003b; Rodríguez et al., 2008). Molinari et al. 2004a suggested that transfer of the explants after Agrobacterium co-cultivation to a medium without selective agent for a week, followed by 25mgl−1 kanamycin for 3 weeks, and then to 50mgl−1 kanamycin was optimal for regeneration of transgenic Swingle citrumelo plants from thin layer explants.
The most important problem of whole transgenic plant production in citrus has been the extraordinary difficulty found in rooting transgenic shoots and embryos, which seriously reduces transformation efficiencies. Probably, the only exceptions are P. trifoliata, grapefruit, and Swingle citrumelo, which could be rooted in MS supplemented with NAA (Kaneyoshi et al. 1994; Luth and Moore, 1999; Molinari et al., 2004a).
As an alternative method for the generation of whole transgenic plants, shoot tip grafting (Navarro et al., 1975) was introduced by the procedure of Peña et al. (1995a, 1995b). Small pieces of the shoots emerging from the explants were assayed for reporter marker activity and, when the result was positive, apical portions were grafted in vitro onto Troyer citrange seedlings. Rootstock preparation was as follows: Troyer citrange (or any other rootstock) seeds were peeled removing both seed coats, disinfected for 10min in a 0.5% sodium hypochlorite solution containing 0.1% Tween-20 and rinsed three times with sterile water. The germination medium was MS salts with 10gl−1 agar, pH 5.7. Seeds were sown individually in tubes and grown in dark at 27°C for 2 weeks. Troyer citrange seedlings were decapitated leaving 1–1.5cm of the epicotyls. The roots were shortened to 4–6cm and the cotyledons and their axillary buds were removed. Then, the regenerated shoot apical ends were placed on the top cut surfaces of the decapitated citrange epicotyls, in contact with the vascular ring, or when larger than 0.4cm, they were inserted into a lateral incision or in a vertical incision along the length of the epicotyl, starting at the point of decapitation (Figure 8). Grafted plants were cultured in a liquid medium composed of MS salts, 100mgl−1 m-inositol, 0.2mgl−1 thiamine-HCl, 1mgl−1 pyridoxine-HCl, 1mgl−1 nicotinic acid, 75gl−1 sucrose, pH 5.7. The cultures were kept at 25°C, 16h of photoperiod, 45μEm−2s−1 of illumination. Shoots with only 0.1cm in length could be used to regenerate transgenic plants following this protocol. A frequency of 100% successful grafts is usually obtained. Scions develop two to four expanded leaves, 3–4 weeks after grafting. A new grafting of the in vitro-grown plants on vigorous rootstocks in the greenhouse allows the rapid acclimatization and development of the plants. Grafted plants are kept in a shadow area and are covered with a closed plastic bag for about 1 month. Then the bag is progressively opened, and when the shoot starts to actively grow, plant is transferred to a greenhouse area with normal illumination. This system is routinely used in our laboratory and has permitted to recover whole transgenic plants from all citrus types tested, even in cases in which the transgene of interest induced malformations in the regenerated shoots (Fagoaga et al., 2005).
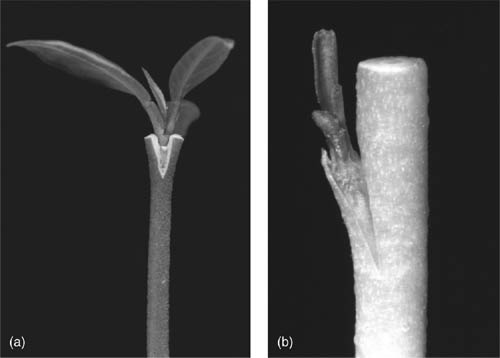
Apical portions from transgenic regenerated shoots are grafted in vitro on citrange seedlings. Regenerated shoot apical ends are placed on the top cut surfaces of the decapitated citrange epicotyls, in contact with the vascular ring, or, when larger than 0.4cm, they are inserted into a vertical incision along the length of the epicotyl, starting at the point of decapitation (a) or in a lateral incision (b)
The grafting system has been demonstrated to be reliable and widely applicable for whole plant production also in other laboratories and for many different citrus genotypes, including P. trifoliata and grapefruit (Kobayashi et al., 1996; Bond and Roose, 1998; Gentile et al., 1998; LaMalfa et al., 2000; Wong et al., 2001; Mendes et al., 2002; Yu et al., 2002; Almeida et al., 2003a, 2003b; Boscariol et al., 2003; Iwanami et al., 2004; Endo et al., 2005). It has been mostly used to recover whole plants from shoots regenerated through organogenesis, but also to regenerate plants from germinated somatic embryos (Fleming et al., 2000; Olivares-Fuster et al., 2003; Guo et al., 2005; Niedz et al., 2003). Moreover, this method could be applied to the recovery of transgenic plants from other woody plants, in which shoots are difficult to elongate and/or root, and escapes regenerate at high frequencies competing with the transgenic shoots.
Alternatively, for certain purposes, rooting of the transgenic shoots allow performing rapid analyses of the effect of the transgene of potential interest even in vitro when the new inserted trait could affect not only to the aerial part of the plant but also the roots (i.e., higher tolerance to salinity, modification of plant size or architecture, etc.). Although development of whole plants is slower and less efficient than performing in vitro grafting, rooting can be obtained by cutting 0.5–1cm regenerated shoots from the explants and transferring them to SRM supplemented with 0.3mgl−1 BAP for 7–10 days, and then to SRM medium supplement with 5mgl−1 indolebutyric acid. At least for Mexican lime and Fino lemon, roots develop within a 3–6 weeks period with 90–100% efficiency (Peña, unpublished results).
2.5 Incorporation of Transgenes of Interest Into Citrus
Although there are many reports on introduction of transgenes of potential interest into citrus, few of them describe how the expression of the transgene affects the phenotype of the modified rootstock or variety genotype. This is due in part to the low number of transgenic plants generated in some cases, to the fact that juvenile tissues were transformed in most cases and consequently fruit characteristics could not be evaluated yet, and also due to the difficulties of performing challenge assays against pathogens with woody plants.
In the first attempts to transform citrus, only marker genes as the selectable marker nptII and the reporter marker uidA were used. Moreover, constitutively expressed promoter and terminator sequences, as those from the 35S gene of Cauliflower mosaic virus (CaMV) or from the nopaline synthase (NOS) gene of A. tumefaciens, were predominantly used. Availability of a genetic transformation system for P. trifoliata (Kaneyoshi et al., 1994) allowed to efficiently incorporate the human epidermal growth factor (hEGF) (Kobayashi et al., 1996) and the rolC gene from A. rhizogenes (Kaneyoshi and Kobayashi, 1999) into this species. In their first paper, Kaneyoshi et al. 1994 used the uidA gene under the control of the 35S promoter, and under the control of the promoter from the rolC gene. As expected, the 35S promoter directed GUS expression in all leaf, stem, and root tissues of the transgenic regenerants. Interestingly, histochemical GUS expression was only detected in phloem cells when the rolC promoter was used. As the precise role of hEGF in vivo was unknown, the work of Kobayashi et al. 1996 only served to demonstrate that it was possible to express human bioactive peptides in transgenic trees. Eleven plantlets were obtained from 282 treated segments (4% transformation efficiency), and seven were regenerated into whole plants by grafting. The highest hEGF achieved was 113pgmg−1, which was considered low.
2.5.1 Tree performance
The following report by Kaneyoshi and Kobayashi 1999 was more interesting from an agricultural point of view since its purpose was to produce dwarf rootstocks able to impart dwarfism to the scion. The rolC gene was used for this purpose under the control of either the 35S promoter or its own promoter. Transgenic plants were produced with both constructs at high efficiency. Most transgenic lines were dwarf, and the degree of dwarfism differed among lines; 35S::rolC transformants were 40% shorter than controls, while rolC::rolC lines ranged from 10% to 120% shortenings. Six transformants (three from each group) were assayed for rooting of cuttings. Five of them rooted better and one rolC::rolC line (with the longest internodes) rooted similarly to controls. As next step, Kaneyoshi and Kobayashi 1999 planned to graft some citrus cultivars onto the transgenic plants and check their potential as dwarfing rootstocks, but no report has been published to date on this aspect. Gentile et al. 1998 regenerated transgenic plants of Tarocco sweet orange and Carrizo citrange with rolABC genes from A. rhizogenes. Although plant phenotypes were not characterized in detail, preliminary observations indicated that they grew with difficulties and showed morphological anomalies, as small and narrow leaves.
Fagoaga et al. 2007 have produced transgenic Carrizo citrange plants overexpressing a citrus gibberellin (GA) 20-oxidase cDNA (CcGA20ox1) in sense or antisense under the control of the 35S promoter to modify plant architecture. CcGA20ox1 is a key enzyme of gibberellin biosynthesis. Expression of the transgenes was assayed by Northern blot and Western blot analyses in both antisense and sense transgenic lines. In sense transgenic lines, the level of CcGA20ox1 protein was high and correlated with plant height and internode length. The leaf area was considerably smaller than that of the control plants. In antisense transgenic lines, down-regulation of CcGA20ox1 resulted in both shorter internodes and dwarfing with larger number of branches. No adverse pleiotropic phenotypic effects were observed in these plants. As expected, taller (sense) and shorter (antisense) phenotypes correlated with higher and lower levels, respectively, of active GA1 in growing shoots of the transgenic plants (Fagoaga et al., 2007). We are currently investigating whether antisense transgenic Carrizo citrange used as rootstock is able to reduce the size of nontransgenic scions. Potential reduction of scion plant stature by down-regulating GA20ox of a well known and widely used rootstock would provide a considerable benefit to citriculture by allowing higher planting density, easier management and fruit harvesting, thus reducing labor costs.
2.5.2 Higher tolerance to abiotic stresses
Soil salinity significantly limits citrus production in several areas worldwide. Carrizo citrange, considered an excellent citrus rootstock, is very sensitive to salt stress, which restrains its use in salty soils. We have successfully transformed plants of Carrizo citrange with the halotolerance gene HAL2 under control of 35S promoter (Cervera et al., 2000a). This gene was originally isolated from yeast and is implicated in salt tolerance mechanisms (Murguía et al., 1995). Plants showing higher transcription levels in Northern blot analyses were chosen to accomplish in vivo salt stress tolerance assays, by using transgenic plants as rootstocks for a sensitive citrus variety. However, transgenic lines did not show higher tolerance to salinity than controls.
The accumulation of proline represents a general response to stress in many organisms, including higher plants, exposed to environmental stresses such as water deficit, high salinity, high temperature, freezing, UV radiation, and heavy metals. Molinari et al. 2004b have incorporated a Δ1-pyrroline-5-carboxylate synthetase mutant gene (P5CS) from Vigna aconitifolia driven by the 35S promoter into Carrizo citrange plants, with the objective of increasing proline accumulation in all tissues and consequently enhancing tolerance to drought stress. In well-watered conditions, transgenic plants accumulated proline in leaves up to eightfold higher than in control plants. As compared to controls of the same age, no apparent differences in growth and morphology were observed in the transgenic Carrizo citrange plants after 5 months in the greenhouse. Ten days after water had been withheld, when water present in substrate was not available to the plant, there was an accentuated fall in photosynthetic rate and increase in the values of stomatal resistance in nontransgenic plants, while the transgenic plants showed comparatively minor changes in these two parameters. The values of stomatal resistance and transpiration during the experiment period showed that transgenic plants were able to support the applied water deficit stress. The adoption of well-established citrus rootstocks with enhanced drought tolerance could represent a promising strategy in maintaining productivity in citrus-growing regions with water deficit.
2.5.3 Rapid cycling citrus trees
Results of genetic improvement programs for citrus are very limited, due, among other reasons, to the extremely long juvenile phases of the trees, which prolong the time required to analyse late traits like fruit features. With the aim to accelerate their flowering time, we have transformed juvenile Carrizo citrange seedlings to constitutively express the Arabidopsis LEAFY (LFY) or APETALA1 (AP1) genes that are sufficient to promote flower initiation in Arabidopsis (Mandel and Yanofsky, 1995; Weigel and Nilsson, 1995). Chimeric genes with their coding regions fused to the 35S promoter were used. Expression of the 35S::LFY and 35S::AP1 transgenes was confirmed by Northern blot hybridization. In contrast to the development of regenerated nontransformed control plants, most of the 35S::LFY transgenic trees developed thin stems with a weeping growth habit and with a rapid reduction in the number and size of thorns. Leaf size was also reduced, and leaves displayed a variable degree of curling in different transgenic lines. The most extreme phenotypes were observed in transgenic plants showing a higher level of accumulation of LFY transcript in leaves. Flowering time was strongly accelerated in many of those plants, which initiated flowering in spring, 12–20 months after their transfer to the greenhouse. Transgenic trees carrying the 35S::AP1 construction displayed more normal growth than the 35S::LFY trees. Many plants showed rapid signs of phase change, evidenced by the reduction in the size and number of thorns. Furthermore, leaves displayed heart-shaped leaflets, typical of adult citrange trees (Peña et al., 2001).

Overexpression of APETALA1 from Arabidopsis in Carrizo citrange transgenic plants induces an early flowering phenotype. Transgenic APETALA1 plants flowering 6 months after sowing (four plants on the right) compared to a nontransformed control plant (plant on the left)
Both types of transgenic citrus plants produced fertile flowers and fruits as earlier as the first year, notably through a mechanism involving a dramatic shortening of their juvenile phase. Furthermore, expression of AP1, being as efficient as LFY in the initiation of flowers, did not produce any severe developmental abnormality. Both types of transgenic trees flowered again in consecutive years and their flowering response was under environmental control (Figure 9). In addition, sexual and nucellar-derived transgenic seedlings had a very short juvenile phase and flowered in their first spring, demonstrating the stability and inheritance of this trait (Peña et al., 2001). This opens the possibility to use independent AP1 transgenic plants as parents in crosses with nontransformed genotypes that would yield 50% of the progeny flowering and setting fruits in 1–2 years, thus providing the opportunity to evaluate fruit features very early and to rapidly advance generations. On the other hand, by retransformation of AP1 transgenic citrus plants it could be possible to rapidly test the effect of the expression of certain transgenes under flower organ or fruit specific promoters as a system to look for biotechnological strategies to develop seedless varieties, modify fruit color and aroma, or favor easy-peeling characteristics. Retransformation of AP1 Carrizo citrange plants has been successfully achieved in Leandro Peña's laboratory (Cervera et al., 2006). In addition, the 35S::AP1 construction has been incorporated into other Citrus genotypes and it also promoted early flowering and fruiting features (Cervera et al., unpublished results).
FLOWERING LOCUS T (FT) is another flowering time gene of Arabidopsis and is characterized as a floral pathway integrator. An FT homolog (CiFT) was found in the expressed sequence tag catalog of a cDNA library from the fruit of satsuma mandarin and its overexpression induced an early flowering phenotype in Arabidopsis. Endo et al. 2005 introduced CiFT under the control of the 35S promoter into P. trifoliata and generated transgenic plants that flowered in general in less than 8 months. Four out of six transgenic lines developed normal fruits with intact seeds. All lines of the 35S::CiFT plants had a leafy inflorescence architecture, in which flowers and leaves concurrently developed. In contrast, wild-type P. trifoliata plants usually develop solitary flowers in the axils of leaves. Differentiation of floral buds starts in early summer and is completed prior to the onset of winter dormancy. In the transgenic plants, the development of flowers was not interrupted by a dormant period. The tree shape of transgenic plants was dwarfed and branched in comparison with that of the controls, and the leaf shape was morphologically altered. Moreover, the leafy inflorescence was often accompanied with gradual changes of thorns into flowers. Nucellar and sexual progeny seedlings from these lines showed extremely early flowering, developing floral buds almost immediately after germination. Although CiFT induced many pleiotropic effects on plant growth and development, early flowering CiFT-P. trifoliata plants could be a helpful tool for functional genomics studies in citrus. It could be also very interesting to verify the effect of 35S::CiFT in Citrus genotypes.
2.5.4 Improvement of fruit quality
Li et al. (2002, 2003) reported the generation of Ponkan and Valencia sweet orange transgenic plants, respectively, through Agrobacterium-mediated transformation of embryogenic calli with a chimeric ribonuclease gene (barnase) derived from Bacillus amyloliquefaciens under the control of an anther tapetum-specific promoter (pTA29). The aim of the work was to produce pollen sterile transformants, and subsequently seedless fruit. More than 20 lines from each genotype were generated. Since transformants were juvenile, several years of cultivation are needed to evaluate possible male sterility. The same can be applied in part for Koltunow et al. 2000 who produced juvenile transgenic Mexican limes containing genes for decreased seed set. However, the juvenile period of Mexican lime is one of the shortest among citrus types. In spite of it, to our knowledge there are no published data on the phenotype of the mature plants and their fruits.
Costa et al. 2002 introduced several carotenoid biosynthetic genes under the control of constitutively expressed promoters into juvenile Duncan grapefruit (see Grapefruit for description of this work). Wong et al. 2001 introduced a 1-aminocyclopropane-1-carboxylate synthase gene (CS-ACS1) isolated from C. sinensis under the control of a double 35S promoter into P. trifoliata and Carrizo citrange seedlings in antisense orientation. Eight and 13 Southern blot-positive plants were generated, respectively. Two and seven of these plants, respectively, expressed the antisense messenger-RNA (mRNA) at detectable levels. Those transgenic lines producing higher levels of antisense mRNA were found to partially repress the increase of ACC accumulation after a chilling treatment. The authors speculated that the reduced level of ACC in transgenic citrus tissues following the chilling treatment could be useful to reduce the damages caused by chilling injury in citrus fruits. However, several years are needed to get mature plants producing enough number of transgenic fruits.
Guo et al. 2005 introduced a pectin methylesterase gene (Cs-PME4) isolated from sweet orange into Valencia sweet orange protoplasts. Cs-PME4 cDNA was cloned in sense between a double 35S promoter and a gfp (e-gfp) marker gene, so the promoter was controlling the expression of both cistrons. Several transgenic plants were recovered but all of them came from the same transformation event. In addition, the plants exhibited a slightly abnormal morphology, likely attributable to the gfp version used. Transgenic plants were juvenile and require years to flower and fruit. Then, it will be possible to investigate whether Cs-PME4 overaccumulation occurs in fruit cells. From a biotechnological perspective, it would be interesting to down-regulate Cs-PME4 with the aim of preventing juice cloud separation.
2.5.5 Resistance to pathogens
Phytophthora citrophthora is the most widely spread oomycete all over the citrus-growing areas and represents one of the major causes of crop losses. Constitutive overexpression of proteins involved in plant defense mechanisms is one of the strategies proposed to increase plant tolerance to fungal pathogens. P23 is a 23-kDa pathogenesis-related protein induced in tomato (Lycopersicon esculetum Mill. cv. Rutgers) plants when these are infected with citrus exocortis viroid and its antifungal activity has been shown in in vitro assays (Rodrigo et al., 1993). Fagoaga et al. 2001 have successfully produced transgenic Pineapple sweet orange plants with a chimeric gene construct comprising the coding region of the P23 gene under the control of the 35S promoter. Nine transgenic lines accumulated the P23 protein, as determined in Western blot analyses, and in general P23 accumulation levels were high in most lines. Transgenic lines constitutively expressing the P23 transgene were challenged with P. citrophthora by using detached bark and whole plant assays. One of them achieved consistently reduced susceptibility in both assays. This line showed plant survival rates higher than the control when whole transgenic plants were inoculated with fungal cultures, since only one of 10 inoculated plants died at the end of the 2-year assay, while 50% of the controls have died 6 months after inoculation. The exact nature of tolerance to P. citrophthora provided by constitutive tomato P23 (PR-5) expression in transgenic orange plants is unknown. These results provide evidence for the antifungal activity in vivo of the P23 pathogenesis-related protein against P. citrophthora and suggest that this may be employed as a strategy aimed at the engineering of Phytophthora disease resistance in citrus (Fagoaga et al., 2001).
CTV is the causal agent of the most important virus disease of citrus in the world. It produces two main field syndromes: common strains cause decline and death of most scion varieties grafted on sour orange rootstock, whereas highly virulent strains additionally cause stem pitting, stunting, low yield, and poor fruit quality of some varieties of sweet orange, limes, and grapefruits, regardless of the rootstock used. Several strategies have been used to engineer plant resistance to viral pathogens. Most are based on the concept of pathogen-derived resistance (PDR), which proposes that the introduction and expression in plants of viral sequences could interfere with the life cycle of the same or a closely related challenging virus, thus providing resistance to infection. Gutiérrez et al. 1997 produced a few transgenic plants of sour orange, Mexican lime, and Carrizo citrange with the p25 major coat protein gene from CTV. However, challenge analysis of these plants has not been reported to date.
Domínguez et al. 2002c generated more than 40 transgenic Mexican lime lines, 25 containing the p25 gene from the severe CTV strain T305, and 17 from the mild strain T317. Transgene integration patterns were usually complex, with almost half of the plants showing T-DNA truncations and a copy number of the p25 transgene ranging from one to six. Accumulation of p25 was detected in most of the transgenic lines (Domínguez et al., 2000). When 8–10 propagations of each transgenic line were graft- and aphid-inoculated with CTV isolates T305 and T300, respectively, two types of responses to viral challenge were observed: most lines developed CTV symptoms similar to those of the nontransgenic controls, but six of them exhibited resistance against the virus. This consisted of a fraction of plants, ranging from 10% to 33%, that were immune to CTV, with the rest showing a significant delay in virus accumulation and symptom onset in at least three consecutive flushes (about 1 year) after inoculation (Domínguez et al., 2002c). These results were reproduced with four of the six transgenic lines in an additional challenge experiments in which propagations were again graft-inoculated with CTV T305: three p25 resistant lines accumulated p25 at high levels, suggesting that a protein-mediated resistance mechanism was operative in these lines, whereas the fourth transgenic line, called SCP.15, showed almost undetectable p25 accumulation and consistent resistance. Moreover, this line exhibited silencing of the uidA transgene, used as reporter in the T-DNA of the transformation vector, as determined by the lack of histochemical GUS expression in leaf pieces. Post-transcriptional gene silencing (PTGS) of the uidA transgene was confirmed in Northern blot hybridization and nuclear run-on analyses (Domínguez et al., 2002a; Domínguez and Peña, unpublished results). When eight plants propagated from the SCP.15 line were graft-inoculated with CTV T305, two were immune to virus challenge and the uidA transgene remained silenced in their leaves along the 1-year-duration of the experiment, but the other six plants became direct antibody sandwich-enzyme-linked immunosorbent assay (DAS-ELISA) positive for CTV and started showing symptoms at the second or third flush postinoculation. Interestingly, infection of these plants was directly related with activation of GUS expression, indicating that CTV might encode a suppressor of gene silencing which could restore GUS expression in infected transgenic plants carrying the uidA silenced. This result also suggested that PTGS of the p25 transgene was involved in the propagations that remained immune to CTV. This was the first demonstration of PDR against a member of the genus Closterovirus in its natural host. In the frame of a collaboration project of the IVIA with the University of Hawaii (M. Melzer, S. Ferreira, and J. Hu) and the USDA (D. Gonsalves), seeds from the most promising p25-transgenic lines were sent to Hawaii in 2004 for a field trial to test the potential resistance of these plants to natural challenge in an area where severe CTV strains and the efficient vector Toxoptera citricida are predominant.
The p25 gene of CTV has also been inserted into sour orange, and transgenic plants accumulating or not the p25 protein have been generated (Ghorbel et al., 2000). However, resistance of these plants to tristeza decline was not evaluated because this syndrome is not usually reproduced under greenhouse conditions and a permit to transfer these plants to the open field could not be obtained yet. The inverted-graft test (Pina et al., 2005) was used as alternative. It consists on grafting the scion genotype onto CTV-infected sweet orange rootstocks in the greenhouse. Those genotypes that are resistant to tristeza decline are able to sprout and develop normally, while susceptible genotypes show serious difficulties in sprouting and usually abscise a few weeks after grafting. Unfortunately, none of the p25-sour orange transgenic lines resulted resistant to tristeza decline in this assay.
In an attempt to develop RNA-mediated resistance against CTV, Mexican lime plants were transformed with two untranslatable versions of the p25 gene from CTV isolate T305: (1) the full-length p25 gene modified by introducing two consecutive stop codons three nucleotides downstream the start codon, and (2) the 3′ half of p25 gene fused to the sgfp marker gene, both under the control of the 35S promoter. Of the 48 transgenic lines obtained, 35 harbored the untranslatable full-length p25 gene and 13 carried the truncated version, as revealed by Southern blot hybridization. Furthermore, run-on assays and cytosine methylation analysis showed that the p25 transgene was post-transcriptionally silenced in some of the lines (Domínguez et al., 2002a). After graft-inoculation with CTV T305, all transgenic plants developed symptoms, though some delay in virus infection and symptom attenuation was observed in the first two flushes postinoculation, especially in lines transformed with the full-length nontranslatable construct. However, in the third and following flushes, all transgenic plants accumulated CTV as the nontransgenic controls (Domínguez et al., 2002b).
Yang et al. 2000 reported production of Rio Red grapefruit transgenic plants containing an untranslatable version of the p25 coat protein gene from CTV and the Galanthus nivalis agglutinin gene, a plant-derived insecticidal gene.
Febres et al. 2003 transformed Duncan grapefruit with several CTV-derived sequences, including p25 from three different strains and a nontranslatable version of the same gene, p27, the replicase gene, and a 400bp segment of the 3′ end of the genomic RNA in either sense or antisense orientation (see Grapefruit for description of these works).
Olivares-Fuster et al. 2003 used two constructs derived from the 3′-UTR of CTV T36, under the control of the 35S promoter, to transform Itaborai sweet orange protoplasts. They were used in sense orientation and were able to transcribe the 393 and 742 3′-terminal nucleotides of the viral genome. Protoplast lines were challenged with CTV, and 4–5 days later viral replication was tested by Northern blot. Two of the clones showed reduced replication and one clone carrying the larger construct showed null replication. Unfortunately, attempts to regenerate whole plants from these clones were unsuccessful. Leandro Peña's group has used a construct derived from the 3′-terminal 550 nucleotides from CTV T36 to transform Mexican lime. It was engineered to express this fragment either in sense or in antisense orientation, under the control of the 35S promoter. More than 20 independent transgenic lines were generated with each construct. After propagation and challenge by graft-inoculation, none of them was immune to infection, though several plants showed delayed infection (Fagoaga et al., unpublished results).
The 3′-terminal gene of CTV codes for a 23-kDa protein (p23), which is an RNA-binding protein that contains a motif rich in cysteine and histidine residues in the core of a putative zinc-finger domain (López et al., 2000). This protein is involved in regulating the balance of plus and minus RNA strands during replication, with the zinc finger domain and the adjacent basic region being indispensable for asymmetrical accumulation of the plus strand (Satyanarayana et al., 2002). Considering its regulatory role in CTV replication, we decided to explore whether overexpression in transgenic plants of this protein could affect the normal CTV infectious process, we have produced more than 50 transgenic lime plants carrying the p23 gene from CTV T36, or a truncated version thereof, both types under the control of the 35S promoter. Constitutive expression of p23 induced phenotypic aberrations resembling symptoms incited by CTV in nontransgenic lime plants, whereas transgenic plants expressing the p23-truncated version were normal. The onset of CTV-like symptoms in p23-transgenic plants was associated with the expression of p23, and its accumulation level paralleled symptom intensity (Ghorbel et al., 2001a).
To determine whether expressing ectopically the p23 gene from a severe and a mild CTV strain induced similar phenotypic effects on Mexican lime, a second set of transgenic limes was produced using the p23 gene from either the severe strain T36 or the mild strain T317. Ectopic expression of the p23 gene induced in Mexican lime aberrations resembling viral leaf symptoms of similar intensity, irrespective of the pathogenicity of the CTV strain from which p23 was obtained, and this was correlated again with accumulation of p23 protein. Transformation with p23-T36 of other CTV susceptible citrus species, including sweet and sour orange, and of the CTV-resistant P. trifoliata, also led to CTV-like symptoms that were not visible when these plants were transformed with a truncated p23 version. The intensity of CTV-like symptoms in citrus species and relatives other than Mexican lime correlated with levels of p23 transcripts, but the p23 protein was barely detectable in these hosts. The higher accumulation of p23 in Mexican lime with respect to sweet and sour orange was also observed in nontransgenic plants inoculated with CTV, suggesting that even minimal levels of p23 cause deleterious effects in the latter two species. In contrast, transgenic expression of p23 in CTV nonhost Nicotiana tabacum and Nicotiana benthamiana species led to consistent accumulation of p23 without phenotypic aberrations. Altogether, these results indicate that p23 is an important CTV pathogenicity determinant that interferes with plant development specifically in citrus species and relatives (Fagoaga et al., 2005). In addition, p23 has been found to act as an RNA silencing suppressor in N. tabacum and N. benthamiana plants (Lu et al., 2004).
In course of the experiments to incorporate p23 into Mexican lime, 3 out of 60 lines carrying the p23 gene of CTV T36 and 2 out of 20 lines carrying that of CTV T317, were visually normal and developed as controls transformed with the empty vector or nontransformed (Figure 10). These five lines displayed characteristics typical of PTGS: multiple copies of the transgene and methylation of the silenced transgene as revealed in Southern blot analyses, low levels of the corresponding mRNA in Northern blots, and accumulation of p23-specific siRNAs as shown in ribonuclease protection assays. When propagations of these silenced lines were graft- or aphid-inoculated with CTV T308 and T300, respectively, some were immune: they neither expressed symptoms nor accumulated virions and viral RNA as estimated by DAS-ELISA and Northern blot hybridization, respectively. Other propagations were moderately resistant because they showed delayed expression of leaf symptom and attenuated stem pitting compared to the controls. The susceptible propagations, in addition to normal symptom expression and elevated virus titer, accumulated p23-specific siRNAs at levels significantly higher than immune or noninoculated propagations, and showed transgene demethylation (Fagoaga et al., 2006).

Engineering resistance to citrus tristeza virus (CTV). Nontransgenic control (left) and p23-silenced transgenic (right) Mexican lime plants graft inoculated with CTV. Transgenic plant shows resistance to viral infection while control plant shows CTV symptoms such as vein clearing and leaf cupping
A characteristic of the p23 transgene-mediated PTGS was that vegetative propagations from the same transgenic line showed different responses against CTV, with some propagations being immune and others susceptible to viral challenge. This variable response among clonal transformants carrying viral-derived transgenes indicates that factors other than the genetic background of the transgenic plant, such as environmental conditions or the developmental stage, play a key role in PTGS-mediated resistance. As mentioned above, Fagoaga et al. 2006 also observed a high accumulation of viral-specific siRNAs in CTV-inoculated nontransgenic (or transformed with the empty vector) Mexican lime plants, indicating a strong natural PTGS-mediated antiviral response in this host. These results indicate that most of the siRNAs detected in susceptible p23-transgenic plants come from degradation of viral RNAs, and that the resistance obtained in immune plants was likely triggered by the small amounts of the transgene-specific siRNAs existing before CTV inoculation. A connection can also be inferred between the high accumulation level of siRNAs in CTV-infected plants and cross-protection induced by mild CTV strains: in line with the accepted PGTS model (Hammond et al., 2000), the siRNAs derived from mild cross-protecting CTV strains could prevent or attenuate the subsequent invasion by a severe CTV strain through their incorporation into an RNA-induced silencing complex targeting for degradation the genomic and subgenomic viral RNAs.
More recently, Batuman et al. 2006 have engineered a construct consisting of the p23 gene plus 3′-UTR from CTV in sense and antisense, separated by the Castor bean catalase intron, under the control of the 35S promoter, which once expressed in transgenic cells should be folded into a double stranded (ds) RNA structure theoretically able to trigger PTGS. It was used to transform N. benthamiana and C. macrophylla. Integration and expression of the transgene was demonstrated by Southern and Northern blot, respectively. Seventy C. macrophylla transgenic plants coming from 35 independent lines were challenged with CTV by graft-inoculation, but only 9 lines showed a delay in viral infection. None showed durable protection against CTV. Nevertheless, transgenic lines accumulated transgene-derived mRNA usually at high level, and neither siRNA accumulation nor transgene methylation was characterized. We have used a similar construct to transform Mexican lime plants, selected those lines carrying a single copy transgene and accumulating siRNAs at high levels, and challenged 6–11 propagations from them with CTV by graft-inoculation. A variable number of propagations from some of these lines were fully resistant to CTV challenge (Cervera et al., unpublished results).
In summary, these studies demonstrate that PDR can be extended to a member of the family Closteroviridae and to its natural hosts. Whether transgenic citrus plants expressing CTV-derived sequences could be an efficient alternative to cross protection for controlling stem pitting CTV strains in the field, and whether resistance to tristeza decline could be incorporated to the sour orange rootstock, remains to be tested.
An alternative strategy to look for resistance against CTV could be using plant-derived resistance genes. General resistance to CTV has been found in P. trifoliata, and a resistance gene (Ctv) has been characterized and mapped (Gmitter et al., 1996; Mestre et al., 1997; Fang et al., 1998). Because of the complex genetics of citrus, it is extremely difficult to introgress this resistance gene into citrus varieties by conventional breeding. However, cloning of this gene is underway in several laboratories (Deng et al., 2001; Yang et al., 2003). A bacterial artificial chromosome (BAC) library developed from P. trifoliata, homozygous to Ctv, was used for a 1.2Mb genome walk spanning the region between Ctv-flanking markers. Sequencing of a set of four overlapping BAC clones in this region, using shotgun sequencing and resolution of their ends by sequencing of additional BAC clones and their use as anchors, further localized Ctv to a 282kb region, comprising 22 predicted genes (Yang et al., 2003). Sequence analysis of the Ctv locus in this region identified 61 SSRs, which were used to further narrow down the locus in the Poncirus genome to 121kb, comprising 10 genes. Each of the 10 genes in this region has been individually cloned in Agrobacterium-based binary vector and used to transform susceptible Ruby Red, Rio Red, and Duncan grapefruit varieties in Eric Mirkov's laboratory (Rai, 2006). Although results from the CTV challenge experiments are still preliminary, transgenic lines carrying and expressing either of the 10 candidate genes were susceptible to CTV infection, suggesting that more than one gene in the locus is involved in resistance to CTV or that the role of other genomic loci has been overlooked (see also Grapefruit).
Citrus mosaic virus (CiMV) is a serious constraint for citrus production in Japan. Infected trees grow poorly and often develop ringspot symptoms on the fruit, which drastically reduce their commercial value. Iwanami et al. 2004 reported the incorporation of the coat protein gene from CiMV into P. trifoliata, the main citrus rootstock in Japan. More than 30 transgenic lines were produced and characterized by Southern blot. Attempts to detect coat protein immunoreaction in the transgenic plants by Western blot failed. More than 20 transgenic lines were propagated and mechanically inoculated with the virus. One line showed resistance to virus challenge (7.1% infection, compared to 65.1% infection in controls at 60 days postinoculation) while the other lines showed responses ranging from susceptibility to more moderate resistance at the same time period. There are plans to transfer the most promising lines to the field and challenge them against the virus under natural conditions.
Citrus canker, caused by the bacterium X. axonopodis pv. citri, is one of the most important diseases in several citrus areas, and there are no ways to control it other than accomplishing very expensive eradication programs in regions where limited number of foci are periodically identified, or applying massive doses of chemicals that only make a partial control and cause adverse effects in the environment. Attacins belong to a class of antimicrobial peptides, which are secreted by several insect species into the haemolymph in response to bacterial infections. Transgenes encoding attacin precursors have been used to reduce susceptibility to Erwinia species in transgenic pear, apple, and potato plants, but the mechanism of resistance is still unknown. Boscariol et al. 2006 introduced the attacin A gene (attA) from Tricloplusia ni under the control of a double 35S promoter into Hamlin sweet orange seedlings. Interestingly, the transgene-derived protein carried a native signal peptide that secreted it to the apoplast, as it was demonstrated by transiently expressing attA-gfp and attA-uidA fusion constructs in onion cells. Propagated plants from eight Southern and Northern blot-positive lines were spray-inoculated with a 106CFU/ml canker bacterial suspension and incubated in a growth room set at 27°C during 1 month, when disease severity was assessed by calculating diseased leaf area in 15 young leaves per transgenic line. Seven lines showed a significant reduction in susceptibility to citrus canker, and in two of them the reduction was of 55% and 60% compared to the control.
Citrus blight is a devastating disease of unknown etiology, which causes general decline of trees in hot and humid areas. A pathogenesis-related (PR) protein of about 12kDa can be detected in trees affected by blight, and it is not found in citrus trees with other disorders and diseases. The p12 gene was isolated from a cDNA library from roots of a tree with citrus blight and used in sense or antisense, under the control of the 35S promoter, to transform Carrizo citrange plants. Stable integration was confirmed by Southern blot, and expression was verified by Northern blot and by Western blot (in sense plants). Accumulation of the p12 protein in sense plants did not provoke any symptom or developmental abnormality, suggesting that this protein is not involved in symptom expression. The precise role of p12 in citrus blight requires further investigation (Kayim et al., 2004).
2.6 Stability of Transgene Expression and Phenotype; and Effects on Growth, Yield, and Quality
A major requisite for evaluating the validity of genetic transformation technology in improvement programs is the stability of the modified genome and the stability of transgene integration and expression over long periods of time, especially in vegetatively propagated and long-lived perennial fruit crops. However, reports on transgenic stability in citrus are almost nonexistent, and most works are focused in introducing a transgene of potential agricultural interest into a given genotype and evaluating the phenotype of the regenerating plants only after few months of growth in the greenhouse and normally just pertaining the trait, which is tentatively modified.
However, somaclonal variation induced during the in vitro culture phase has been extensively reported in greenhouse experiments and field trials of transgenic plants. It has been used in citrus as a strategy to generate genetic variation that could be potentially useful in improvement programs (Grosser et al., 2002), but somaclonal variation could also affect plant phenotypes producing abnormal morphologies, which has been correlated with genomic changes as chromosomal deletions and alterations in ploidy. In addition, expression of transgenes can be affected by numerous causes, as transgene loci number, genomic context of the integrated loci or position effects, abnormal configurations of the integrated T-DNA, or even environmental conditions that may contribute to differences in expression between plants with unlikely integration patterns.
Cervera et al. 2000b maintained a collection of 70 transgenic citrange plants grown in 50l-pots in a screenhouse in order to investigate, for a period of 4–5 years, (1) the origin of morphological variants in the transgenic population, (2) how factors related to T-DNA integration, regeneration process and expression of transgenes may be influenced by transformation conditions, (3) whether transgenes are stably integrated and expressed over long time periods in citrus plants grown under natural environmental conditions, and (4) whether correlation between integration patterns and transgene expression could be established in these plants (Cervera et al., 2000b).
Transgenic plants used for this analysis were generated earlier (Peña et al., 1995b; Cervera et al., 1998c). Briefly, A. tumefaciens EHA 105 containing the binary plasmid p35SGUSINT was used as vector for transformation of epicotyl segments from in vitro-grown seedlings. Two co-cultivation media were used: in the first case it contained BAP at 1mgl−1 as growth regulator (type 1), and in the second, co-cultivation was performed in the medium rich in auxins (2mgl−1 2,4-D, 2mgl−1 IAA, and 1mgl−1 2-iP as phytohormones) (type 2). All the transgenic plants were confirmed as nptII- and uidA-positive by Southern blot analysis. First of all, isozyme analysis allowed to confirm that the same banding pattern was found for all the samples, which corresponded exactly to the pattern shown by the female parent from which the starting seeds were taken. These results indicated that no zygotic plants had been recovered from our transformation experiments and that all the plants were nucellar, maintaining the Carrizo citrange maternal genotype, with the exception of differences in transgenes integration loci.
Four phenotypic variants were detected among the transgenic population. Several morphological features made these plants distinguishable from the maternal phenotype: they developed thicker and broader leaves, with darker green color, and usually with broader petiole wings, and showed a slower growth. Ploidy tests of the whole plant collection demonstrated that all the plants in the population were diploid except these four plants, which were tetraploid. Transgenic tetraploids could have been originated either by a process of polyploidization during in vitro culture or more likely by the regeneration of transgenic shoots from tetraploid plant tissue, since more than 10% of the Carrizo citrange seedlings germinated in vitro are tetraploid.
Somaclonal variation may be common after exposition of explants to auxins, which is used as stimulus for cell de-differentiation, division, and consequently callus induction. This de-differentiated state makes cells more likely to integrate foreign DNA (Peña et al., 2004b) but at the same time more predisposed to genomic alterations. Nevertheless, we did not find phenotypical differences between plants of type 1, obtained after transformation on a culture medium without auxins, and plants of type 2 that were obtained after transformation on a culture medium rich in auxins and thus after regeneration through a prominent callus phase.
The follow up of the population of transgenic plants by means of histological and fluorimetric GUS analyses confirmed the stable expression of the uidA transgene in all the plants over a period of 4–5 years. Patterns of expression were comparable for each line in successive histochemical analyses, although differences of 40% on measured values could be detected in fluorimetric assays, making the quantification of transgene activity over years very difficult. These fluctuations in time could be attributed to the developmental and physiological state of the plants. Almost one third of the population exhibited a typical pale blue staining pattern, recurrent in histochemical analyses performed during several years that correlated with very low values of expression measured by fluorescence analyses. These plants seemed to be undergoing a process of transgene silencing. A correlation between the pale blue color in leaf pieces and PTGS phenomena in citrus was corroborated in subsequent experiments (Domínguez et al., 2002a, 2004). Any transgenic plant with complete silencing was not detected among the population, which may be reasonable since the recovery of transgenic plants was performed after a histochemical GUS assay of regenerating shoots, and only those showing a detectable GUS blue staining were shoot-tip grafted and allowed to progress into whole plants. This selection scheme was possibly delimiting the recovery of plants in which total silencing phenomena could be occurring.
The most frequent number of T-DNA inserts was one and two, but plants having possibly more than six uidA copies were also found. According to the data from Southern blot analyses, it is suggested that at least 35% of the analyzed plants had integrated multiple T-DNA rearranged inserts at one locus. A significant tendency to low uidA expression levels was confirmed for transgenic citrus plants with more than two T-DNA copies, but levels of expression were highly variable for plants having only single copy. Our data agree in general with the results described by other authors, since although it has been frequently reported that single copies of a transgene are generally more stably expressed than multiple or rearranged insertions, a clear-cut correlation has not been found. Copy number, position effects, and organization of a given insert could account as a whole for the highly variable levels of expression displayed by the transgenic plants.
To investigate more deeply the actual incidence of transgene silencing in citrus, we decided to use a population of transgenic Mexican lime plants regenerated under uidA/nptII selection (type A), and a second one regenerated without marker selection but confirmed as transgenic by PCR (type B) (Domínguez et al., 2002a). Irrespective of the recovery scheme used, most of the transformants (at least 59%) showed T-DNAs arranged as tandem repeats. The high frequency at which these complex structures were found, and their configuration as direct repeats (DRs) or inverted repeats (IRs), either on the right border (RB) or on the left border (LB), supports the hypothesis that the T-DNA is made double stranded prior to integration and that repeats are formed by extrachromosomal homologous recombination.
Regeneration of transgenic limes under nonselective conditions resulted in the production of plants with silenced transgenes at high frequency. Furthermore, silencing affected all the transgenes of the T-DNA in all silenced lines. When organization of T-DNA was analyzed in silenced and nonsilenced lines, it was observed that IRs were exclusive of silenced transformants, establishing a clear-cut correlation between both the phenomena. About 30% of the lines regenerated under nonselective conditions were silenced, whereas none of the lines recovered after nptII and uidA selection showed transgene silencing. These results indicated that under selective conditions only transgenic events expressing the marker genes could be detected, thus precluding the possibility of recovering transgenic plants with silenced transgenes along the whole T-DNAs. Moreover, more than 5% of the regenerants obtained without marker selection were actually transgenic plants, opening the way for the production of citrus transformants without marker genes by PCR screening.
Gene silencing in citrus was further investigated by Domínguez et al. 2004. In this report, all Mexican lime regenerants obtained after kanamycin selection that resulted either GUS-positive or GUS-negative were analyzed by Southern blot. Interestingly, one-fourth of the GUS-negative plants were not escapes but transgenic plants showing uidA silencing, indicating that the transformation efficiency was underestimated when it was based in reporter marker gene expression.
Although genetic instability is usually a nondesirable trait in transgenic plants, Trainin et al. 2005 proposed the use of genetic transformation as a tool to induce mutagenesis in citrus by transposon tagging that could be used to identify mutants in genes of interest. Unless silenced, an intact transposon introduced into a plant will keep transposing and generating mutations for years converting transformed trees into mutation machines. With this purpose, Trainin et al. 2005 transformed Duncan grapefruit plants with a construct consisting of the uidA gene controlled by the 35S promoter but with the Ac transposable element cloned between the promoter and the marker gene, so GUS activity would result only if Ac excision occurred. However, this system had major drawbacks because transposition could not be controlled that caused in most cases tiny sectors if the transgenic plants, making detection of any mutant phenotype very difficult.
To investigate the effects of transgene incorporation and expression on tree growth and development, and fruit yield and quality, it is necessary to perform field trials. Unfortunately, there are only two field trials with citrus trees so far in which genetically modified plants are growing in the field over years under diverse environmental conditions. A release of transgenic citrus plants under controlled field conditions is being performed at the IVIA (Valencia, Spain) since 1997. The release site is located in an experimental field with an area of 1638m2. There are 130 trees, including 16 transgenic plants of Pineapple sweet orange, 16 transgenic plants of Mexican lime, and 16 transgenic plants of Carrizo citrange. There are two plants from eight independent transgenic lines for each case. In all cases, the transgenes integrated were 35Spro::uidA::NOSter and NOSpro::nptII::NOSter, providing GUS expression and resistance to kanamycin, respectively. In addition, there are 8 nontransgenic control plants from each of the species and an external border of 58 nontransgenic trees of Clemenules clementine (Figure 11).

Release of genetically modified citrus plants under controlled field conditions at the IVIA. (a) Schematic diagram of the trial. There are 130 trees, including 16 transgenic plants of Pineapple sweet orange (Pi), 16 transgenic plants of Mexican lime (Ml), and 16 transgenic plants of Carrizo citrange (Cc) (2 plants from 8 independent transgenic lines numbered from 1 to 8; gray circles). In addition, there are 8 nontransgenic control plants from each genotype (black circles). There is also an external border of 58 nontransgenic trees of Clemenules clementine (CN; white circles). (b) Photograph taken 10 years after the field trial was established. It shows the Pineapple sweet orange row (left), the Carrizo citrange row (centre), and the Mexican lime row (right). Most proximal and surrounding trees correspond to the Clemenules clementine border
The purpose of the release is to investigate (1) morphological and phenological characteristics of the transgenic trees, (2) expression of the transgenes in different tissues and organs, (3) stability of the transgenes, (4) transmission of the transgenes to the progeny, and (5) possibility of transgene dispersal through the pollen to nontransgenic monoembryonic citrus trees (Clemenules clementine). The trial was approved by the Spanish Ministry of Environment (permit Nr. B/ES/96/15) and was in accordance with Article 9 of Directive 90/220/EEC of the European Union. This was the first release in the world of transgenic citrus plants to the field. Until now, transgenic plants are morphologically and phenologically normal, as nontransgenic controls, and transgenes are stably expressed over different seasons and in different plant tissues and organs. Tree phenology and fruit quality have been evaluated during three consecutive years for each tree. Phenological calendar was made by observation and description of phenological stages of development every 2 weeks. Fruit analysis is being made once a year when fruit is ripe and full colored. The parameters analyzed to determine fruit quality are weight, volume, caliber, color, acidity, Brix, juice weight, juice volume, % solid weight (juice weight/fruit weight) and maturity index (Pons and Peña, unpublished results).
A second field trial is being performed in Weslaco (Texas, USA) with Rio Red grapefruit transgenic plants (see Grapefruit). In addition, seeds from Mexican lime transgenic plants carrying a CTV-derived transgene encoding the major coat protein gene p25 were sent to Hawaii in 2004 to carry out a field trial to test the potential resistance of these plants to CTV under natural challenge conditions, as part of a collaboration project of the IVIA with the University of Hawaii (M. Melzer, S. Ferreira, and J. Hu) and the USDA (D. Gonsalves).
2.7 Specific Regulatory Measures Adopted
The European Union currently regulates the activities performed with genetically modified organisms (GMOs) through two basic Directives: Directive 98/81/CE, on the confined utilization of GM microorganisms, including transgenic plants, and Directive 2001/18/CE (repealing the above-mentioned Directive 90/220/EEC) on the deliberate release into the environment of GMOs. Several other regulations have been adopted later, as those related to GMOs in human food and animal feed, other on the labeling and traceability of GMOs, or other on cross-border movement of GMOs.
Both these European Directives were incorporated to the Spanish legislation through the Law 9/2003 on the confined use, deliberate release, and commercialization of GMOs. Regulation 178/2004 approved and established the general framework for the development and execution of the Law 9/2003. In Spain, competencies on confined utilization and deliberate release rely on Regional Governments, and commercialization entirely depends on the National Government. At the practical level, confined utilization of transgenic citrus plants, either in laboratories, phytotrons, or special greenhouses (those in which transgenic pollen release is prevented), is usually considered at the type 1 level (lowest risk in the scale of 1–4) by the competent authorities. If this is the case, it only requires a notification of the activities that are being carried out but does not need any specific authorization by the competent regulators. Regarding deliberate release of transgenic plants, much more detailed information is requested in any submitted notification, including data on the genetically modified plant (identity of the recipient, description of modified traits, type of genetic modification, etc.), information relating to the experimental release (purpose of the release, geographical location, size, etc.), potential environmental impact of the release, description of any measures taken by the notifier for the control of risks including isolation designed to limit dispersal, and planned field trials designed to gain new data on the environment and human health impact of the release (where appropriate). Notifications are publicly available. In Spain, there is a Biosafety National Committee, which is a technical board mostly composed of well-recognized independent experts that recommends to the competent National and Regional Authorities on approval or rejection.
It is important to point out that the Article 4 of the Directive 2001/18/CE says that “Member States and the Commission shall ensure that GMOs, which contain genes expressing resistance to antibiotics in use for medical or veterinary treatment are taken into particular consideration when carrying out an environmental risk assessment, with a view to identifying and phasing out antibiotic resistance markers in GMOs, which may have adverse effects on human health and the environment. This phasing out shall take place by the December 31, 2004 in the case of GMOs placed on the market and by December 31, 2008 in the case of GMOs authorized for deliberate release.”
In Valencia region, the legislation on confined utilization and deliberate release of GMOs was approved in 2006, meaning that during the precedent 8 years Leandro Peña's group could not submit any notification on these issues because there was no competent authority in our region. From now on, we are preparing five new notifications for deliberate release of transgenic citrus trees, which have been modified in disease resistance, tree performance, and fruit quality aspects.
3 Future Road Map
3.1 Expected Products
There are citrus areas seriously threatened by diseases caused by bacteria. It is the case of Huanglongbing, induced by the bacterium Candidatus L. asiaticum, which has impeded the development of citrus industries in Southeast Asian countries. It is also a limiting factor for increasing citrus productivity in China. The bacterium affects all citrus types making trees unproductive, and there are no means of efficient control other than almost permanent insecticidal treatments to fight against the psyllid vector. Candidatus L. asiaticum and the apparent variant Candidatus Liberibacter americanus were first detected in Sao Paulo state (Brazil) in 2004 and an eradication program was started 1 year later. In about 1 year, more than half million affected trees have been removed. Candidatus L. asiaticum was also found in Florida (USA) in 2005 and the disease is spreading from south to north without control. In this area, the situation is even worse due to the impossibility to eradicate citrus canker, caused by the bacterium X. axonopodis pv. citri, and affecting all economically important varieties cultivated in the region. Therefore, looking for resistance mainly against Liberobacter spp. but also against X. axonopodis pv. citri is a major priority for the most important citrus industries in the world. Probably, the only opportunity for getting durable resistance against these diseases could come from the incorporation of transgenes able to efficiently protect the most relevant variety and rootstock genotypes grown in these areas from the bacteria and/or their vectors.
There are other pests and diseases caused by other bacteria, viruses, fungi, nematodes, etc. that are not so serious but limit production and fruit quality in certain citrus areas depending on the pathogen. Among them, much effort has been invested in attempting to incorporate transgenic resistance to CTV into citrus genotypes used as rootstocks and varieties. It can be predicted that this will continue as an important objective, and that strategies based on either PDR or plant-derived resistance will be further investigated.
Since markets of developed countries demand fresh fruit of increasing quality and with less agrochemical treatments, and also better and richer juice, more research will focus in understanding genetic control of metabolic pathways regulating carotenoid, flavonoid, limonoid, and monoterpene/essential oil biosynthesis with the aim of modifying fruit color and aroma, increasing vitamin content and reducing bitterness. In the same way, a better knowledge of citrus maturation and of determinants of sugar and acid content is important to attempt modulation of these traits in transgenic fruits. Seedlessness is another trait that has been successfully achieved in annual crops, and similar strategies could be used to afford this in citrus. Most strategies related to transgenic improvement in citrus fruits would require the use of tissue-specific promoters.
Considering that citrus trees are composed of scion and rootstock genotypes and the lack of current acceptance of transgenic foods by the consumer, it is logical to think that genetic modification of rootstock traits will be emphasized in the near future. In this sense, increasing tolerance to abiotic stresses (salinity, drought, etc.), to rootstock diseases (CTV, Phytophthora spp., nematodes, etc.), and adapting tree architecture to managing schemes requiring less labor and land, could become research areas of major interest.
In spite of the increasing number of laboratories working on development of genetic transformation systems for citrus and on the search for potentially useful transgenes derived from widely diverse source organisms, the lack of basic knowledge on citrus and citrus pathogens biology and the difficulties of working with genetically complex tree species, make very difficult to produce new improved transgenic genotypes of real agricultural importance.
3.2 Addressing Risks and Concerns
To our knowledge, the only transgenic citrus field trial in which environmental risk is being assessed is that performed at the IVIA, and researchers are exclusively investigating the frequency of transgenic pollen dispersal. Since 2001, the number of transgenic seeds expressing the uidA gene has been yearly assessed in fruits produced by the nontransformed monoembryonic Clemenules clementine border trees surrounding the transgenic trees. Clementines are self-incompatible and parthenocarpic, meaning that they can produce fruits without seeds. Seeded fruit results only in case of cross-pollination. As border trees are very close to the transgenic trees (3–6m), these are the most probable pollinators of clementine flowers. Even under this situation, the frequency of GUS-positive seeds is being less than 1% year by year (Pons and Peña, unpublished results).
In addition, there are characteristics of citrus biology and cultivation that should be strongly considered when environmental risks are assessed in transgenic field trials, and that depends on the citrus area of the world and the transgenic genotypes that are going to be evaluated. Commercial citrus varieties are propagated vegetatively by grafting of well-known genotypes onto well-known rootstocks. In our Mediterranean conditions and considering the citrus genotypes used, it is not possible that transgenic plants could become weeds. There are no wild citrus species and relatives in Europe, so there are no possibilities of compatible interactions between transgenic and wild plants. The situation would be completely different in Southeast Asia regarding this issue.
Citrus cultivars grown in some citrus areas are sexually compatible with many transgenic citrus genotypes that would be desirably produced. Under natural conditions, cross-pollination between transgenic lines and cultivated genotypes would be theoretically possible in many cases. Pollination in citrus is exclusively performed by insects, bees being the most successful pollinators. In areas where citrus is commercialized as fresh fruit, most varieties are sterile or self-incompatible, but the former are usually cross-compatible, leading to seed development. As presence of seeds in the fruit drastically reduces its price, affecting possible commercialization, cross-pollination in citrus is usually prevented by farmers by using different cultural practices and treatments. Most citrus species are parthenocarpic. In contrast, in areas in which fruit industry is based on juice production, cross-pollination and presence of seeds is not a matter of concern. In any case, if cross-pollination occurs, transgenes will only be expressed in the seed, which is never consumed and is not used for propagation of varieties. In the incidental case that transgenic seedlings could germinate in an orchard, they could be removed by farmers as it is usually done with any citrus seedling germinating in any orchard in most citrus areas. Moreover, these seedlings would never flower before being removed because citrus seedlings are juvenile and thus, they need several years to start flowering.
With the aim to assess the potential risk of horizontal gene transfer between GM-citrus plant material and food-associated bacteria, Weiss et al. 2007 investigated the effect of conditions required for orange juice processing on the stability of DNA from transgenic Pineapple sweet orange. Results showed that genomic DNA from orange juice suffered degradation within 2 days of storage, indicating that current standard industrial procedures to pasteurize orange juice as well as its acidic nature caused a strong degradation of genomic DNA below sizes reported to be suitable for horizontal gene transfer.
3.3 Expected Technologies
The primary function of genetic transformation in citrus is the development of new improved varieties and rootstocks based on otherwise genotypes of excellent quality but deficient in one or a few characteristics. However, genetic transformation has been usually attempted and applied to a restricted list of economically important genotypes. In this sense, there are a few or no reports on transgenic satsuma and clementine mandarins, navel and Pera sweet oranges, Eureka and Lisbon lemons, Rangpur lime or sour orange. More importantly, most of the work on citrus transformation has been performed with juvenile material, which will need many years to start flowering and fruiting and several years more to fully lose the juvenile characteristics. To gain a real profit from genetic transformation as a tool for citrus improvement, development of efficient transformation systems for mature tissues becomes an obligate task. Currently, Leandro Peña's group works almost exclusively in mature citrus transformation, but few laboratories are using this approach. It could be claimed that juvenile material is highly responsive and that it could be used at least to investigate gene function, but as the main target of citrus genetic improvement for the industry of most citrus-growing areas is usually the fruit, transformation of mature plants is essential even for functional genomics studies. We developed a general basic procedure for genetic transformation of mature Pineapple sweet orange and are adopting it to many other citrus genotypes of interest. The mature transformation system highly relies on the use of plant material in excellent sanitary and ontological state, which is cultivated under extremely clean and very well controlled environmental conditions. Even with this, contamination of the plant material with saprophytes during the in vitro culture phase is still a limitation of the procedure. Other than this, transformation efficiency is still low for many genotypes of interest and more effort should be put in improving it.
Most transgenic improvement projects directed to fruit genetic modification depend on the use of tissue/organ-specific promoters. Moreover, appropriate transgene expression levels at specific developmental stages and under various environmental induction conditions could be ideal for certain improvement strategies aimed to increase disease resistance or higher abiotic stress tolerance. Rapid and reliable systems to evaluate regulatory sequences are required, especially for fruit tissues. Transient transformation procedures through either A. tumefaciens vectors or particle gun systems could be good alternatives to stable transgenic expression for testing promoter elements. Transient transformation could also be useful to test heterologous transit peptides that would direct the transgenically expressed protein to a given subcellular target site.
RNA interference-inducing hairpin vectors, in which target sequences are expressed simultaneously in sense and antisense (Watson et al., 2005) are excellent tools to knock down putative genes of unknown function and thus decipher their possible role in citrus growth and/or development. They could be used for both genetic improvement projects and delineation of basic gene functions. At the same time, new transformation vectors are being designed, as those minimizing transgene expression fluctuations through the use of transgene flanking matrix attachment regions (MARs) (Allen et al., 2005), those precluding integration of plasmid vector sequences of prokaryotic origin that flank transgene expression cassettes (Kuraya et al., 2004), or those allowing integration of large DNA inserts containing multiple genes, such as BIBAC (Binary-BAC) and TAC (transformation-competent artificial chromosome) vectors (Hamilton et al., 1996; Liu et al., 1999), to facilitate positional cloning and functional analysis of linked genes and for engineering complex metabolic pathways.
A European Union Directive forbids specifically the presence in transgenic plants of transgenes conferring resistance to antibiotics used for medical or veterinary purposes, due to concerns related to possible horizontal transfer of these genes to gastrointestinal bacteria. Although this is not affecting directly to nptII, it has been proposed its substitution by using only reporter marker genes or using herbicide resistance genes as alternatives for transgenic selection. In any case, the presence of marker genes in GMO foods prevents their public acceptance. In plants propagated by seeds, it is possible to remove the marker gene once transgenic plants have been recovered, by means of co-transformation with different transformation vectors and segregation of marker genes from the gene of interest in the transgenic sexual progeny. This is not feasible in vegetatively propagated plants and trees that possess very long juvenile periods.
An alternative comes from the use of the manA gene that encodes PMI (Haldrup et al., 1998). When mannose is added to the plant tissue culture medium, it is transformed to mannose-6-phosphate, which cannot be metabolized by plant cells. Transgenic cells expressing manA could be able to transform mannose-6-phosphate into fructose-6-phosphate, which would be used by plant cells as carbon source. Therefore, transgenic cells accumulating PMI could have a clear advantage (positive selection) over nontransformed cells, when explants are exposed to a tissue culture medium containing mannose and deprived of any other carbon source. This system has already been successfully used for juvenile sweet orange and Carrizo citrange transformation (Boscariol et al., 2003; Ballester et al., 2008).
Another attractive possibility is offered by the multiautotransformation (MAT) vector system that combines positive selection, using the ipt gene, with a site-specific recombination and DNA removal system that generates marker-free plants (Ebinuma et al., 1997; Sugita et al., 1999). The ipt gene is located in the Ti plasmid of A. tumefaciens and encodes the enzyme ipt, which catalyzes the production of a precursor of several cytokinins (Ebinuma et al., 1997). Cytokinins are widely used to stimulate organogenesis in many cultured plant tissues, including citrus. The second interesting element of the MAT vector is the site-specific recombination system R/RS from Zygosaccharomyces rouxii (Sugita et al., 1999), in which the recombinase R removes the DNA fragment placed between two recognition RS sites from the transgenic cells after transformation. In this vector, RS sites flank both the ipt marker and the R recombinase transgenes. After site-specific recombination and excision of the DNA fragment between RS sites, ipt marker-free transgenic plants may be obtained. Ballester et al. 2007 have successfully used this system in sweet orange and Carrizo citrange and are currently further exploring their possibilities to generate marker-free transgenic citrus plants that carry only the transgene of interest (Ballester et al., 2008).
A third possibility is regeneration without selection and screening of all regenerants through PCR analysis. Using this system in Mexican lime, more than 5% of the regenerants resulted to be transgenic (Domínguez et al., 2002a, 2004). We are further exploring this procedure with other citrus genotypes.
Transgene stacking could have an outstanding interest in citrus genetic improvement programs because it would allow incorporating a second transgene in an already improved transgenic line. More transgenes could be pyramided into the transgenic line showing the desired phenotype for the first and second transgenes and so on. The main advantage of this approach is that it is possible to check individual expression and phenotype conferred by each transgene before proceeding to the next transformation round. However, because of the long juvenility of citrus, the process can take extremely long time if more than 2 or 3 transgenes have to be introduced. Alternatively, we have used AP1-transgenic plants for evaluating the possibilities of transgene stacking in citrus. AP1 overexpression extremely shortens the juvenile period in citrus (Peña et al., 2001). AP1-Carrizo citrange nucellar seedlings were successfully retransformed with a gfp vector and GFP-positive transgenic plants flowered and set fruits within 1 year after sowing, as AP1-Carrizo citrange controls (Cervera et al., 2006; Cervera et al., unpublished results). This could be then a valuable material and strategy to attempt functional genomics studies related to aspects of fruit and flower development by using reverse genetics. Furthermore, it can be used as a tool to rapidly evaluate putative fruit-specific promoter elements by stable transgenic expression. In addition, it could be an excellent tool for genetic improvement because transgene expression and phenotype evaluation related to modification of fruit characteristics could be readily achieved in less than a couple of years. However, for this purpose the AP1 strategy should be combined with a gene removal system, as the R recombinase/RS target sequence mentioned above or other similar recombinase system, to eliminate the AP1 transgene once it has fulfilled its early fruiting promotive function.
Recombinase systems represent a very interesting option for many different purposes in citrus transformation, because they could allow removal of marker genes and removal of transgenes of interest once their presence is not needed anymore in the plant (Dale and Ow, 1991). Moreover, they have been used to site-specific insertion of transgenes at specific loci by homologous recombination (Srivastava and Ow, 2002). However, this system and others proposing homologous recombination in the nuclear genome have still important limitations, related for instance to unexpected chromosomal rearrangements, unintended recombination, and generally very low efficiency (Gilbertson, 2003). Alternatively, chloroplast transformation through homologous recombination represents a feasible technology not only to get stable and uniform transgene expression, but also to achieve very high transgene product accumulation since it permits the introduction of thousands of copies of foreign genes per plant cell. Furthermore, transgene dispersal through pollen is avoided since the chloroplast is maternally inherited in most plants. The main obstacle of this approach is that efficient chloroplast transformation is restricted to very few plant species (Daniell et al., 2005).
3.4 Intellectual Property Rights (IPR), Public Perceptions, Industrial Perspectives, Political, and Economic Consequences
Genetic improvement through transformation requires active research in many fronts, including plant biology and physiology, phytopathology, biochemistry, molecular biology, genetics, and genomics, plus a good knowledge of the crop and its problems and the support of the industry to be able to develop new genotypes of real importance.
Multinational companies are able to cover all these aspects and concentrate in a few crops and improvement in a few traits to develop new products so important as for instance insect-resistant and glyphosate-tolerant corn, cotton, and soybean varieties. Their research lines are not only planned to decipher biological problems and publish their results in scientific journals but mainly to discover new patentable tools for genetic improvement of genotypes that will become also patentable or registrable and consequently will be part of their property. Many strategies for transgenic plant improvement are based on genes or sequences and regulatory sequences as well as on procedures for transformation or for marker elimination already patented by big companies. In contrast, most of the research on citrus transformation is being performed by small laboratories from academic or agricultural institutions, depending basically on Ph.D. students, with low-funded 3-year projects, without any patent policy, with very low support from the local citrus industry, and requiring very expensive facilities. In this context, it is easy to speculate that any future important development on citrus transgenic improvement will come from a company interested in this technology or from a committed public institution involved in citrus research and with big support from the industry, able to support the elevated costs of maintaining international patents and of mandatory biosafety tests required by the current legislation of most countries.
However, it is difficult for private companies to invest in transgenic improvement of citrus now, because public perception in developed countries (especially in Europe and Japan) about GM-food is in general very negative and there are no signs of change of this situation in the coming future. It could be proposed to work on transgenic rootstock improvement, until scion improvement is accepted by the consumer. In any case, it remains to be seen whether fruit from a tree composed of a transgenic roostock and a nontransformed variety is considered transgenic or not by the legislators and, more important, by the consumers. It is sad to see that more than 40 years after the first use of genetic engineering and 20 years after the generation of the first transgenic crop plants, the general public is unable to understand the benefits of the technology, and prefers to believe in catastrophic and unreasonable opinions from people with clearly defined political interests than in commitments and integrity of scientists working in public institutions. It is a matter of time that people will realize that genetic transformation is an excellent technology for crop improvement, in many senses much better and safer than other commonly used strategies. It is hard to predict nowadays whether public will need years or decades to accept these GM foods. In the meantime, genetic transformation is probably the most efficient approach to make reverse genetics in citrus to investigate gene function and thus to gain better understanding in metabolic processes and plant–pathogen-environment interactions. This will be essential for the future of citrus genetic improvement. In addition, the transgenic technology will be necessarily improved and most of the current concerns and risks on pollen dispersal, presence of marker genes, unintended transgene silencing, etc., will likely be overcome by new scientific and technological developments.
4 Acknowledgments
The authors wish to thank J.A. Pina, C. Ortega, and A. Navarro for their excellent technical assistance. This research was supported by grants from the Ministerio de Educación y Ciencia (MEC-CICYT), Instituto Nacional de Investigaciones Agrarias, and Generalitat Valenciana, and is currently being granted by project AGL2006-03673/AGR.
- AFLP
- amplified fragment length polymorphism
- BAC
- bacterial artificial chromosome
- BAP
- 6-benzylaminopurine
- BIBAC
- Binary-BAC
- CAPs
- cleaved amplified polymorphic sequences
- CTV
- Citrus tristeza virus
- CaMV
- Cauliflower mosaic virus
- CiMV
- Citrus mosaic virus
- Cs-PME4
- pectin methylesterase gene
- DAS-ELISA
- direct antibody sandwich-enzyme-linked immunosorbent assay
- DRs
- direct repeats
- FT
- FLOWERING LOCUS T
- GMOs
- genetically modified organisms
- IAA
- indole-3-acetic acid
- IPR
- Intellectual Property Rights
- IRs
- inverted repeats
- LB
- Luria Broth
- LB
- left border
- MARs
- matrix attachment regions
- MAT
- multiautotransformation
- MEC-CICYT
- Ministerio de Educación y Ciencia
- NAA
- α-naphthalene acetic acid
- NOS
- nopaline synthase
- P5CS
- Δ1-pyrroline-5-carboxylate synthetase mutant gene
- PAT
- phosphinothricin acetyl transferase
- PCR
- polymerase chain reaction
- PDR
- pathogen-derived resistance
- PEG
- polyethylene glycol
- PMI
- phosphomannose isomerase
- PR
- pathogenesis-related
- PTGS
- Post-transcriptional gene silencing
- RAPD
- random amplified polymorphic DNA
- RB
- right border
- RFLP
- restriction fragment length polymorphism
- RGCs
- resistance gene candidates
- SCAR
- sequence-characterized amplified region
- SNPs
- single nucleotide polymorphisms
- SRM
- shoot regeneration medium
- SSR
- single sequence repeat
- TAC
- transformation-competent artificial chromosome
- TCS
- tomato cell suspension
- T-DNA
- transfer-DNA
- UV
- ultraviolet
- attA
- attacin A gene
- cat
- chloramphenicol acetyltransferase
- gfp
- green fluorescent protein gene
- hEGF
- human epidermal growth factor
- hpt
- hygromycin phosphotransferase
- ipt
- isopentenyl transferase gene
- mRNA
- messenger-RNA
- nptII
- neomycin phosphotransferase II
- rol
- root loci



