Enhancement of the complement activating capacity of 17-1A mAb to overcome the effect of membrane-bound complement regulatory proteins on colorectal carcinoma
Abstract
Adjuvant immunotherapy with 17-1A mAb directed against colorectal carcinoma is found to be effective in patients. However, 52 % of the patients treated with mAb 17-1A showed recurrence within 7 years. This high recurrence rate might be due to inhibition of complement activation by membrane-bound complement regulatory proteins (mCRP). The effect of these complement regulatory proteins might be reduced by blocking mCRP, or be overcome by activating more complement at the tumor cell membrane. In this study the complement-activating capacity of the 17-1A mAb was enlarged by conjugating it to cobra venom factor (CVF) or C3b. The most important C3 regulatory protein, CD55, was blocked using a bispecific mAb directed against the 17-1A / Ep-CAM antigen and CD55. Up to a 13-fold increase in C3 deposition was observed due to 17-1A-CVF and 17-1A-C3b, as compared to 17-1A. CD55 was shown to partly inhibit complement activation by these conjugates. The effect of the bispecific anti-17-1A / Ep-CAM*anti-CD55 mAb was compared with 17-1A conjugates with CVF or C3, and bispecific mAb were shown to be equally or more efficient in complement activation than the 17-1A-CVF or 17-1A-C3b conjugates. Therefore, 17-1A conjugates and anti-17-1A / EpCAM*anti-CD55 bispecific mAb may be promising immunotherapeutic agents for patients with colorectal cancer.
Abbreviations:
-
- Ep-CAM:
-
Epithelial cell adhesion molecule
-
- mCRP:
-
Membrane-bound complement regulatory proteins
1 Introduction
Colorectal carcinoma is the third leading cause in cancer death among men and women 1. In spite of adjuvant chemotherapy after surgical resection of the primary tumor, outgrowth of distant metastases is difficult to prevent. Adjuvant monoclonal antibody (mAb)-mediated immunotherapy may provide an effective method to prevent or reduce the spread of tumor cells. These mAb activate the classical pathway of complement activation, what can result in direct complement-mediated lysis as well as attraction and activation of leukocytes at the tumor site.
Immunotherapy with complement activating mAb to treat solid tumors has not been successful yet 2. Treatment of smaller tumor masses such as micro-metastases, which may occur after resection of the primary tumor, might be more successful. Indeed, a significant decrease in recurrence of colorectal carcinoma was observed when immunotherapy with 17-1A mAb directed againstthe 17-1A-antigen / epithelial cell adhesion molecule (Ep-CAM) 3, was applied after resection of the primary tumor 4. However, still 52 % of these patients showed recurrence of the disease after 7 years, suggesting that protective mechanisms prevent an efficient elimination of mAb-opsonized tumor cells. One of these mechanisms may be the expression of membrane-bound complement regulatory proteins (mCRP) on tumor cells 5 – 10.
The most important mCRP on tumor cells are CD46, a cofactor for factor I-mediated cleavage of C3b and C4b, CD55 which accelerates the decay of C3 and C5 convertases, both inhibiting the formation of the chemoattractants C3a and C5a and the deposition of iC3b on the cell surface, and CD59 that inhibits the formation of the membrane attack complex, thereby preventing direct complement-mediated lysis 11.
Several strategies can be deployed to overcome the effect of mCRP. The inhibitory effect of mCRP can be partly overcome by activating an increased amount of complement. To achieve this, cobra venom factor (CVF) or C3b can be conjugated to mAb 12, 13. CVF is a functional and structural homologue of complement component C3b that activates the alternative pathway of complement and can form stable C3 and C5 convertases in human serum. In contrast to CVF, C3b is not resistant to the soluble complement regulatory protein factors H and I and lacks the complement-depleting effect of CVF. Increased amounts of C3b or iC3b on the tumor cell membrane as a result of these conjugates may lead to an increase in complement-dependent cellular cytotoxicity (CDCC) and complement-dependent cytotoxicity (CDC).
In addition to overwhelming the effect of mCRP by massive complement activation the function of these mCRP can be blocked. To achieve tumor cell-specific inhibition of mCRP, bispecific mAb directed against a tumor-associated antigen and a mCRP can be used 14, 15. When CD46 or CD55 is inhibited in its function, an increased deposition of C3b and iC3b might occur, resulting in an increased inflammatory reaction at the tumor site.
In this study 17-1A mAb (anti-Ep-CAM) were used as a model, to investigate the effect of CVF or C3b conjugates and bispecific mAb. It was investigated whether conjugation of CVF or C3b to 17-1A mAb in vitro resulted in an increased activation of the complement system. In addition, the effect of these conjugated mAb was compared to the effect of a bispecific mAb directed against the tumor-associated antigen 17-1A / Ep-CAM and CD55.
2 Results
2.1 Expression levels of mCRP and 17-1A / EP-CAM
The expression levels of mCRP and 17-1A / EP-CAM were determined for seven colorectal carcinoma cell lines by flow cytometry. 17-1A / Ep-CAM expression was determined with both the low-affinity 17-1A mAb (IgG2a) and the high-affinity 323 / A3 mAb (IgG2a) (Table 1). These mAb are directed against competing epitopes on the Ep-CAM antigen 3. With the high-affinity 323 / A3 mAb, a high Ep-CAM expression was shown on LS180, SW1116, T84 and SW837. A relative low Ep-CAM expression was observed on CaCo-2 and SW1463 cells. Colo 320 was the only colorectal cancer cell line lacking Ep-CAM. All cell lines expressed mCRP. In general, CD55 was expressed at a lower level than CD46 and CD59. The highest expression was observed for CD59. From these seven cell lines, two were selected on the basis of their different levels of Ep-CAM expression: SW837 with a high and CaCo-2 with a low Ep-CAM expression, to investigate the effect of mAb conjugates and bispecific mAb on complement activation.
|
Cell line |
17-1A / Ep-CAM |
CD46 |
CD55 |
CD59 |
|
|||
|---|---|---|---|---|---|---|---|---|
|
|
(17-1A mAb) |
n |
(323 / A3 mAb) |
n |
|
|
|
n |
|
LS180 |
334 + / − 68a |
3 |
1003 + / − 304 |
3 |
25 + / − 51 |
22 + / − 9 |
154 + / − 54 |
7 |
|
SW1116 |
n. d. |
− |
991 |
1 |
92 |
31 |
61 |
1 |
|
T84 |
286 |
1 |
858 + / − 67 |
4 |
97 + / − 19 |
19 + / − 9 |
286 + / − 102 |
4 |
|
SW837 |
238 + / − 16 |
3 |
812 + / − 184 |
6 |
111 + / − 16 |
27 + / − 12 |
139 + / − 31 |
6 |
|
SW1463 |
n. d. |
− |
387 + / − 187 |
3 |
91 + / − 28 |
12 + / − 1 |
93 + / − 25 |
3 |
|
CaCo2 |
119 + / − 6 |
3 |
380 + / − 112 |
5 |
84 + / − 24 |
119 + / − 30 |
294 + / − 69 |
5 |
|
Colo 320 |
0 + / − 1 |
3 |
0 + / − 1 |
3 |
17 + / − 3 |
11 + / − 3 |
76 + / − 32 |
3 |
- a) Expression levels were determined by flow cytometry as described in Sect. 4.7 and expressed in MESF values (× 104).
2.2 17-1A-CVF conjugates
To examine whether the complement activating capacity of 17-1A mAb could be enhanced, conjugates of 17-1A and CVF were made. The 323 / A3 mAb was, due to its high affinity, used as a positive control for complement activation. When the amount of C3 deposited on the tumor cell membrane was compared between SW837 and CaCo-2 cells opsonized with 17-1A-CVF or 17-1A, a 4- to 13-fold increase in C3 deposition was observed, respectively (Fig. 1 a). When investigating complement-mediated lysis, CaCo-2 cells with the low Ep-CAM expression were lysed by neither 17-1A, 17-1A-CVF nor 323 / A3 (Fig. 1 b). For SW837 cells a clear increase in lysis was observed, comparing 17-1A-CVF with 17-1A, although the amount of lysis did not reach the amount caused by 323 / A3.
Because the level of C3 deposition and lysis caused by 17-1A and 17-1A-CVF were low compared to 323 / A3, the effect of mCRP on inhibiting complement activation was investigated on SW837 and CaCo-2 cells. Indeed, blocking of either CD46 or CD55 with anti-CD46 or anti-CD55 mAb resulted in an increased amount of C3 deposition. CD55 proved to be more efficient than CD46 in blocking complement activation of 17-1A-CVF conjugates. No synergistic effect was observed when anti-CD46 and anti-CD55 were combined (Fig. 2 a).
This increase in C3 deposition resulted in an increased amount of lysis of SW837 in the presence of either CD46, CD55 or CD59. CaCo-2 was only lysed in the presence of 17-1A-CVF and mAb against all three mCRP (CD46, CD55 and CD59). In general, there was a positive relation between the amount of C3 deposited on the tumor cell membrane and the amount of complement-mediated lysis (Fig. 2 b). When CD59 was blocked, the amount of complement-mediated lysis was dependent on the amount of Ep-CAM expressed by the tumor cells. The highest amount of lysis was reached in the presence of all three anti-mCRP mAb.
The 17-1A and 323 / A3 mAb activate the classical pathway of complement. Conjugates with CVF were shown also to activate the alternative pathway of complement as shown by an increase in complement-mediated lysis, in the presence of Mg-EGTA. In contrast, 323 / A3 and 323 / A3 conjugated to an irrelevant protein, yeast alcohol dehydrogenase (ADH), did not cause alternative pathway complement activation (results not shown).
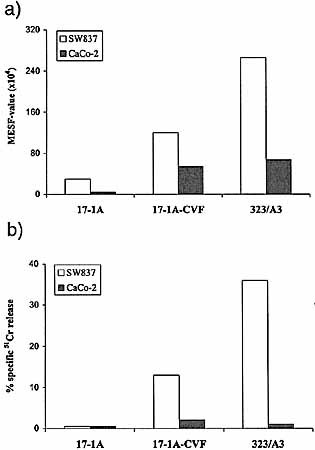
a) 17-1A-CVF results in an increased amount of C3 deposition on SW837 and CaCo-2. b) 17-1A-CVF results in an increased amount of complement mediated lysis of SW837 and CaCo-2 cells. 323 / A3 is used as a positive control for complement activation. Amount of deposited C3 was measured by flow cytometry as described in Sect. 4.8 and expressed in MESF values (× 104). Amount of complement-mediated lysis is determined by a 51Cr-release assay as described in Sect. 4.9 and is expressed in the percentage of specific 51Cr release.
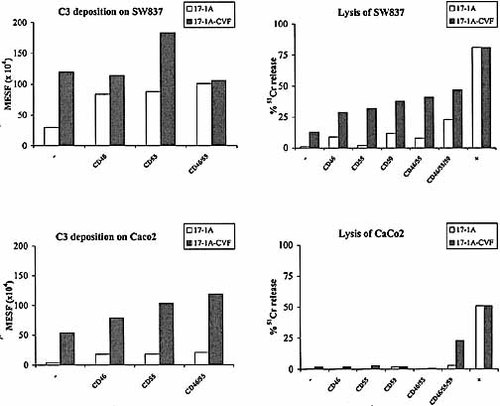
Inhibition of mCRP leads to an increased amount of C3 deposition on, as well as complement-mediated lysis of, SW837 and CaCo-2 when complement is activated by 17-1A or 17-1A-CVF. The blocked mCRP is indicated under the X-axis. Amount of deposited C3 was measured by flow cytometry as described in Sect. 4.8 and expressed in MESF values (× 104). Amount of complement-mediated lysis is determined by a 51Cr-release assay as described in Sect. 4.9 and is expressed in the percentage of specific 51Cr release. As a positive control for complement-mediated lysis heterologous complement (BRC) was used.
2.3 17-1A-C3b conjugates
The effect of conjugates of 17-1A and C3b was also investigated and compared to the complement activating capacity of 17-1A-CVF. 17-1A-C3b induced more than 10-fold the amount of C3 deposition as compared to 17-1A (Fig. 3). This increase is in the same range as for 17-1A-CVF. Also the plateau values were of similar height. The pattern of C3 deposition induced by 17-1A-C3b was comparable to that induced by 323 / A3.
To exclude the possibility that the observed effects were induced by either N-succinimidyl-3(2-pyridyldithio) proprionate (SPDP) or an arbitrary protein coupled to SPDP we used 323 / A3-SPDP and 323 / A3-ADH as a control. No increase in complement activation by either 323 / A3-SPDP or 323 / A3-ADH as compared to 323 / A3 was observed (results not shown).
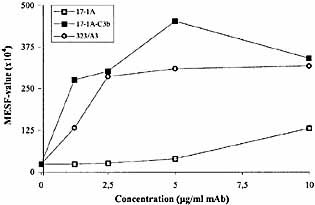
17-1A-C3b results in an increased amount of C3 deposition on SW837 cells as compared to 17-1A. Amount of deposited C3 was measured by flow cytometry as described in Sect. 4.8 and expressed in MESF values (× 104). 323 / A3 was used as a positive control.
2.4 Bispecific mAb
To inhibit the function of mCRP on the tumor cell surface a bispecific mAb directed against Ep-CAM was developed. For this purpose, because of the presence of a monovalent anti-tumor binding site in bispecific mAb, the high-affinity 323 / A3 mAb was chosen. The other arm consisted of anti-CD55, because blocking of CD55 resulted in the largest increase of complement activation (Figs. 2 and 4). A threefold higher amount of deposited C3 was observed with anti-17-1A / EpCAM*anti-CD55 bispecific mAb (323 / A3*MBC1) than with 323 / A3. In addition, the amount of C3 deposition as a result of 323 / A3*MBC1 was also higher as the amount of C3 deposition induced by a combination of the parental mAb (Fig. 5). When the levels of C3 deposition were compared between the different agents, 17-1A-CVF, 17-1A-C3b and 323 / A3*MBC1, the bispecific mAb showed the highest plateau level of C3 deposition.
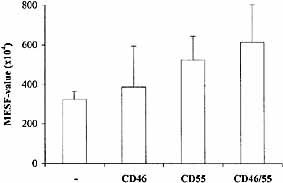
Blocking CD55 results in an increased amount of C3 deposition when complement is activated by 323 / A3. Deposited C3 was measured by flow cytometry as described in Sect. 4.8 and expressed in MESF values (× 104). Anti-mCRP mAb did not activate the complement system themselves. The mean plus SD of three experiments is shown.
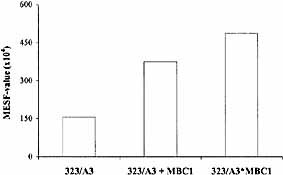
Complement activation by bispecific mAb 323 / A3*MBC1 results in an increased amount of C3 deposition as compared to 323 / A3. Amount of deposited C3 was measured by flow cytometry as described in Sect. 4.8 and expressed in MESF values (× 104). Results of a representative experiment are shown.
3 Discussion
The current investigation showed that the complement activating capacities of immunotherapeutic mAb can be enlarged. Bispecific mAb and conjugates of 17-1A mAb and CVF or C3b were tested for increased complement activating capacities.
Conjugation of CVF to 17-1A resulted in an increase in C3 deposition as well as in complement-mediated lysis. The low affinity of 17-1A lead to a low density of mAb on the tumor cell membranes, resulting in little complement activation as is also demonstrated before 16. The increase in complement activation due to 17-1A-CVF was comparable to that due to 323 / A3, which is of a 40-fold higher affinity 17. In vivo high-affinity mAb to tumor-associated antigens may evoke damage to normal host tissue as a result of cross-reactivity, while the low-affinity 17-1A mAb has been shown not to be toxic in vivo 4. Previously, the effect of 17-1A-CVF was examined on gastrointestinal cancer cell lines. In that study an increased C3a release due to 17-1A-CVF, as compared to 17-1A, was observed. However, complement-mediated lysis was not increased 18. It was suggested that the expression of mCRP might be responsible for the latter observation. The present study showed that blocking of single mCRP can result in C3 deposition as well as complement-mediated lysis. This discrepancy may be due to a difference in expression levels of antigen 16 and mCRP 19 on the used cell lines. Also in our study CaCo-2 cells, expressing low amounts of Ep-CAM, did not show complement-mediated lysis, unless all three mCRP were inhibited and complement was activated by 17-1A-CVF or 323/ A3. With respect to C3 deposition, the additional effect of 17-1A-CVF on cells with a low antigen expression such as CaCo-2 was more obvious than for the SW837. This suggests that CVF conjugates have the largest effect when antigen expression is limited. The observation that the expression of the tumor-associated antigen is often low on tumor cells 20, implies that mAb immunotherapy with mAb-CVF conjugates in these cases may lead to sufficient complement activation.
Also 17-1A-C3b was able to activate more complement as compared to 17-1A. Previously, Reiter et al. reported similar findings for a mAb directed against the mouse and human transferrin receptor, expressed on human leukemic and mouse lymphoma cells 13. In contrast to CVF, C3b is susceptible to inactivation by fH and fI in serum. The estimated half-life of the alternative C3 convertase C3bBb formed with C3b conjugated to mAb in serum is approximately 20 min 13. This might lead in vivo to inactivation of the convertase before reaching thetumor site. A way to circumvent this problem can be to mutate C3 to prolong its half-life in serum 21. In vitro this approach has been successful for hematopoietic cells. Because convertases formed with CVF are not sensitive to inactivation by fH and fI, 17-1A-CVF conjugates might in vivo be more stable than 17-1A-C3b conjugates. It has been shown that CVF, despite its complement depleting capacities is not toxic when injected in mice or rats 22, 23. CVF, presumably will be immunogenic in humans, as it is in rabbits 24, which may result in a fast clearance after repeated exposure.
An increase in complement-mediated elimination of tumor cells may besides increasing the complement activating capacities of tumor-associated mAb, also be achieved by blocking mCRP on the tumor cell. For this purpose tumor cells need to be targeted specifically. Systemic blocking of mCRP in vivo has been shown to lead to a decrease in blood pressure in rodents, and to a decreased number of circulating leukocytes 25. Tumor-specific inhibition of mCRP might be achieved by a bispecific mAb, directed against a tumor antigen and a mCRP 14, 15. To reduce binding to normal tissue expressing mCRP the mAb arm directed against the tumor antigen should be of high affinity to ensure tumor-specific homing. The mAb arm directed against the mCRP should be of low affinity to prevent binding to mCRP-expressing normal tissue. C3 / C5 convertase regulators are the most attractive candidates. Inhibition of CD46 or CD55 potentially increases an inflammatory response by inducing an increased production of chemoattractants C3a and C5a and an increased C3 deposition. From our data, CD55 seems to be the most effective mCRP in regulating C3 deposition on colorectal cancer cells. Other in vitro studies performed with other cancer types support an important immunoregulatory role of CD55 14, 26. For this reason a bispecific mAb directed against 17-1A / Ep-CAM and CD55, 323 / A3*MBC1, was developed. In agreement with results obtained with bispecific mAb directed against renal and cervical carcinoma cells 14 (K. A. Gelderman, unpublished observations), 323 / A3*MBC1 in vitro resulted in an increased amount of C3 deposition as compared to 323 / A3. Compared to 17-A or 17-1A-conjugates these bispecific mAb were at least equally efficient.
In conclusion, conjugates of mAb and CVF or C3b appear to have a similar effect on complement activation as bispecific mAb. This effect seems to be dependent on mAb affinity and level of antigen expression on the tumor cells. Although the in vivo efficacy of bispecific mAb or conjugates with CVF or with C3b has to be substantiated in a proper animal model, all three reagents demonstrate an increase in complement activating capacity in vitro. So in case of low affinity of the mAb or a low density of the tumor antigen, these mAb conjugates or bispecific mAb may nevertheless result in sufficient complement activation and induce a local inflammatory response.
4 Materials and methods
4.1 Antibodies
The following antibodies were used: 17-1A (IgG2a; Centocor, Leiden, The Netherlands) and 323 / A3 27 (IgG1 and IgG2a), both directed against Ep-CAM. GB24 28 (IgG1; a kind gift of Prof. Atkinson, Washington University, St Louis, MO), directed against CD46; MBC1 (IgG1; hybridoma cells were kindly provided by Prof. B. P. Morgan, University of Wales, Cardiff, GB) and Bric216 (IgG1, IBGRL, Bristol, GB), both directed against CD55; Bric229 (IgG2b; IBGRL) directed against CD59; goat anti-mouse-IgG / M-FITC (DAKO, Glostrup, Denmark) and goat-anti-Human-C3c-FITC (Nordic Immunologicals, Tilburg, The Netherlands). 323 / A3(IgG2a)*MBC1 (bispecific mAb), quadroma cells producing this bispecific mAb were developed in our own department as described by Blok et al. 14.
4.2 Cell lines
The following colorectal carcinoma cell lines were used: LS 180, SW1116, T84, SW837, SW1463, CaCo-2, Colo 320 (all obtained from the ATCC, Rockville, MY). Cells were cultured in DMEM (Life technologies, Rockville, MD) containing 22.5 mM Hepes, supplemented with 10 % heat-inactivated fetal calf serum (FCS), 2 mM glutamine, 50 U / ml penicillin and 50 μg / ml streptomycin (complete medium).
4.3 Complement source
Normal human serum (NHS) was prepared from freshly collected human AB + serum. Rabbit serum (baby rabbit complement, BRC; Pel Freez, Rogers, Arkansas) was used as a source of heterologous complement. All sera were aliquoted and stored at − 70 °C until use.
4.4 Cobra venom factor
CVF was purchased as lyophilized cobra venom (Naja naja kaouthia) (Latoxan, Valence, France). In short, cobra venom was first separated by gel filtration (S300 column, Pharmacia, Woerden, the Netherlands) and subsequently by ion-exchange on a DEAE-column (Pharmacia) and a CM-cellulose column (Pharmacia), respectively. The degree of purity was determined by SDS-PAGE analysis. Functional activity was evaluated using a guinea pig hemolytic assay (described below).
4.5 Hemolytic assays
CVF hemolytic activity was measured using 20 μl guinea pig erythrocytes (3 × 108 / ml) (Harlan, Horst, the Netherlands) suspended in D-glucose gelatin veronal buffer containing 0.5 mM MgCl2, 0.15 mM MgCl2 and 0.1 M Mg-EGTA pH 7 (DGVB ++), 20 μl CVF-containing fraction and 20 μl guinea pig serum (Harlan) were incubated for 30 min at 7 °C. After adding 1 ml of PBS and centrifugation for 7 min at 2,000 rpm the amount of released hemoglobin was measured by measuring the absorption at 412 nm.
C3 was isolated according to the method of Reiter et al. 13. C3 was trypsinized before conjugation to obtain C3b. C3 hemolytic activity was measured using 50 μl (5 × 108 / ml) EAs (sheep erythrocytes coated with rabit anti-goat Ab) suspended in DGVB ++, 50 μl of a 1 / 100 dilution of human C3-depleted serum and 50 μl C3 (1 mg / ml). After adding 1 ml of PBS and centrifugation for 7 min at 2,000 rpm the amount of released hemoglobin was measured at 412 nm.
4.6 Conjugates
Conjugation of CVF, C3b or ADH (Sigma Aldrich, Zwijndrecht, the Netherlands) to mAb was performed as described by Juhl et al. 12. In short, 5 mg of antibody and 2.5 mg of CVF / C3b / ADH were incubated with SPDP (Sigma Aldrich). mAb-SPDP was reduced with dithiothreitol and allowed to form conjugates with CVF-SPDP, overnight at room temperature. Conjugates were isolatedby gel filtration (S300 column, Pharmacia) and positive fractions were pooled. Purity of the conjugates was determined by SDS-PAGE on a 7.5 % gel under nonreducing conditions and by high pressureliquid chromatography (HPLC) with a superdex200 column (Pharmacia). Only fractions containing mAb : CVF at a ratio of 1 : 1 were used in the experiments. The percentage of conjugates in these fractions was approximately 20 – 25 %. Protein concentrations were determined spectrophotometrically at 280 nm using E = 1.2 for mAb conjugates 12.
4.7 Flow cytometry
Cells (2.5 × 105) were incubated with 100 μl of a mixture of primary antibodies diluted in PBS / 0.5 % BSA (30 min, 4 °C). Cells were washed twice with PBS / 0.5 % BSA and incubated with 100 μl of a mixture of secondary antibodies (30 min, 4 °C). After washing, the cells were resuspended in 250 μl PBS / 0.5 % BSA containing 1 μg / ml propidium iodide (PI) to stain dead cells. Samples were measured on the FACSCalibur (Becton Dickinson, San Jose, CA). Ten thousand living cells were counted. FITC-positive cells were measured on FL1 : BP530 / 30 nm (green fluorescence). PI-positive (dead) cells were measured on FL3: LP 650 nm (red fluorescence). Fluorescence compensation was used to correct for spectral cross-talk between the fluorescent signals. Data are expressed in molecules of equivalent soluble fluorescence (MESF) values. MESF values were calculated on the basis of a flow cytometry standardization kit, Quantum 25 FITC (Flow Cytometry Standards Europe, Leiden, The Netherlands).
4.8 C3c deposition
C3d deposition was assessed by flow cytometric analysis as described above. After incubation with primary mAb (30 min, 4 °C), cells were incubated with 10 % normal human serum (NHS) diluted in DMEM containing 1 mM Ca2 + and 0.5 mM Mg2 + (20 min, 37 °C). C3c deposition was detected using GaH-C3c-FITC (30 min, 4 °). Ten thousand living cells were counted. C3 deposition was expressed in MESF values.
4.9 51Cr-release assay
The total reaction volume of the 51Cr-release assay was 125 μl and all dilutions were performed in DMEM complete medium. Twenty-five microliters of 51Cr-labeled target cells (1.500 cells / well) were mixed with 50 μl mAb in round-bottom microtiter plates and incubated for 30 min at 37 °C. As a homologous complement source 50 μl of NHS was added (final concentration of 10 %). As a control for complement activation BRC (final concentration of 2.5 %) was used as a heterologous complement source. Wells were incubated for 4 h, after which 100 μl supernatant was counted for 51Cr release. Maximal release was defined as the release obtained by the addition of 10 % (v/v) Triton X-100 to the target cells. Spontaneous release was obtained by incubating the target cells with medium. The percentage of specific release was calculated as 100 × (counts experimental release – spontaneous release) / (maximal release – spontaneous release). All tests were performed in triplicate.
4.10 Isotype-specific ELISA
To test bispecificity of 323 / A3*MBC1, a bi-isotypic ELISA was used. Microtiter plates (96-well) were coated for 2 h at 37 °C with rat anti-mouse (RAM) IgG1 (Sanbio) at a concentration of 2.5 μg / ml in 0.1 M NaHCO3, pH 9.6. Serial dilutions of culture medium from quadroma clones, in phosphate-buffered saline containing 0.05 % Tween 20 (PBS / T) and 1 % bovine serum albumin (BSA) were incubated in the coated microtiter plates for 1 h at room temperature. Bi-isotypic mAb was detected using goat anti-mouse (GAM) IgG2a conjugated with horseradish peroxidase (HRP) (Southern Biotechnology Associates). Cross-reactivity with coated rat Ig was blocked with 10 % rat serum in PBS / T. The assay was developed with 1 % 3,5,3′,5′-tetramethyl-benzidine in 0.11 M sodium acetate (pH 5.5, 100 μg / ml) containing 0.1 % freshly added H2O2. The concentration of monospecific IgG1 (parental mAb) was measured with a GAM-horseradish peroxidase κ chain conjugation (Sanbio). To measure the concentration of monospecific IgG2a (parental mAb) the procedure was repeated with a GAM-IgG2a coating (Southern Biotechnology Associates, Inc.). For determination of the mAb concentration IgG1 and IgG2a standards, obtained from Centocor (Leiden, The Netherlands), were used.
Acknowledgements
This study was sponsored by the Dutch Cancer Society, grant number RUL 99-2001.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




