Immunodomination results from functional differences between competing CTL
Abstract
The presence of dominant epitopes suppresses generation of CTL activity toward other non-dominant epitopes found on the same antigen-presenting cell (APC). This phenomenon, termed immunodomination, drastically restricts the diversity of the repertoire of CTL responses. Under various experimental conditions we assessed the in vivo expansion by tetramer staining and function by expression of O-glycans and intracellular perforin of CTL specific for a dominant (B6dom1) and a non-dominant (HY) H2Db-restricted epitope. Immunodomination abrogated expansion rather than differentiation of HY-specific CTL. When immunodomination was precluded because HY was presented alone or because high numbers of antigen-bearing APC were present, the numbers of HY-specific T cells detected after antigen priming were similar to those of B6dom1-specific T cells. The main difference between T cells that recognized B6dom1 versus HY was functional rather than quantitative. The key feature of T cells specific for B6dom1 is that they show striking up-regulation of molecules involved in CTL effector activity rather than accumulating to particularlyhigh levels, as assessed by tetramer staining. These results support the emerging concept that following antigen priming, CTL populations of similar size can display important differences in effector function, and suggest that these functional differences are instrumental in shaping the repertoire of CTL responses.
Abbreviation:
-
- MiHA:
-
Minor histocompatibility antigen
1 Introduction
The functioning of the adaptive immune system found in jawed vertebrates has been shaped by 450 million years of co-evolution with viruses and bacteria 1, 2. Recognition of MHC class I/peptide complexes by CD8+ CTL has emerged as a crucial and effective, effector mechanism against intracellular pathogens 3. For a peptide to be immunogenic, it must be efficiently processed and presented in association with MHC molecules to CTL with a complementary TCR. Remarkably, confrontation with complex pathogens usually results in the generation of cytotoxic activity focused on a single, or at most a few, epitopes 4–6. A priori, such limited diversity of CTL response repertoire appears hazardous for the host. Viruses, such as human immunodeficiency virus, hepatitis C virus, influenza A virus and lymphocytic choriomeningitis virus may be initially controlled by CTL but subsequently escape through mutation of the relevant epitope 7–11. Constraints related to antigen processing and to the availability of complementary TCR are not sufficient toexplain why, during the course of an immune response, the number of epitopes recognized by cytotoxic CD8+ cells is so drastically restricted. Indeed, studies of CTL responses to viruses, tumor antigens, and minor histocompatibility antigens (MiHA) have shown that the diversity of CTL response is further limited by immunodomination: the presence of some dominant epitopes suppresses generation of CTL specific for other immunogenic non-dominant epitopes 12–16. Non-dominant epitopes elicit CTL responses when they are presented alone but they are neglected when presented with dominant epitopes.
Suppression of CTL cytotoxic response toward immunodominated epitopes is not due to competition for binding to MHC class I molecules. In fact, immunodomination is frequently observed between peptides that are presented by different class I allomorphs such as Kb and Db on APC 5. Moreover, immunodomination is not associated with decreased expression of non-dominant peptides at the cell surface in the presence of dominant peptides 12, 17. Implicit in the definition of immunodomination is the notion that whether or not an epitope will elicit a response depends on its own characteristics as well as on those of co-presented epitopes. Therefore, since epitope A may dominate B but be dominated by C, its designation as dominant or not is relative and potentially ambiguous. Comparison of B6dom1 and HY MiHA has, therefore, been an instructive paradigm to study immunodomination 12, 14, 15, 18, 19. Indeed, these two epitopes lie at opposite ends on the dominance scale in H2b mice. HY elicits CD8 cytotoxic response when presented alone but not when presented with one or numerous autosomal MiHA 12, 20. On the contrary, that B6dom1 is always dominant means that it induces CD8 cytotoxic effectors when presented with hundreds of MiHA 12, 15. Both B6dom1 and HY are H2Db-associated nonapeptides. HY is encoded by the Uty gene, whereas B6dom1 is encoded by the H7 locus at the telomeric end of chromosome 9 15, 19, 21–23. Studies of CTL responses to B6dom1 and HY showed that immunodomination is found only when both dominant (B6dom1) and non-dominant (HY) epitopes are presented on the same APC, and does not occur when they are presented concomitantly on separate APC 12. This observation has been confirmed with other antigen combinations 17, 24 and it indicates that immunodomination results from competition between responding CTL populations that recognize epitopes on the same APC 14.
This work aimed at deciphering the mechanism of immunodomination: how the presence of a dominant epitope (B6dom1) prevents generation of a cytotoxic response to a non-dominant determinant (HY) presented on the same APC.
2 Results
2.1 Recognition of B6dom1 prevents expansion of HY-specific CD8+ T cells
In vitro studies have shown that T cell activation can elicit a diverse array of effector activities and that there is a hierarchy of thresholds to activate T cells for different functions 25, 26. In the case of naive CTL, induction of perforin-dependent cytotoxicity is the most stringently controlled function and is elicited only by strong agonists that induce optimal TCR signals. Thus, antigen presentation can result in expansion of CD8+ T cells that display no effector function or that produce cytokines such as IL-2 and IFN-γ but are not cytotoxic 16, 25, 27–29. In previous studies of CTL immunodomination, the influence of dominant epitopes on response to non-dominant epitopes has been assessed essentially using cytotoxicity assays. Such estimations of cytotoxic activity allow no inference to be drawn as to whether immunodomination prevents expansion of CTL specific for non-dominant antigens or whether it simply precludes development of cytotoxic activity by these CTL. To address this fundamental issue, we used tetramer labeling to estimate expansion of B6dom1-(dominant) and HY-(non-dominant) specific T cells 5–30 days after priming with 20×106 spleen cells. Staining of B10 splenocytes as well as B6dom1- and HY-specific cell lines showed that B6dom1/Db and HY/Db tetramers were highly specific and sensitive reagents (Fig. 1).
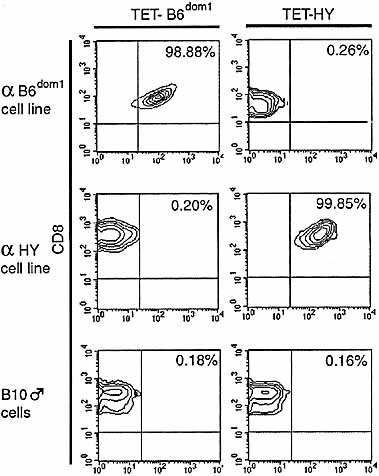
Staining of splenocytes and antigen-specific cell lines with B6dom1/Db and HY/Db tetramers. Labeling with anti-CD8 antibody and tetramers was performed on B6dom1- and HY-specific CD8+ cell lines. Splenocytes from B10 male mice (gated on CD8+ cells), that express B6dom1 and HY MiHA, served as additional negative controls.
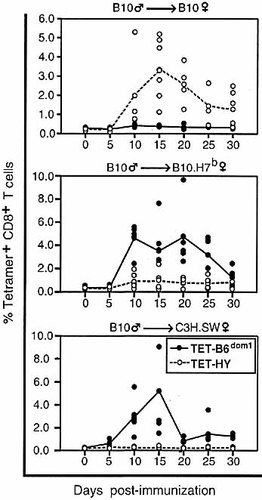
Recognition of B6dom1 prevents expansion of HY-specific CD8+ T cells. Percentage of B6dom1- and HY-tetramer+ cells were assessed in three combinations of priming cells/recipient mice (incompatible MiHA). Upper panel: B10 male cells injected in B10 female mice (HY); middle panel: B10 male cells in B10.H7b female mice (HY + B6dom1); and lower panel: B10 male cells in C3H.SW female mice (HY + B6dom1 + numerous other MiHA). The number of MiHA differences between C3H.SW and B10 mice is estimated to be over 40 48, 49. Each dot represents one individual. Lines indicate the mean percentage of B6dom1- and HY-tetramer+ cells at various times with three to seven mice per group.
Expansion of B6dom1- and HY-tetramer+ cells was as-sessed in three combinations of priming cells/recipient mice which differed in (i) HY, (ii) HY and B6dom1, or (iii) HY + B6dom1 + numerous (more than 40) other MiHA (Fig. 2). In mice primed solely against HY, we detected expansion of antigen-reactive CD8+ T cells that peaked at about day 15 (Fig. 2). This indicated that there are no inherent constraints to the expansion of CD8+ T cells specific for this non-dominant MHC class I-associated epitope. Results were strikingly different after priming with APC expressing both HY and B6dom1. In that case, expansion of HY-specific CD8+ T cells was almost totally abrogated (p=0.03, HY alone versus HY with B6dom1). Expansion of B6dom1-specific T cells was affected little, if at all, when numerous other MiHA were present on the priming APC, whereas in the latter case no expansion of HY-specific T cells was detected. Together, these data show that immunodomination prevents expansion of CTL that recognize the non-dominant epitope HY. This finding excludes the alternative hypothesis that immunodomination might result in a quantitatively normal expansion of functionally defective HY-specific CTL. Furthermore, our data show that the presence of a single dominant epitope is sufficient to inhibit expansion of HY-specific CTL, whereas the presence of more than 40 other epitopes does not significantly affect expansion of B6dom1-specific CTL.
2.2 Immunodomination is regulated by the T cell/APC ratio
Compelling evidence indicates that immunodomination results from competition between responding CTL populations that recognize dominant versus non-dominant epitopes. The essence of this competition, however, remains elusive. Recent studies using hematopoietic chimeras with variable frequency of antigen-specific T cells (TCR transgenic CD4 T cells specific for an I-Ek-restricted pigeon cytochrome peptide) have demonstrated an inverse relationship between the abundance of antigen-specific T cells and their level of expansion after antigen priming 30. At low frequency almost all T cells proliferated but at higher frequency of antigen-specific T cells, the proportion of cycling cells decreased irrespective of the number of antigen-bearing APC. This suggests that there may be homeostatic regulation or some limitation of the total number of effector T cells generated during the course of immune response. Accordingly, expansion of antigen-responsive T cells would cease when the number of effector T cells reaches a given threshold. This could theoretically restrict the diversity of the repertoire of CTL responses and be instrumental in immunodomination when several epitopes are presented simultaneously. Alternatively, the fact that immunodomination takes place only when the dominant and the non-dominant epitopes are present on the same APC suggests that the crux of immunodomination is competition for APC resources 12, 15, 24. To address this, we estimated expansion of antigen-specific T cells in a model where the number of APC expressing HY and/or B6dom1 was increased by several orders of magnitude. Splenocyte suspensions containing 5×106 CD8+ T cells were injected into irradiated recipients presenting selected MiHA differences, and the number of tetramer+ cells was assessed on days 9 and 15 (Fig. 3). HY and B6dom1 share the same tissue distribution profile. Both antigens are ubiquitous and well expressed on hematopoietic APC, which have an essential role in priming of naïve CTL 23, 31–33. Thus, in this model injected T cells were confronted with large quantities of antigen-positive APC. Irradiated syngeneic recipients served as control to evaluate the homeostatic expansion of tetramer+ T cells in the absence of HY and B6dom1 antigens. Under these conditions, we found that the numbers of HY- and B6dom1-specific CD8+ T cells were similar, regardless of whether their cognate antigens were presented alone, together, or with multiple MiHA. In fact, the greater the number of MiHA discrepancies between the host and injected T cells, the larger was the accumulation of HY- and B6dom1-specific T cells. Thus, when T cells encountered large numbers of APC co-expressing HY and B6dom1 no immunodomination was found. This strongly supports the concept that immunodominance results from competition for APC resources.
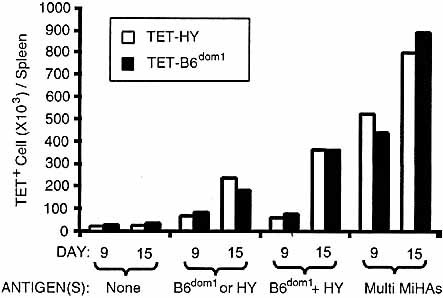
Expansion of B6dom1- and HY-specific CD8+ T cells after adoptive transfer to irradiated recipients. Spleen cell suspensions containing 5×106 CD8+ T cells were injected via the tail vein in irradiated (10 Gy) recipients. Donors and recipients were either syngeneic (B10 male cells → B10 male hosts) or different for (i) HY (B10 female cells → B10 male hosts); (ii) B6dom1 (B10.H7b male cells → B10 male hosts); (iii) HY and B6dom1 (B10 female cells → B10 male hosts); or (iv) HY + B6dom1 + numerous other MiHA (C3H.SW female cells → B10 hosts). Absolute numbers of CD8+ tetramer+ cells were estimated on days 9 and 15 after transfer. Bars represent the mean values for two mice per time.
2.3 Effector function of CTL specific for B6dom1 and HY epitopes
Why do T cells specific for B6dom1 compete successfully for APC when presented with epitopes such as HY? One possibility might be that B6dom1-specific T cells simply outnumber HY-specific T cells at the APC surface. This could happen if B6dom1-specific T cells had higher precursor frequency or proliferated more rapidly than HY- specific T cells. Results depicted in Fig. 2 and 3 argue against this. Indeed, when immunodomination was precluded because HY was presented alone or because high numbers of antigen-bearing APC were present, the numbers of HY-specific T cells detected after antigen priming were similar to those of B6dom1-specific T cells. Hence, if the difference between CD8+ T cells recognizing B6dom1 and HY is not a question of numbers, it should be one of function. Therefore, to determine whether B6dom1-specific T cells displayed superior effector function, we assessed expression of O-glycans recognized by the 1B11 antibody on tetramer+ T cells 15 days after priming against either HY or B6dom1. 1B11 monoclonal antibody is specific for epitopes on CD43 and CD45RB glycoproteins expressed on effector CD8+ T cells 34, 35. Expression of cell surface O-glycan epitopes is specifically up-regulated on effector, as opposed to naive and memory CTL 36. Strikingly, when labeled with 1B11 antibody, the proportion of positive cells and the mean fluorescence intensity of 1B11+ cells were ∼77%/168 U for B6dom1-specific T cells, but only ∼29%/74 U for HY-specific T cells. Furthermore, B6dom1-specific T cells had a much higher intracellular perforin content than HY-specific T cells (Fig. 4). These data support the idea that the critical issue involved in immunodomination is a question of CTL function rather than CTL numbers. CTL that compete successfully for APC resources display superior effector function as evidenced by 1B11 labeling and perforin content.
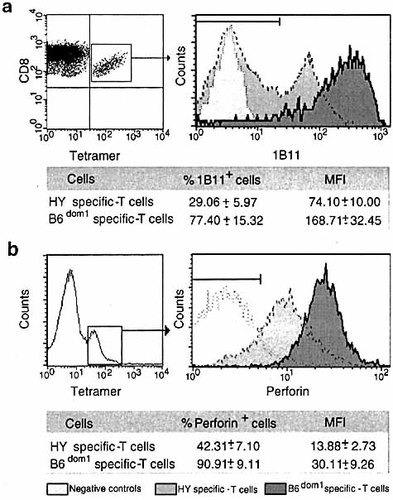
Expression of cell surface O-glycan epitopes and intracellular perforin content. (a) Staining with 1B11 antibody was assessed on spleen tetramer+ cells 15 days after priming against HY (B10 female mice injected with B10 male cells), or B6dom1 (B10.H7b male mice injected with B10 male cells). (b) Intracellular perforin staining. Splenocytes from mice primed against HY or B6dom1 (as in a) were incubated for 5 h with irradiated B10 male spleen cells expressing HY and B6dom1 antigens in culture medium supplemented with brefeldin A, then permeabilized with saponin, and stained with anti-perforin antibody. For both (a) and (b), flow cytometry data from one representative experiment are shown and the mean results ± SD of three experiments are presented. Splenocytes from unprimed mice served as negative controls.
2.4 Immunodomination prevails in the absence of perforin-mediated killing
Given the evidence that perforin-mediated killing is the main though not the sole mechanism responsible for elimination of APC in vivo 19, and that CTL responsible for immunodomination show greater intracellular perforin content (Fig. 4), we evaluated whether immunodomination would disappear in the absence of perforin. Here, the underlying assumption is that by killing APC, CTL specific for immunodominant determinants might reduce the duration of antigen presentation below a critical threshold and consequently impede the generation of CTL responses toward non-dominant antigens. This would be consistent with reports showing that the duration and/or the level of proliferation of several antigen-reactive CTL is increased in perforin-deficient mice 37, 38. To test our prediction we assessed expansion of HY-tetramer+ CD8+ T cells when HY was presented alone (B6 or B6.PKO male cells) or with numerous other MiHA (C3H.SW male cells) to either wild-type (B6) or perforin-deficient (B6.PKO) female mice. As expected, presentation of HY alone resulted in expansion of tetramer+ CD8+ T cells in both wild-type and perforin-deficient mice (Fig. 5). The key finding was that immunodomination was not abrogated in perforin-deficient animals: no HY-tetramer+ T cells were detected in wild-type and perforin-deficient mice primed with C3H.SW male cells. Thus, immunodomination cannot be accounted for solely on the basis of perforin-mediated killing of APC.
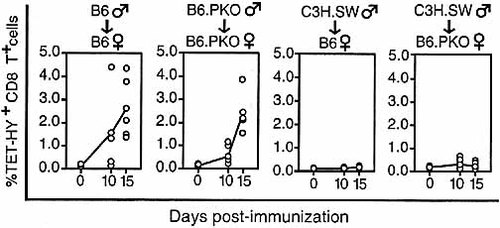
Immunodomination prevails in perforin-deficient mice. Expansion of HY-tetramer+ CD8+ T cells when HY was presented alone (B6 male cells) or with numerous other MiHA (C3H.SW male cells) to wild-type (B6) or perforin-deficient (B6.PKO) female mice. Each dot represents one individual. Lines indicate the mean percentage of HY-tetramer+ cells at various times with three to six mice per group.
3 Discussion
Our model has two noteworthy features. CD8+ T cell responses were studied in naive mice with a normal T cell repertoire: they were not transgenic, and their T cell repertoire was not modified by passively transferred T cells. Besides, antigens used for priming were normal endogenous epitopes expressed at physiological levels: APC were not transgenic, transfected, or peptide-coated. Our relatively uncontrived model suggests several points. When two or more MHC class I-associated epitopes are found on the same APC, the presence of a single dominant antigen, B6dom1, is sufficient to cause immunodomination. Yet B6dom1 itself is resistant to immunodomination when co-presented with more than 40 other epitopes. Immunodomination prevents expansion rather than differentiation of CD8+ T cells that recognize a non-dominant epitope, HY, and may be regulated by the CTL/APC ratio. Of special interest, the key difference between T cells that recognize B6dom1 and those recognizing HY is qualitative or functional rather than quantitative. The notable feature of T cells specific for B6dom1 is that they show striking up-regulation of molecules involved in CTL effector activity and not that they accumulate to particularly high levels (as assessed by tetramer staining).
One surprising finding was that, when immunodomination was precluded (Fig. 2 and 3), the rate of accumulation of HY-tetramer+ T cells was similar to that of B6dom1-tetramer+ T cells. Thus, the dominant status of B6dom1 does not depend on an intrinsically higher proliferative activity of cognate CD8+ T cells. The crucial difference between HY- and B6dom1-specific T cells seems somewhat related to their CTL effector activity estimated on the basis of two parameters: the expression of O-glycans recognized by 1B11 antibody and the intracellular perforin content. Compared with B6dom1-tetramer+ T cells, HY-tetramer+ T cells can accumulate at similar levels, yet are defective in acquiring effector function. This observation is consistent with recent evidence that, although T cell proliferation and acquisition of effector function are often correlated, they are not mechanistically linked 30. Supporting this idea, activated CD8+ T cells have been shown to vary in their lytic potential, a discrepancy determined by the nature of the epitope which they recognize 16, 28. Thus, even though HIV-specific CD8+ T cells can be present at very high frequencies in HIV-infected subjects, these CTL remain crippled in terms of cytotoxic activity relative to CMV-specific CTL 29, 39. Our study suggests that, aside from its impact on eradication of intracellular pathogens, development of effector function by CTL is responsible for immunodomination, and is thereby instrumental in shaping the repertoire of CTL responses. How does the nature of a given epitope regulate the development of effector function in cognate CTL? Previous studies with B6dom1 provide evidence that epitope density may be a key determinant because of its influence on the efficiency of TCR triggering 15. This contention is supported by the fact that, while B6dom1/Db complexes do not interact with their respective TCR with higher affinity than HY/Db complexes, the former epitopes are much more abundant at the surface of APC: 1,000 versus 10 copies per cell for B6dom1 and HY, respectively 15.
The fact that immunodomination is abrogated when the quantity of antigen-bearing APC is increased suggests that its essence is competition for APC resources (Fig. 3). This is consistent with studies of Kedl et al. 24 in a model based on immunization of mice with peptide-coated dendritic cells and adoptive transfer of TCR transgenic OT1 T cells specific for the dominant Kb-associated ovalbumin peptide. These authors showed that injection of OT1 CD8+ T cells inhibited the response of host antigen-specific T cells to two ovalbumin-derived peptides, and that this inhibition could be overcome by the injection of large numbers of antigen-pulsed dendritic cells. Our work extends these results in a non-transgenic model in which mice with a normal T cell repertoire were confronted with endogenous epitopes expressed at physiological levels.
How does competition for APC resources lead to the selective expansion of CTL specific for dominant epitopes and to the neglect of non-dominant epitopes? We consider the following three observations. When immunodomination occurred, expansion of CTL that recognize HY was aborted and not merely delayed (Fig. 2). Immunodomination was prevented when APC were present in very high numbers (Fig. 3). CTL specific for B6dom1 displayed superior effector function (Fig. 4) but not a higher intrinsic proliferative potential (Fig. 3). These data argue against two proposed mechanisms for CTL competition which hinge on the assumption that CTL specific for dominant epitopes might simply monopolizeaccess to the APC surface without impairing APC number or function 15, 24. First, it has been conjectured that, because of more rapid or extensive expansion, CTLspecific for dominant epitopes might simply outnumber other CTL at the APC surface 15, 18. Clearly, the rate of expansion of CTL populations in our experiments was not consistent with the prediction that B6dom1-specific CTL are endowed with a higher proliferative potential than their competitors. A second hypothesis was that by sticking to APC, CTL that recognize the dominant epitope could physically deny access by other CTL to the APC surface or to APC molecules recruited at the site of the immunological synapse 24. However, it is hard to reconcile this premise with evidence that antigen-specific naive T cells are rare, enter lymphoid organs asynchronously, and maintain vigorous migration upon cognate interactions with APC 40. Since each T cell-APC encounter is short-lived, with a median duration between 6 and 12 min, it is difficult to imagine how CTL specific for a given epitope could monopolize access to the surface of antigen-bearing APC during a period of several days if the latter APC remain functional.
We surmise that to abort the priming of competitor CTL, B6dom1-specific CTL must rapidly curtail the number or the function of antigen-bearing APC. In doing this, because of their superior effector activity, they reduce the time of interaction between competent APC and CTL below the duration threshold required to prime CTL that recognize non-dominant epitopes. First, this could be achieved by killing antigen-bearing APC. Supporting reports demonstrate that some 19, 41, but not all 24, CD4+ and CD8+ T cells rapidly eliminate antigen-bearing APC in vivo. One report 24 made use of exogenous peptide-coated APC where interactions with CTL apparently had no influence on thefate of APC. This approach may lead to an overestimation of APC survival since the half-life of peptide-MHC class I complexes at 37°C is usually a matter of a few hours 12, 15. Notably, we showed that CTL specific for B6dom1 rapidly eliminate APC in vivo, whereas CTL specific for HY do not 19. Our demonstration that B6dom1-specific CTL are endowed with superior effector activity (Fig. 4) provides a cogent explanation for this discrepancy. Nevertheless, here we furnish conclusiveevidence that perforin-mediated killing by itself is not required to cause immunodomination (Fig. 5). However, this observation should not be construed as a demonstration thatimmunodomination does not involve APC killing because other effector pathways such as involving ligands of the TNF family, might contribute to the rapid demise of antigen-bearing APC in vivo 19. Second, CTL specific for a dominant epitope could, because of their high effector activity, rapidly lead to exhaustion of APC. This assumption is consistent with evidence that within 24 h after antigen presentation, naive CD8+ T cells are able to trigger dendritic cell maturation 42, a process that can rapidly lead to dendritic cell exhaustion within a few hours 43. Demonstration that immunodomination is contingent on the rapid physical or functional demise of antigen-bearing APC will require identification of the molecular pathway(s) in-volved.
Regardless of whether it is caused by APC killing or APC exhaustion, the main consequence of immunodomination is to restrict the diversity of the repertoire of CTL responses. Deng et al. 13 raised the interesting possibility that the raison d'être of this limitation may be to lessen the risk of collateral autoimmune damage by cross-reactive CTL. Collectively, our datasuggest that immunodomination entails selective expansion of CTL with the best effector function, favoring quality over quantity.
4 Materials and methods
4.1 Mice
The following strains of mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and bred at the Guy-Bernier Research Center: C3H.SW, C57BL/6J (B6), C57BL/6-Pfptm1 Arp (B6.PKO), and C57BL/10J (B10). B10.C-H7b (47N)/Sn (B10.H7b) breeder mice were a kind gift of Dr. D. C. Roopenian (The Jackson Laboratory). Mice used between 6 and 16 weeks of age were maintained in specific pathogen-free conditions according to the standards of the Canadian Council on Animal Care.
4.2 Immunization and adoptive transfer
Mice were primed by i. p. injection of 2×107 spleen cells. In adoptive transfer experiments, spleen cell suspensions containing 5×106 CD8+ T cells were injected via the tail vein in irradiated (10 Gy) recipients.
4.3 Peptides
B6dom1 (AAPDNRETF) and HY (WMHHNMDLI) peptides were synthesized by the Sheldon Biotechnology Centre (Montreal, Canada). Purity, as determined by reversed phase-high performance liquidchromatography and mass spectrometric analysis, was above 97%.
4.4 MHC class I/peptide tetramers
MHC class I (H2Db)/peptide (B6dom1 or HY) tetramers were produced as previously described 44, 45. Recombinant β2-microglobulin and theextracellular domain of the MHC class I heavy chain containing the BirA recognition sequence in-frame at its C terminus were overexpressed in Escherichia coli as insoluble aggregates that formed inclusion bodies. Purified inclusion bodies were solubilized in urea, and monomeric H2Db complexes were refolded around peptide by dilution of denaturing buffer. Monomeric complexes were recovered by anion exchange chromatography over a Mono Q HR column (Pharmacia, Uppsala, Sweden). H2Db/peptide complexes were biotinylated using BirA enzyme (Avidity, Denver, CO) as described 46. Tetramers were generated by mixing the biotinylated monomeric complexes with NeutrAvidin-PE (Molecular Probes, Eugene, OR) at a 6:1 molar ratio. Then, biotinylated tetramers were purified by gel filtration over a Superdex 200 HR column (Pharmacia). Purified tetramers were stored at 1 mg/ml at 4°C and were frequently tested on B6dom1- and HY-specific T cell lines to document maintenance of staining capacity and signal intensity. B6dom1- and HY-specific CD8+ T cell lines have been previously described 22, 23.
4.5 Cell staining and flow cytometry
Cell suspensions were stained with allophycocyanin-conjugated anti-CD8 antibody (PharMingen, Mississauga, Canada), sometimes with 1B11 antibody (PharMingen), in PBS/BSA 0.1% for 25 min at 4°C,washed, and then incubated with 1 μg PE-labeled tetramers at 37°C for 40 min. TCR expression was monitored for antigen-specific T cell lines with FITC-conjugated anti-TCRα β antibody (PharMingen). Cells were analyzed on a FACSCaliburTM (Becton Dickinson, Mountain View, CA) using CellQuestTM software. Intracellular perforin labeling was performed as previously described 47. Briefly, splenocytes from mice primed in vivo against either B6dom1 or HY were incubated for 5 h with irradiated B10 male spleen cells in culture medium supplemented with 2 μg/ml brefeldin A (Sigma, Oakville, Canada), washed and permeabilized with 0.1% saponin (Sigma), and stained with anti-perforin antibody (clone KM585; Kamiya Biomedical, Seattle, WA) 29. Results for group means were compared using Student's t-test.
Acknowledgements
We thank Dr. R. P. Sékaly for the gift of plasmid clones, Dr. J. D. Altman for advice on the preparation of H2Db-peptide tetramers, Drs. N. Labrecque and P. Santamaria for thoughtful review of the manuscript and Ms. J. Kashul for editorial assistance. Grant 011189 from the National Cancer Institute of Canada supported this work.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




