The role of MyD88 and TLR4 in the LPS-mimetic activity of Taxol
The first two authors contributed equally to this work.
Abstract
Taxol can mimic bacterial lipopolysaccharide (LPS) by activating mouse macrophages in a cell cycle-independent, LPS antagonist-inhibitable manner. Macrophages from C3H/HeJ mice, which have a spontaneous mutation in Toll-like receptor 4 (TLR4), are hyporesponsive to both LPS and Taxol, suggesting that LPS and Taxol may share a signaling pathway involving TLR4. To determine whether TLR4 and its interacting adaptor molecule MyD88 are necessary for Taxol's LPS mimetic actions, we examined Taxol responses of primary macrophages from genetically defective mice lacking either TLR4 (C57BL/10ScNCr) or MyD88 (MyD88 knockout). When stimulated with Taxol, macrophages from wild-type mice responded robustly by secreting both TNF and NO, while macrophages from either TLR4-deficient C57BL/10ScNCr mice or MyD88 knockout mice produced only minimal amounts of TNF and NO. Taxol-induced NF-κB-driven luciferase activity was reduced after transfection of RAW 264.7 macrophages with a dominant negative version of mouse MyD88. Taxol-induced microtubule-associated protein kinase (MAPK) activation and NF-κB nuclear translocation were absent from TLR4-null macrophages, but were preserved in MyD88 knockout macrophages with a slight delay in kinetics. Neither Taxol-induced NF-κB activation, nor IκB degradation was affected by the presence of phosphatidylinositol 3-kinaseinhibitors. These results suggest that Taxol and LPS not only share a TLR4/MyD88-dependent pathway in generating inflammatory mediators, but also share a TLR4-dependent/MyD88-independent pathway leading to activation of MAPK and NF-κB.
Abbreviation:
-
- dnMyD88:
-
Dominant negative MyD88
-
- Hsp:
-
Heat shock protein
-
- LBP:
-
LPS binding protein
-
- NOS2:
-
Inducible nitric oxide synthase
-
- PI3K:
-
Phosphatidylinositol 3-kinase
-
- TIR:
-
Toll/IL-1 receptor domain
-
- TLR:
-
Toll-like receptor
1 Introduction
Taxol is a product of the Pacific yew with potent anti-tumor activity 1, 2. Taxol's anti-mitotic actions are due to its ability to bind and stabilize microtubules, which prevents proper cell division during mitosis 3–5. In 1990, we unexpectedly discovered that Taxol can induce down-regulation of the type I TNF receptor and release of TNF from mouse macrophages in a manner indistinguishable from lipopolysaccharide (LPS) 6. The LPS-mimetic activities of Taxol have since been extended to includeactivation of NF-κB 7 and microtubule-associated protein kinases (MAPK) 8, up-regulation of LPS-induced genes, secretion of cytokines, and activation of anti-Leishmania and anti-tumor activity 9. Some of these LPS-mimetic activities can be inhibited by inactive LPS analogues 10. The LPS-mimetic effect of Taxol is absent in macrophages from C3H/HeJ mice 6 that carry a mutation in the lps gene locus on chromosome 4 11–13. Genetic analysis of recombinant inbred mice showed that the genes controlling responses to LPS and LPS-like response to Taxol are closely linked 6. Taxol has since been used as a tool to investigate molecular mechanisms by which macrophages respond to LPS.
Macrophages are exquisitely sensitive to LPS and they respond in a robust manner by up-regulating a battery of host defense mechanisms 14–19. This response is critical for combating bacterial invasion, yet when uncontrolled, can lead to systemic inflammation, septic shock, and death 20–22. For over a decade, a serum LPS-binding protein (LBP) and a glycosylphosphatidylinositol (GPI)-anchored membrane protein CD14 have been recognized to have a role in recruitment of LPS to the macrophage surface 23–25, but the molecules responsible for transducing LPS signal across the membrane remained elusive until recently.
The gene encoded by the lps locus was identified as Toll-like receptor-4 (TLR4) 26, 27. Toll was originally identified as a Drosophila protein that not only is involved in dorso-ventral patterning during development, but also is required for host defense against fungal infection 28, 29. In mammals, TLR4 belongs to a family of type 1 transmembrane proteins with an extracellular leucine-rich repeat domain and an intracellular domain homologous to Toll and the IL-1 receptor. The TLR4 allele in C3H/HeJ mice has a point mutation in the cytoplasmic tail, resulting in a substitution of proline 712 by histidine 26, 27. Another LPS-hyporesponsive strain of mice, C57BL/10ScCr, has a chromosomal deletion encompassing the TLR4 locus 26, 27. Additional support for TLR4 as an indispensable component for LPS-mediated macrophage activation comes from studies with TLR4 knockout mice 30, which are hyporesponsive to LPS.
There is a growing body of evidence indicating that TLR4 and other TLR share the same signaling cascade as the IL-1 receptor 31–33. An adaptor protein, MyD88, is first recruited to the receptor, followed by activation of IL-1 receptor-associated kinases and TNF receptor-associated factor 6, that leads to NF-κB activation. MyD88 is an important mediator of macrophage activation by both IL-1 and LPS since dominant negative versions of this adaptor protein block their signaling 34–36. Furthermore, cells from MyD88 knockout mice respond poorly to IL-1, IL-18, and components of Gram-positive and Gram-negative bacteria 37–39. However, LPS-induced MAPK activation and NF-κB nuclear translocation were delayed but preserved in MyD88 knock-out cells 37, indicating the presence of a MyD88-independent signaling pathway for LPS.
Recently, a role for the signaling molecules Akt, Rac1 and phosphatidylinositol 3-kinase (PI3K) has been demonstrated in the activation of NF-κB by heat-killed bacteria via TLR2 in a MyD88-independent manner 40. The PI3K pathway has been implicated in LPS-mediated macrophage activation 41, 42. It is not clear whether PI3K is also involved in Taxol-mediated macrophage activation and whether PI3K participates in the MyD88-independent actions of LPS or Taxol.
Taxol is membrane permeable and its activity does not require LBP or CD14 43. As noted earlier, macrophages from C3H/HeJ mice do not respond to the LPS-mimetic effect of Taxol 6. It is not clear whether this is due to the absence of a positive signal by TLR4, or alternatively, to a negative signal by mutant TLR4, which has been postulated to have a co-dominant role in LPS responses 12, 44, 45. Here we test the roles of TLR4, MyD88 and PI3K in Taxol-mediated macrophage activation using primary macrophages from TLR4-null C57BL/10ScNCr mice 26, 27 and MyD88 knockout mice 38 and using specific inhibitors of PI3K.
2 Results
2.1 Macrophages lacking TLR4 do not respond to Taxol in producing TNF and NO
TNF was induced after 24-h exposure of wild-type C57BL/10ScSn macrophages to Taxol in a concentration-dependent manner (Fig. 1A). In contrast, macrophages from TLR4-null C57BL/10ScNCr mice produced much reduced levels of TNF in response to Taxol (Fig. 1A). NO was monitored by measuring nitrite levels in the medium 48 h later. Like LPS, Taxol is a poor stimulus of primary macrophages to induce NO when acting alone. However, in the presence of a trace amount of interferon-γ (IFN-γ), Taxol-induced nitrite was detected in the conditioned medium from wild-type but not from TLR4-null mice under the same conditions (Fig. 1A). We next compared steady state levels of TNF and inducible nitric oxide synthase (NOS2) mRNA in Taxol-stimulated macrophages from C57BL/10ScSn (TLR4-normal) and C57BL/10ScNCr (TLR4-null) mice. Fig. 1B shows that induction of TNF and NOS2 expression in response to Taxol was only observed in macrophages from wild-type mice but not from TLR4-null mice.
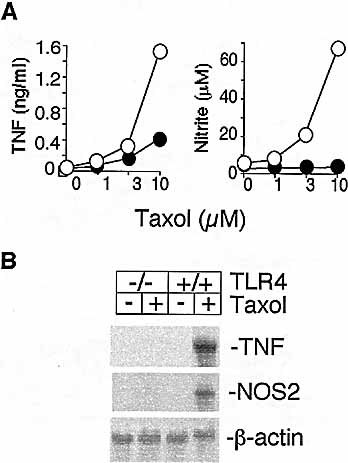
Macrophages from C57BL/10ScNCr mice are deficient in responding to the LPS-mimetic actions of Taxol. Macrophages from C57BL/10ScSn (○) and C57BL/10ScNCr (•) mice were exposed to Taxol in the presence (for nitrite) or absence (for TNF) of 1 U/ml IFN-γ. (A) Nitrite accumulation and TNF in the conditioned medium were determined. Results are means of triplicate determinations. Bars for the standard error fall within the symbols. (B) Total RNA from cells treated with or without Taxol (10 μ M, 18 h) was analyzed by Northern blotting with cDNA probes for mouse TNF, NOS2 or β-actin on the same membrane. One of three similar experiments is shown.
2.2 Macrophages from MyD88 knockout mice do not respond to Taxol in producing TNF and NO
We performed similar experiments to those described in Sect. 2.1, except that primary macrophages were from MyD88-deficient and matched wild-type mice. Increasing amounts of nitrite and TNF were secreted from wild-type macrophages in response to Taxol, but much lower levels were detected from MyD88 knockout macrophages under the same conditions (Fig. 2A). Induction of TNF and NOS2 gene expression in response to Taxol was not observed in MyD88 knockout macrophages (Fig. 2B).
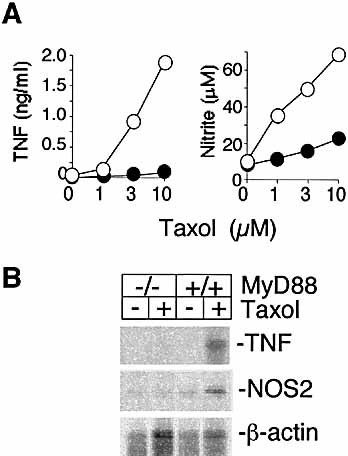
Macrophages from MyD88 knockout mice are deficient in the LPS-mimetic actions of Taxol. Macrophages from wild-type (○) and MyD88 knockout (•) mice were tested as described in Fig. 1. One of four similar experiments is shown.
Taken together, these results indicate that TLR4 and MyD88 are necessary for Taxol to induce inflammatory mediators such as TNF and NO in macrophages.
2.3 Dominant negative MyD88 (dnMyD88) but not wortmannin blocks Taxol-induced NF-κB activity in RAW 264.7 cells
NF-κB plays an important role in regulating transcription of numerous inflammatory cytokines. Activation of NF-κB is often used as a read-out for LPS signaling. To determine whether MyD88 affects NF-κB activation by Taxol, we co-transfected RAW 264.7, a macrophage-like cell line, with plasmids containing NF-κB-driven luciferase and a dnMyD88. This provided an independent test of MyD88 function in macrophage's response to Taxol. A cell line was used because primary macrophages are refractory to transfection. RAW 264.7 cells were transfected with reporter plasmid together with equal total amounts of DNA consisting of empty pFLAG-CMV2 and pdnMyD88 in different ratios. DnMyD88 blocked Taxol-induced signaling as measured by luciferase activity in a concentration-dependent manner. In contrast, phorbol myristate acetate (PMA)-induced activity was not affected by dominant negative MyD88 (Fig. 3A).
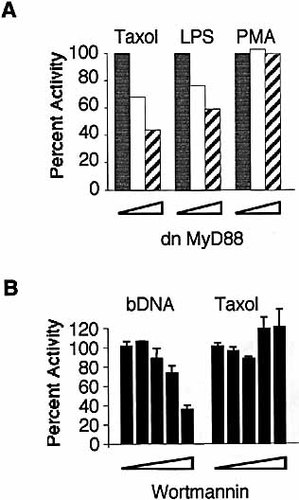
Taxol-induced signaling in mouse macrophages is inhibitable by dominant negative MyD88, but not by PI3K inhibitor wortmannin. (A) RAW 264.7 cells were transiently transfected with 6 μ g DNA: 3 μ g NF-κB-luciferase reporter DNA, together with pdnMyD88 (0 μ g, closed bars; 1 μ g, open bars or 3 μ g, hatched bars) plus a compensatory amount of pFLAG-CMV2 vector. After 36 h, the cells were stimulated with 10 μ M Taxol, 100 ng/ml LPS, or 100 ng/ml PMA for 3 h and the luciferase activity assayed. Induction of luciferase activity without dnMyD88 was designated as 100%. The actual fold inductions were 10.2±1.3, 43.4±6.6 and 2.4±0.4 (n=5) fold for Taxol, LPS and PMA, respectively. One of five similar experiments is shown. (B) Transfection was done as in (A) except only the NF-κB-luciferase reporter DNA was transfected. Cells were preincubated with 0.1, 1, 10 or 100 nM wortmannin for 1 h before addition of Taxol (10 μ M) or single-stranded bacterial DNA (100 μ g/ml) for 8 h. Induction of luciferase activity without wortmannin was designated as 100%. The actual fold inductions were 13.1±0.1 and 13.8±0.5 (n=3) for single-stranded bacterial DNA and Taxol. One of three similar experiments is shown.
To determine whether PI3K played a role in Taxol-induced NF-κB activation, wortmannin, a specific inhibitor of PI3K, was added before Taxol stimulation. Fig. 3B shows that wortmannin inhibited bacterial DNA-induced but did not affect Taxol-induced luciferase activity, suggesting this activation is PI3K independent.
2.4 Taxol promotes NF-κB nuclear translocation in MyD88-deficient but not in TLR4-deficient macrophages
Nuclear translocation of NF-κB is a prerequisite for its activation and is often used to indicate the activation state of this transcription factor. Participation of TLR4 and MyD88 in this process was examined by measuring Taxol-induced translocation of NF-κB from the cytosol to the nucleus in wild-type and in TLR4- or MyD88-deficient macrophages using an electrophoretic mobility shift assay (EMSA). Taxol-induced NF-κB DNA binding activity was minimal in TLR4-null macrophages, but was normal from MyD88 knockout cells, albeit with slower kinetics than that of wild-type cells (Fig. 4). Supershift analysis with antibodies specific for p50 and p65 confirmed that the complex detected is the p50/p65 heterodimer (Fig. 4A). In addition, wortmannin (100 nM) or Ly294002 (1 μM), another PI3K inhibitor, could not block nuclear translocation of NF-κB in wild-type macrophages (Fig. 4B).
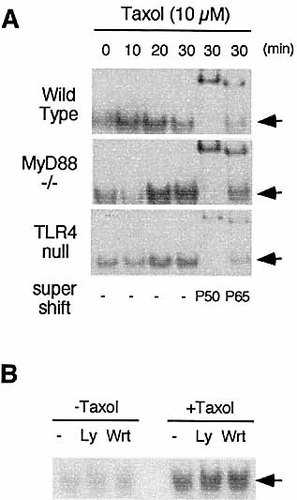
Taxol-induced NF-κB nuclear translocation in MyD88- or TLR4-deficient macrophages. (A) Primary macrophages (107) from wild-type, C57BL/10ScNCr (TLR4 null) or MyD88 knockout mice were treated with 10 μ M Taxol for the time indicated. Nuclear extracts were prepared. Nuclear extract (5 μ g) was incubated with a labeled probe containing NF-κB binding sites for 20 min in the presence or absence of antibodies for p50 or p65. NF-κB DNA binding activity was determined by EMSA. Arrows indicate the position of p50/p65 heterodimer. (B) Wild-type macrophages were pretreated with wortmannin (Wrt) or Ly 294002 (Ly) for 30 min.
Nuclear translocation of NF-κB occurs following the degradation of its inhibitor IκB. To test whether there is a role for MyD88 in IκB degradation in response to Taxol, macrophages from wild-type and MyD88-deficient mice were incubated with Taxol, and IκB integrity was examined in the lysates. Fig. 5A shows that macrophages from MyD88-deficient mice were able to degrade IκB in response to Taxol but with slower kinetics than the wild-type cells. To test whether PI3K is involved in MyD88-independent activities of Taxol, IκB degradation was examined in MyD88-deficient macrophages treated with wortmannin or Ly294002. Neither PI3K inhibitor affected IκB degradation (Fig. 5B).
Taken together, these results showed that both TLR4 and MyD88 are required for Taxol to activate transcriptional activity of NF-κB. In contrast, Taxol-induced nuclear translocation of NF-κB only required TLR4 but not MyD88. PI3K was not involved in either MyD88-dependent or MyD88-independent Taxol-mediated NF-κB activation.
2.5 Taxol activates MAPK and JNK in MyD88-deficient but not in TLR4-deficient macrophages
The ability of Taxol to activate MAPK and JNK was examined in wild type, TLR4-, and MyD88-deficient cells. Dual phosphorylation of MAPK and JNK, an indication of the activation of these kinases, was detected in wild-type macrophages after 15 min of treatment with Taxol (Fig. 6). In MyD88-deficient cells activation of MAPK and JNK was not detected until 30 min after Taxol treatment. The degree of dual phosphorylation in these cells, however, reached the same extent as that of wild type cells (Fig. 6). In contrast, TLR4-null macrophages exhibited no detectable dual phosphorylation of MAPK by Taxol. Their JNK had a high background of activation, and could not be further activated by Taxol (Fig. 6).
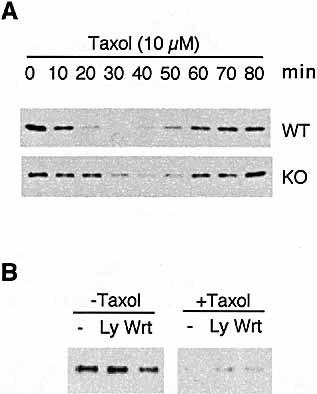
Delayed kinetics of Taxol-induced IκB degredation in MyD88-deficient macrophages. (A) Macrophages from wild-type (WT) or MyD88 knockout (KO) mice were treated with 10 μ M Taxol for the time indicated. The lysates (30 μ g/sample) were immunoblotted with antibody against IκB. (B) Macrophages from MyD88 knockout mice were preincubated with medium alone, 100 nM wortmannin or 1 μ M LY294002 for 30 min followed by a 40-min incubation with or without 10 μ M Taxol. Immunoblot was generated as in (A).
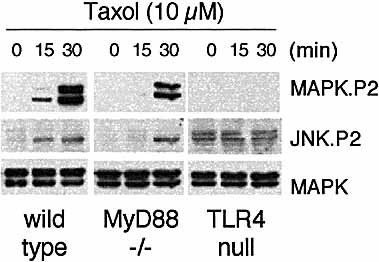
MAPK and JNK activation by Taxol in MyD88- or TLR4-deficient macrophages. Primary macrophages from wild-type, C57BL/10ScNCr (TLR4 null) or MyD88 knockout mice were treated with 10 μ M Taxol for the time indicated. The lysates (30 μ g/sample) were immunoblotted sequentially with antibodies specific for dual phosphorylated MAPK (MAPK.P2), JNK (JNK.P2) and total MAPK.
2.6 Taxol retains anti-mitotic effects on TLR4- and MyD88-deficient macrophages
Taxol caused microtubule bundling to the same extent in wild-type and TLR4-or MyD88-deficient cells (Fig. 7). This confirms that Taxol's LPS-mimetic effects are dissociable from its effect on microtubules 6, 43, 46.
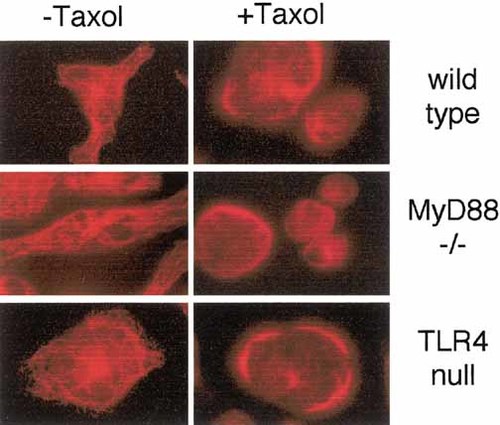
Taxol causes microtubule bundling in macrophages lacking either TLR4 or MyD88. Primary macrophages from wild-type, C57BL/10ScNCr (TLR4 null) or MyD88 knockout mice were treated with or without 10 μ M Taxol for 2 h and stained for α-tubulin.
3 Discussion
Our results show that both TLR4 and its downstream effector MyD88 are required in macrophages for Taxol's LPS mimetic activity in inducing TNF and NO, two important pro-inflammatory mediators. Neither TLR4 nor MyD88, however, is required for Taxol to bundle and stabilize microtubules. Kawasaki et al. reported recently that co-transfection of mouse TLR4 and MD-2 but not either plasmid alone into a mouse B cell line 47 or human 293 cells 48 was permissive for Taxol-induced NF-κB activation. A single mutation of Gln22 in mouse MD-2 dramatically reduced this activity but had little effect on their ability to confer LPS responsiveness 48. These data together with those described here suggest an indispensable role for TLR4 in Taxol's LPS-mimetic activity, and that TLR4/MD-2 is needed for Taxol to initiate a signal.
It is still not clear how TLR4 senses the signal from LPS or Taxol. The only known ligand for Drosophila Toll is spaetzle, a cleaved protein fragment generated by a protease cascade that becomes activated upon infection. A mammalian homologue for spaetzle has been postulated 49 but not identified. Two recent studies favor the notion that TLR4 itself can recognize and sense the active moiety of LPS 50, 51. By transfecting LPS-nonresponsive cells with TLR4 from a different species and exposing them to a lipid A analogue with species-specific pharmacology, these authors showed that the origin of transfected TLR4 dictates whether the cells respond to the lipid A analogue as agonist or antagonist 50, 51. These studies, however, did not exclude a species-dependent ability of TLR4 to associate differentially with distinct LPS-binding molecules supplied by the host cell. Using fluorescence resonance energy transfer techniques, Jiang et al. showed that LPS triggers a physical proximity between TLR4 and CD14, which may allow direct recognition of LPS or LPS/CD14 by TLR4 52. However, Taxol does not bind CD14 and its LPS-mimetic activity does not require CD14 43. The fact that LPS and Taxol share a highly conserved TLR4 pathway yet bear no structural resemblance argues against the conventional notion that activation of TLR4/MyD88 signaling pathway is simply initiated by stimulus/TLR4 recognition.
The lipophilic nature of Taxol enables it to cross the plasma membrane. Although the majority of labeled Taxol was found to be associated with microtubules 4, its LPS-mimetic effects do not rely on this interaction 6, 46. Thus, other cellular targets besides tubulin must exist to mediate Taxol's LPS-mimetic activity. We have recentlyidentified heat shock proteins (Hsp) of the 70- and 90-kDa families as additional Taxol targets in mouse macrophages 53. Geldanamycin, an Hsp90-inactivating drug, inhibited the activation of NF-κB and induction of TNF by both Taxol and LPS 53. The presence of Hsp70 and 90 in the extracellular space is thought to act as a signal of stressed or damaged cells, and is able to initiate innate immune responses 54, 55. Activation of macrophages by Hsp60 has been demonstrated and suggested to be dependent on TLR4 56. One can postulate that a Taxol/Hsp90 complex may induce the same endogenous ligand for TLR4 that is also induced by LPS/CD14. Alternatively, Hsp90 may interact with TLR4 in aninactive state, and Taxol/Hsp binding induces an active conformation of TLR4 that favors the recruitment of MyD88. Further experiments are needed to differentiate these possibilities.
Taxol-induced NF-κB transactivation of a luciferase gene reporter was inhibited by dnMyD88 (Fig. 3) yet MyD88-deficient cells seemed to respond to Taxol almost normally in inducing nuclear translocation of this transcription factor (Fig. 4). These seemingly discordant results emphasize the complexity of NF-κB activation. Most studies employ EMSA to indicate NF-κB activity, based on the presumption that NF-κB is primarily regulated by nuclear translocation 57. It has been reported recently that under certain conditions this activity is necessary but not sufficient for transactivation by NF-κB 58, 59. Our results demonstrated that, while MyD88 is required for Taxol-induced transcription of NF-κB-driven genes, NF-κB nuclear translocation occurs in the absence of MyD88, albeit with a slightly slower kinetics than in the presence of MyD88. It is possible that the precise timing and early induction of IκB degradation and translocation of NF-κB is particularly important in the induction of proinflammatory mediators in wild-type macrophages. MyD88 is necessary for this early response. Alternatively, there may be a MyD88-dependent contribution to NF-κB transactivation distinct from NF-κB nuclear translocation and DNA binding in Taxol signaling.
MyD88 is regarded as an essential mediator of numerous innate immune pathways leading to NF-κB activation that are initiated by surface receptors containing a Toll/IL-1R (TIR) domain. Recruitment of MyD88 to IL-1R 60 participates in IL-1 signaling. Mutation of a conserved proline in the TIR region of TLR generates a dominant negative phenotype 26, 27, 61 associated with an inability of TLR4 62 or TLR2 63 to recruit MyD88 upon activation. Dominant negative MyD88 inhibits NF-κB transactivation involving IL-1R 64, TLR2 61, and TLR4 (Fig. 3) 65. Paradoxically, MyD88 knockout cells can carry out nuclear translocation of NF-κB in response to LPS 37 and Taxol (Fig. 4), but not to IL-1 38. Together with MyD88-independent activation of MAPK by Taxol (Fig. 6) these data iterate that TLR4 can transduce LPS and Taxol signals to downstream molecule(s) other than MyD88. Such a possibility has been recently reported for TLR2 that involves PI3K 40. We could not find any inhibitory effect of wortmannin or Ly294002 on Taxol-induced signals reported here. The inability of these PI3K inhibitors to block Taxol-induced IκB degradation in MyD88-deficient cells rules out the possibility that PI3K may represent an alternative signaling pathway for MyD88 in Taxol's LPS mimetic actions on macrophages.
In summary, we have shown that Taxol and LPS not only share a TLR4/MyD88-dependent pathway(s) in generating inflammatory mediators, but also share a TLR4-dependent/MyD88-independent pathway(s)leading to activation of MAPK and nuclear translocation of NF-κB. PI3K seems to be involved in neither MyD88-dependent nor MyD88-independent signaling by Taxol. Exploring the mechanisms by which Taxol activates TLR4 should continue to shed light on early events in the macrophage response to LPS.
4 Materials and methods
4.1 Mice
MyD88 knockout mice were generated as described 38. Heterozygous fertilized ova were injected into a pseudopregnant female. Heterozygous offspring were mated to produce bothhomozygous knockout and wild-type mice. In the initial breeding, several of the homozygous knockout mice died suddenly. Pasteurella pneumotropica, a Gram-negative bacterium, was isolated from the spleens and lungs of four such mice. MyD88-deficient mice were thereafter housed in specific pathogen-free conditions, and given autoclaved food and a broad-spectrum antibiotic, enrofloxacin (0.4 mg/ml), in the drinking water. Wild-type mice were housed in the same conditions but without the antibiotic. Exposure to 40 μ g/ml enrofloxacin overnight in vitro has no effect on macrophage responses to LPS or Taxol (data not shown). Genotypes were determined by PCR and Southern blot 38 prior to use in experiments and again post-mortem using genomic DNA from tail biopsy. Mice of both sexes (8–12 weeks) were used for collection of primary macrophages. Female adult C57BL/10ScNCr (LPS-hypo-responsive) and C57BL/ScSn (LPS-normoresponsive) mice were purchased from NCI (Frederick, MD) and Jackson Laboratories (Bar Harbor, ME), respectively.
4.2 Cells
Primary mouse macrophages were harvested from the peritoneal cavity 4 days after intraperitoneal injection with 2 ml of 4% Brewer's thioglycollate broth (BBL, Sparks, MD). There was no difference in the macrophage content of peritoneal cells between wild-type and MyD88-deficient mice (94.8±1.9% for wildtype and 91.7±1.8% for knockout, n=8). The murine macrophage-like cell line RAW 264.7 was purchased from American Type Culture Collection (Rockville, MD). Cells were cultured in RPMI 1640 supplemented with 10% heat-inactivated FCS (HyClone, Logan, UT), 2 mM L-glutamine, 200 U/ml penicillin, and 200 μ g/ml streptomycin in 5% CO2/95% air at 37°C. Complete medium was monitored for LPS contamination by the chromogenic Limulus amebocyte lysate assay (BioWhittaker, Walkersville, MD) and found to contain <25 pg LPS/ml.
4.3 Secretion of nitrite and TNF
Cells were plated in 96-well plates at 1×105 cells/well in 150 μ l of complete medium and treated with indicated concentrations of stimuli. Nitrite was assayed by mixing 48 h-conditioned medium with an equal volume of Griess's reagent (1% sulfanilamide, 0.1% naphthylethylenediamine dihydrochloride, 2.5% H3PO4). Absorbance at 550 nm was recorded. Nitrite content of similarly incubated cell-free medium was subtracted as background. For mouse TNF ELISA (Duoset, R & D Systems, Minneapolis, MN), culture supernatants were collected at 24 h and tested according to the manufacturer's instructions.
4.4 Northern blot
Total RNA (20 μ g) from cells treated with or without 10 μ M Taxol for 18 h was run on 1% agarose gel, transferred onto nylon membranes, and hybridized with labeled probes overnight.
4.5 Construction of plasmids
The expression plasmid containing dnMyD88 (pdnMyD88) was generated by inserting a PCR-amplified cDNA fragment of mouse MyD88 (amino acids 152–296, the TIR domain) into the pFLAG-CMV2 expression vector (Sigma). For the luciferase reporter construct, PCR was used to generate an NF-κB responsive portion of the E-selectin promoter (-241 to –54), from genomic DNA of 293 cells. The PCR product was subcloned into pBluescript (Stratagene, La Jolla, CA). The plasmid was digested with KpnI and HindIII to release the E-selectin promoter and generate compatible restriction sites and ligated into the pGL3 enhancer (Promega, Madison, WI) upstream of the luciferase reporter gene.
4.6 Transfection/luciferase assay
RAW 264.7 (107 cells/10-cm plate) were collected and incubated in 1 ml serum-free medium with plasmid DNA and 250 μ g/ml DEAE-dextran in a centrifuge tube for 45 min at 37°C,followed by dropwise addition of DMSO to a final concentration of 10% (1 min). Cells were then washed and plated in complete medium for 36 h before addition of stimuli. For assays using wortmannin,the drug was added 1 h before stimuli. Lysates were prepared and analyzed using the luciferase Reporter Kit (Promega) according to the manufacturer's instructions. Relative light units (RLU) were normalized according to protein lysate concentrations.
4.7 Immune blotting for MAPK activation/IκB degradation
Thioglycollate broth-elicited peritoneal macrophages stimulated with Taxol were lysed. Equal amounts (30 μ g) of lysate protein were fractionated on SDS-PAGE, transferred to nitrocellulosemembranes, and immune blotted with antibodies against dual phosphorylated MAPK or JNK for MAPK activation or with antibody against IκB for IκB degradation.
4.8 Electrophoretic mobility shift assay
Thioglycollate broth-elicited peritoneal macrophages were treated with 10 μ M Taxol at 37°C for various times, and nuclear extracts prepared. Nuclear translocation of NF-κB was examined by incubating 5 μ g nuclear extract with a labeled DNA probe containing NF-κB binding site A from the NOS2 promoter, and fractionating the mixture on a native gel as described 7.
4.9 Microtubule bundling
Thioglycollate broth-elicited peritoneal macrophages adherent to 13-mm coverslips were fixed in methanol at –20°C for 2 min. The microtubule network was visualized by staining the cells with an anti-α-tubulin antibody and donkey anti-mouse IgG conjugated with Texas red.
4.10 Materials
Monoclonal antibody against α-tubulin was from ICN (Costa Mesa, CA). Mouse rIFN-γ with a specific activity of 5.2×107 U/mg protein was a gift of Genentech (South San Francisco, CA). Antibodies for NF-κB p50 (sc-114), p65 (c-20) and IκB-α (c-21) were from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies against dual phosphorylated MAPK and JNK were from Promega, Madison, WI. All other reagents including Taxol and LPS derived from E. coli (0111;B4) were from Sigma. Single-strand bacterial DNA was prepared as described 66. Wortmannin and Ly294002 were from Calbiochem (La Jolla, CA).
Acknowledgements
We thank Ming Deng and Sae Kyung Lee for technical assistance and Carl Nathan for critical reading of the manuscript. This work was supported by NIH grant (AI30165, A.D.) and pre-doctoral fellowships (E.F.B., C.A.B.) from the Cancer Research Institute. The Department of Microbiology and Immunology gratefully acknowledges the support of the William Randolph Hearst Foundation.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




