Impaired IgE response in SWAP-70-deficient mice
Abstract
Protein SWAP-70 was initially isolated from nuclei of activated B cells and was implicated in the immunoglobulin class switch process. After B cell activation the protein translocates from thecytoplasm to the nucleus, and may serve to signal nuclear processes. We have generated mice deficient in SWAP-70 and found three main differences when compared to wild-type mice: (i) their B lymphocytes are two- to threefold more sensitive to γ-irradiation than B cells of wild type; (ii) SWAP-70-deficient mice developed autoantibodies at a much higher frequency; and (iii) the CD40 signaling pathway is compromised in the mutant mice. CD40-dependent switching to the IgE isotype is reduced five- to eightfold in vitro. In SWAP-70-deficient mice, IgE levels prior to immunizationwere six- to sevenfold lower than in wild-type mice, and after immunization three- to fourfold lower. CD40-induced proliferation was transiently increased in the mutant. LPS-induced switching to other isotypes, however, and LPS-induced proliferation were normal. We propose that SWAP-70 serves a specific role in the CD40 signaling pathway, in particular in the IgE response.
Abbreviations:
-
- NA-PK:
-
DNA-dependent protein kinase
-
- PI:
-
Propidiom iodide
1 Introduction
Specific B cells are activated by antigen and various coreceptors, including CD40. A broader range of B cells is stimulated by lipopolysaccharide (LPS) 1–3.Both processes induce a switch in the production of immunoglobulin class, although different isotypes are generated 4–6. LPS favors the switch to IgG2b and IgG3,while CD40 together with IL-4 favors switching to IgG1 and IgE. The nuclear factors that define isotype specificity are only partially known. For example, it has been extensively demonstrated that so-called germ-line transcripts from I regions 5′ of the respective heavy chain constant region exons are required 5. These germ-line transcripts are activated by specific signal transduction pathways. In one instance, CD40 induces the formation of p50-RelA/p50-RelB dimers, and induces specific transcripts, whereas in another instance LPS leads to p50/c-Rel dimers, which generate different transcripts 7. Factors that determine which members of the NF-κB family are specifically induced, however, remain to be identified, and not for all isotypes, including the switch to IgE, are such pathways completely known.
While detailed knowledge is accumulating on the general process of B cell activation and signaling cascades 1, less is known about the nuclear events in immunoglobulin isotype class switch recombination, the process of looping out, excising, and rejoining 8 recombinogenic sequences, the so-called S regions, which are located 5′ of each heavy chain constant region exon (except Cδ). A few proteins have been identified to provide necessary activities in the reaction, and thus are likely components of a putative recombination complex. The DNA-dependent protein kinase (DNA-PK) with its two associated factors, the heterodimeric Ku proteins Ku70 and Ku86, are required in vivo to generate Ig isotypes other than IgM 9–11. While formally the direct involvement of DNA-PK in the actual recombination reaction has not been proven, such role seems very likely given the functions of the holoenzyme in DNA double-strand break repair and V(D)J recombination 12. Mice lacking mismatch repair enzymes Msh2, Pms2, or Mlh1, are also somewhat deficient in class switching 13, 14, but the specific contribution of these enzymes to class switch recombination has not yet been defined. If, as a model proposes 15, overhanging nonhomologous DNA strands are to be removed during class switch recombination, mismatch repair proteins may act at this step 16. Recently, a cytidine deaminase was found tobe required for both class switching and somatic hypermutation in complexes 5, 6. The mitochondrial maturation is similar mice and humans 17, 18, but the precise role of this enzyme in both processes remains to be determined.
In a biochemical screen for nuclear proteins that may mediate immunoglobulin class switch recombination in activated murine B cells, we identified a novel protein, SWAP-70, that does not belong to any described protein family 19. The protein contains an unusual combination of domains and motifs: an extended coiled-coil region near the C terminus, a pleckstrin homology domain that is also found in certain signaling molecules, three nuclear localization signals, a nuclear exit signal, and a supposedly calcium-binding EF-hand motif 19, 20. Thus, SWAP-70 carries features of both cytoplasmic signal-transducing proteins and nuclear proteins. In most cell types SWAP-70 protein expression is undetectable. So far we found it only in mast cells (Gross et al., submitted) and B cells 19, 21, where upon activation its expression is rapidly increased. SWAP-70 interacts with several other proteins and protein complexes, including the B cell antigen receptor complex 20. SWAP-70 translocates from the cytoplasm to the nucleus and associates with a number of nuclear proteins, among them DNA repair proteins like poly(ADP)ribose polymerase and DNA-dependent protein kinase) (19; Jessberger et al., unpublished observations). Some of these proteins are known to be involved in class switch recombination and may serve—directly or indirectly – to activate or regulate the recombination machinery. To study the function of SWAP-70 in immunoglobulin class switching, B cell activation, and DNA repair and recombination, we generated a SWAP-70-deficient mouse. Here we report on the radiation sensitivity, ability to generate specific immunoglobulin isotypes, and on the extent of self-tolerance of such mice.
2 Results and discussion
2.1 The SWAP-70–/– mouse
Mice deficient in the SWAP-70 protein were generated by removing the first exon and part of the 5′ UTR in both alleles (Fig. 1A, B). In the homozygous SWAP-70ko/ko mice, immunoblotting with an affinity-purified polyclonal antibody raised against the full-length protein 19 showed that SWAP-70 protein is expressed neither in activated nor in resting splenic B cells (Fig. 1C).
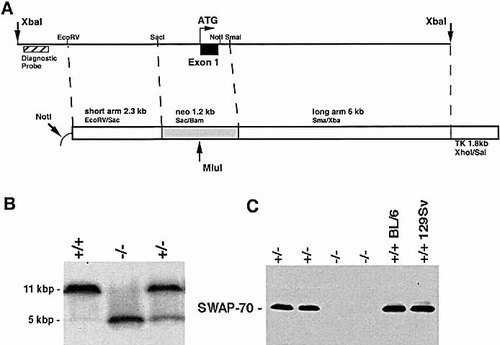
(A) SWAP-70 gene targeting strategy. (B) Southern blot analysis of the targeted locus. Genomic DNA from wild-type (+/+), heterozygous (+/–), and homozygous (–/–) SWAP-70-deficient mice was digested with XbaI and MluI, and probed with a XbaI/NcoI fragment from the promoter region of SWAP-70. (C) Immunoblot analysis of SWAP-70 expression in LPS-activated splenic B cells, derived from the indicated genotypes (BL/6 = C57BL/6; 129Sv = 129/SvJ).
The SWAP-70ko/ko mice are of normal phenotype, apparently healthy, reproduce normally, and the size of spleen, thymus and other organs is as in wild type. Also, we found no difference in the number of B cells expressing IgM, or IgM plus IgD, as analyzed by flow cytometry (not shown). For the analysis reported here, we used mice of congenic 129SvJ background.
2.2 Radiation sensitivity
The presumptive function of SWAP-70 in activating nuclear processes like DNA recombination and repair in B cells prompted us to analyze the sensitivity to γ-irradiation of cultures of B cells and non-B cells from either wild-type or SWAP-70ko/ko mice. In particular, we looked for the fraction of apoptotic cells in these cultures at various time points after irradiation (Fig. 2). We stained cells with either anti-annexin antibodies, which detect cells of an early stage of apoptosis 22, or with propidium iodide (PI), for cells of an advanced apoptotic stage 23; or with both agents. Thymocytes were taken from 4-week-old mice, cultivated and irradiated shortly thereafter; kidney fibroblasts were obtained as described 24; and proliferating pre-B cell cultures were established from bone marrow on stromal cells in the presence of IL-7 25. All experiments were repeated at least three times with cells from different mice, and cells cultured for the same length of time were compared.
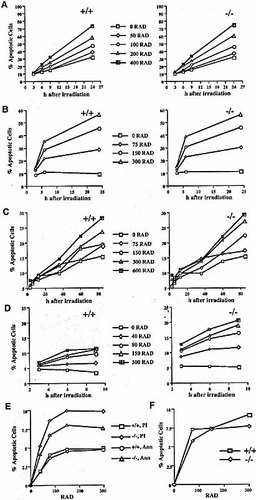
Irradiation sensitivity of wild-type (+/+) and SWAP-70ko/ko (–/–) cells. Cultures of primary cells were irradiated with the indicated dose, further cultured for the indicated times and the fraction of apoptotic cells measured by anti-annexin and PI staining. The number of apoptotic cells at zero time point (background) was the same for both genotypes and subtracted from the experimental values. (A) Thymocytes; (B) fibroblasts; (C) proliferating pre-B cells; (D and E) splenic B cells, activated by LPS for 2 days; in E, annexin and PI staining were performed individually. (F) Splenic B cells, activated by anti-CD40/IL-4 for 2 days.
SWAP-70ko/ko thymocytes, fibroblasts, and proliferating pre-B cells showed no increased sensitivity to γ-irradiation as compared to wild-type cells (Fig. 2A–C). In contrast, splenic B cells, activated by lipopolysaccharide (LPS), were 2-to 2.5-fold more sensitive when derived from SWAP-70ko/ko mice (Fig. 1D); however, heterozygous cells were like wild type (not shown). Also, the numbers of both, annexin-positive, PI-positive and of annexin/PI double-positive cells, were increased in SWAP-70ko/ko B cells (Fig. 2E). The involvement of SWAP-70 in the response to γ-irradiation is in line with the original isolation of SWAP-70 from B cell nuclear extracts in an assay for DNA recombination 19. The level of increased sensitivity, 2- to 3-fold, is similar to that of cells derived from SCID mice, which are well known for their irradiation sensitivity and DNA repair deficiency 24. In B cells, SWAP-70 may signal and activate DNA repair of double strand breaks that are caused by γ-irradiation. Its association with known DNA repair enzymes supports this hypothesis. Unexpectedly, splenic B cell cultures that were activated by anti-CD40 and IL-4 did not show an increase in irradiation sensitivity (Fig. 2F). We take this as a first indication that the role of SWAP-70 in the CD40 pathway differs from that in LPS activation. As a possible explanation, CD40-but not LPS-signaling may activate an irradiation response that is not dependent on SWAP-70. Alternatively, differences in CD40-triggered proliferation, e.g. hyperproliferation of SWAP-70ko/ko B cells upon CD40 activation (see below), may balance an increased radiation sensitivity of the mutant, i.e. irradiation-induced apoptotic signals may in part be overridden.
2.3 Somatic hypermutation
Because of the increased radiation sensitivity we thought it possible that another phenomenon that is connected to DNA repair – hypermutation at the immunoglobulin loci – might be affected. We put the SWAP-70-deficient mice on a κ light chain-deficient background, leaving the mice with only λ chain to incorporate into immunoglobulin 26. In these mice we analyzed the sequences of the λ1 gene in cells that had switched their immunoglobulin class, i.e. the μ-negative, λ-positive cells. The pattern (not shown) and the frequency of mutations were similar in mutant (53 mutations in 6,625 bp, or 0.8%) and wild-type mice (57 mutations in 9,826 bp, or 0.6%).
2.4 B cell proliferation and antibody response
We then investigated the effect of SWAP-70 deficiency on proliferation. We activated splenic B cells with either LPS or anti-CD40 as described before 19, 21. Proliferation was determined by incubating the cells at different time points for 4 h with [3H]thymidine, and subsequent measurement of its incorporation into DNA. All experiments were done in triplicates of individual spleen cell cultures from at least five mice. There was no difference between wild type and SWAP-70ko/ko in the proliferative response to LPS at days 1 to 3 after stimulation (Fig. 3A). In contrast, upon activation of the cells with anti-CD40 we observed the SWAP-70ko/ko cells to faster enter into a highly proliferative state (Fig. 3B). While the SWAP-70ko/ko cells were proliferating only a little faster than wild-type cells at 28 h, at 52 h after stimulation they showed a two- to threefold higher level of [3H]thymidine incorporation than wild type. Later, at the 68 and 76 h time points, the wild type cells had reached the same level of proliferation activity as the SWAP-70ko/ko cells. We consider these data as another indication for a specific role of SWAP-70 in the CD40 signaling pathway.
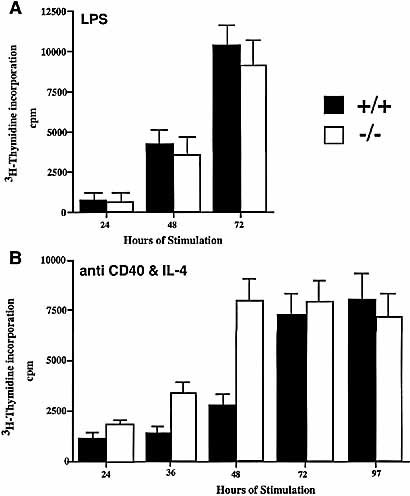
Proliferation of wild-type (+/+) and SWAP-70ko/ko (–/–) splenic B cells. (A) Induction by LPS. (B) Induction by anti CD40 and IL-4.
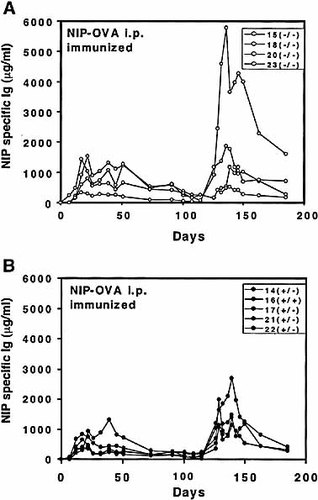
Memory response in SWAP-70-deficient mice. Mice were immunized with NIP-OVA at day 0 and at day 118. NIP specific Ig in sera from (A) SWAP-70ko/ko mice (–/–); and from (B) SWAP-70+/ko (+/–) and wild-type mice (+/+).
Because proliferation in SWAP-70 deficient B cells seems to be more easily triggered we investigated whether this fact has any influence on a specific primary or secondary immune response. We compared littermates of ko/ko, ko/+, and +/+ genotypes after immunization with NIP coupled to ovalbumin. Fig. 4 shows the total IgG titers of the specific antibody response followed over 180 days. Although the primary response tends to come on a little stronger in SWAP-70ko/ko mice (Fig. 4A) than in wild type and SWAP-70+/– mice (Fig. 4B), the secondary responses were similar in both types of mice.
2.5 Immunoglobulin class switching
As SWAP-70 has initially been found in a screen for proteins involved in immunoglobulin class switch recombination 19, we tested the ability of SWAP-70ko/ko cells to perform class switching. Spleen cells from 5-week-old mice were stimulated by various agents to induce the switch to different isotypes. Production of the various isotypes was measured by ELISA of the cell culture supernatants. We used LPS to induce the switch to (predominantly) IgG2b and to IgG3, LPS combined with IL-4 to induce the switch to IgG1, and anti-CD40 combined with IL-4 for the switch to IgG1 and IgE. Wild-type and SWAP-70ko/ko cells produced same amounts of IgG2b and IgG3 (Fig. 5). The SWAP-70ko/ko cells, however, were about threefold less efficient in producing the IgG1 isotype if induced by anti-CD40/IL-4. The smaller, about twofold reduction seen in IgG1 production upon LPS/IL-4 stimulation may perhaps reflect a contribution of SWAP-70 to the IL-4 branch of signaling in this experiment. The most dramatic effect of absence of SWAP-70 was seen in IgE production, which was reduced five- to eightfold (Fig. 5). Thus, while signaling solely by LPS appears to be normal in SWAP-70-deficient cells, CD40 signaling is compromised. In particular, the switch to IgE is reduced.
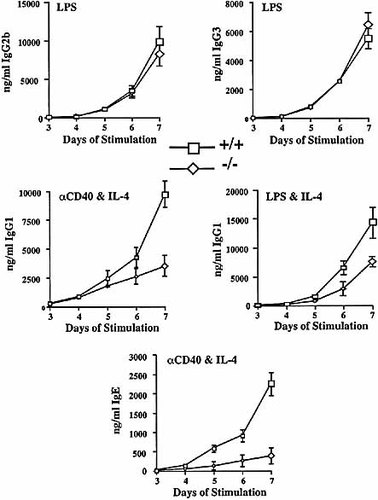
Class switching of splenic B cell cultures from wild-type (+/+) and SWAP-70ko/ko (–/–) mice. Immunoglobulin in the tissue culture supernatant was measured by ELISA at the indicated time points after commencement of stimulation, and the modes of activation are shown.
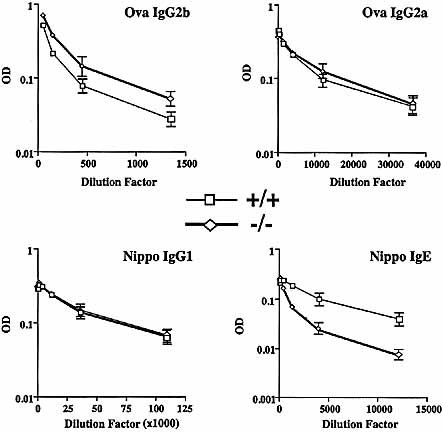
Immunoglobulin isotypes measured in sera from wild-type (+/+) and SWAP-70ko/ko (–/–) mice after immunization with either ovalbumin or N. brasiliensis, as indicated. OD = arbitrary optical density unit.
To determine whether the deficiency in CD40 signaling and switching to the IgE isotype also occurs in the animal, we used the nematode Nippostrongylus brasiliensis as an immunogen that elicits a strong CD40 dependent response and favors switching to the IgE isotype 27. In a control immunization, ovalbumin was used to assay switching to IgG2a, and IgG2b. The experiments were repeated twice in 5-week-old mice. Serum was taken before immunization (day 0), and 2 weeks after immunization, and analyzed by ELISA (Fig. 6 and Table 1). The pre-immune IgE titers were 6- to 7-fold lower in SWAP-70ko/ko mice compared to wild type (Table 1). Pre-immune levels of IgG2b, however, were almost identical, and levels of IgG1 only reduced 1.4-fold. The IgG1 response to the parasite was similar in wild-type and SWAP-70ko/ko mice, showing a 1.6-fold increase in titer. Also, the IgG2a and IgG2b responses to ovalbumin were similar in wild-type and mutant mice. Microscopic inspection of germinal centers formed in spleen and lymph nodes showed no difference between the two genotypes (not shown). After 2 weeks, IgE levels in the SWAP-70ko/ko mice were 3 to 4-fold less than in wild-type mice. These data reflect the results we obtained with spleen cell cultures.
|
N. brasiliensis |
|||
|---|---|---|---|
|
Strain |
Day |
IgE (μg/ml) |
IgG1 (μg/ml) |
|
wt |
0 |
5.0 ± 3.0 |
595.5 ± 196.5 |
|
|
14 |
116.8 ± 12.8 |
947.1 ± 430.4 |
|
ko |
0 |
0.7 ± 0.16 |
425.8 ± 107.5 |
|
|
14 |
26.5 ± 15.3 |
680.3 ± 243.4 |
Our findings demonstrate that for the function of SWAP-70, the mode of B cell activation seems to be especially critical if Ig class switching is concerned, and strongly suggest a requirement for SWAP-70 in CD40 signaling. This became most evident for the switch to the IgE isotype. The specific deficiency of SWAP-70ko/ko cells to produce IgE isotype antibody in vitro and in vivo may have also implications for a possible modulatory role of SWAP-70 in the allergic response.
SWAP-70 was originally isolated from both, LPS- or antiCD40/IL-4-induced cells, and the protein found up-regulated in both settings 19, 21, yet no switch phenotype of the SWAP-70ko/ko B cells was observed upon LPS activation. We speculate that SWAP-70 may be involved in LPS-triggered B cell activation but may here be redundant. The increased radiation sensitivity of LPS-activated SWAP-70ko/ko B cells at least argues for an involvement of SWAP-70 in this mode of B cell activation.
2.6 Autoantibodies
Altered CD40 signaling, as well as sensitivity to ionizing radiation, have been suggested to cause autoimmune responses 28–31. Therefore, we looked for the presence of autoantibodies in sera of wild-type and SWAP-70ko/ko mice. Spleen and kidney sections were prepared from RAG-2ko/ko mice, incubated with sera of sample mice, and presence of autoantibodies in these sera was visualized by incubation with anti-mouse Ig-FITC antibodies as secondary reagent. As RAG-2ko/ko mice do not develop antibodies on their own, all signals must be derived from the sera tested. An example for the read out for two sera each of wild-type and SWAP-70ko/ko mice is shown in Fig. 7A. With the exception of one 25-week-old wild-type mouse all mice tested were 22 week old. Out of 42 wild-type mice, only 2 had autoantibodies. Among the 39 SWAP-70ko/ko mice, however, 17 clearly showed autoantibodies. These autoantibodies were further characterized in regard to their isotypes. As summarized in Fig. 7B, 6 were IgG2a; 7 IgG2b, 1 IgG3 and 3 had mostly IgM autoantibodies. We also analyzed the intracellular staining for all 17 autoantibodies by co-staining with PI, and comparing with anti histone H1 staining for the nuclei. Of the 17 autoantibodies, 14 recognized nuclear antigens, and only 3 cytoplasmic antigens. Among the latter we found the one IgG3 and two of the IgM autoantibodies. All IgG2a and IgG2b autoantibodies recognize nuclear antigens and thus represent the typical isotype for such autoantibodies. The tendency of SWAP-70-deficient mice to develop autoantibodies can be explained by a putative lower threshold for signaling, as indicated by the faster entry into CD40-triggered proliferation. Faster onset of CD40-induced proliferation in SWAP-70-deficient cells may thus contribute to the development of autoimmune antibodies. This finding may implicate SWAP-70 in autoimmune diseases that are associated with B cell hyperactivation.
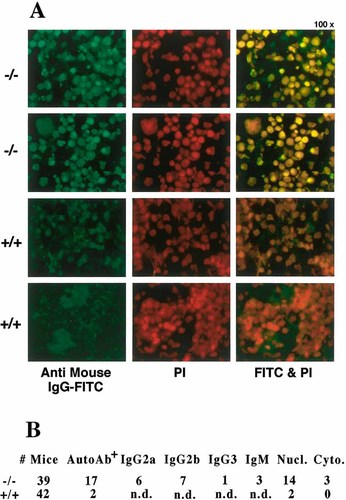
Production of autoantibodies in wild-type (+/+) and SWAP-70ko/ko (–/–) mice. (A) Immunofluorescence of RAG-2ko/ko spleen sections, incubated with test sera and FITC-labeled anti-mouse IgG antibody as secondary reagent; staining with PI visualizes the nuclei. (B) Summary table of all mice analyzed for autoantibodies.
Finally, the fact that SWAP-70 is linked to the antigen receptor 21 and, as shown here, tied into the CD40 signaling pathway suggest a function in one or several events after triggering by antigen. Because SWAP-70 is absent in plasma cells 19, 21, it clearly is not involved in secretion, leaving proliferation, class switching and hypermutation as three prominent processes besides house-keeping functions. But SWAP-70-deficient mice show no defects in house-keeping functions, and there is no difference in hypermutation. However, there is a clear effect on radiation sensitivity, proliferation, autoantibody formation, and specific heavy chain class switching. The common denominator for these functions can only be speculated about, but it may be triggering of DNA repair and recombination, i.e. DNA dynamic processes.
3 Materials and methods
3.1 Generation of SWAP-70-deficient mice
An 11kb XbaI/XbaI fragment containing SWAP-70 exon 1 was subcloned from a BAC clone obtained from Genome Systems Inc. The targeting construct (Fig. 1A) was designed to replace a 2.7-kb SacI/SmaI fragment containing exon 1 of the SWAP-70 gene with a phosphoglycerate kinase (PGK)-neo resistance cassette. The targeting vector, which also contained the HSV-tk gene, was linearized with NotI and transfected into 129/SvJ embryonic stem (ES) cells (clone RW-4; Genome Systems Inc.). Cells resistant to G418 and fialuridine were selected and several clones identified by Southern blotting (Fig. 1B) using as a probe a XbaI/NcoI fragment from the promoter region of SWAP-70. The targeted ES cell clones were injected into C57BL/6 blastocysts. The resulting chimeric mice were first bred with C57BL/6 and subsequently with 129/SvEMS mice. Further breeding over more than five generations yielded a 129/SvEMS strain deficient in SWAP-70. For all the experiments reported here, we compared 129/SvEMS wild-type and 129/SvEMS SWAP-70ko/ko mice.
3.2 Infection, immunization and antibody responses
Measurements of IgG1 and IgE responses were performed 14 days after infection with N. brasiliensis according to Barner et al. 27. For ovalbumin immunization, a solution of 50 μg/ml ovalbumin mixed with an equal volume alu-gel-S, was diluted 1:1 with PBS and 100 μl injected each intraperitoneally and subcutaneously. Sera were taken at day 14 after immunization, and analyzed by ELISA on ovalbumin-coated plates. For NIP immunization littermates were injected i.p. with 100 μg NIP-OVA with complete Freund's adjuvant. Secondary injections were donewith 10 μg antigen in incomplete Freund's adjuvant. NIP-specific antibody concentrations in sera were measured by an indirect ELISA assay. Plates were coated with 1 μg/ml NIP-BSA in borate buffered saline. NIP-binding antibody from hybridoma 17.2.25 (IgG1) was used as a positive control and as a standard for the IgG1 isotype and total NIP-specific Ig.
3.3 Hypermutation
Comparison of the λ1 alleles derived from FACS-sorted μ-negative, λ1-positive B cells of mice heterozygous (ko/+) or homozygous (ko/ko) for the disrupted Swap70ko allele was done by cDNA sequencing of a 317 nt sequence containing most of Vλ1. PCR amplification was done from cDNA using 5 pmoles of the Vλ1 5′ primer 5′ GGAATTCCTGCACTCACCACATCACCTGG and Cλ1 3prime; primer GGATCCTACCTTCCAGTCCACTGTCACC. The reactions were done with Pfu polymerase. The PCR fragments were cloned into the Invitrogen pCRTM II vector. Double-stranded DNA was prepared from clones and sequenced.
3.4 ELISA, measurement of apoptosis, and proliferation assay
Measurement of antibody in tissue culture supernatant was done by coating ELISA plates with isotype-specific antibodies (5 μg/ml solutions; clone 841C for anti IgE), blocking the plates with 10% BSA in PBS, applying the tissue culture supernatant and standard antibody (200 ng/ml) dilutions (steps of 1:50), and probing with an biotinylated isotype-specific antibody. The color reactionwas developed and read according to standard procedures. For determining apoptosis, cells were either stained with antibodies specific for annexin V (to measure early stages of apoptosis), or with PI at 50 μg/ml for advanced stages of apoptosis according to protocols supplied by the manufacturer. The proliferation assay used incorporation of [3H]thymidine (5 Ci/mmol; Pharmacia-Amersham Inc.) given in a pulse of 4 h. All experiments were done at least three times and run in triplicates.
Acknowledgements
S.K. and M.W. were supported by NIH grant GM37699 and by a Transgenic Mouse Grant of the Howard Hughes Medical Institute.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




