Preferential Th1 profile of T helper cell responses in X-linked (Bruton′s) agammaglobulinemia
Abstract
X-linked agammaglobulinemia (XLA) is a primary immunodeficiency of the B-cell compartment caused by a defective gene encoding for the tyrosine kinase (btk) essential for B cell differentiation. Affected males undergo recurrent pyogenic infections and deficient immunoglobulin production. Peripheral blood T cells from 6 XLA patients and 6 matched healthy controls were stimulated with either PHA or tetanus toxoid (TT) and T cell clones obtained were compared for their cytokine profile. In the series of PHA-induced or TT-specific CD4+ T cell clones derived from XLA patients, the Th1 profile was predominant (63 and 65 %, respectively). Upon stimulation with TT, the proportion of activated T cells from XLA that expressed the IFN-γ -associated LAG-3 activation molecule was higher than in control T cells (51 vs. 25 %), whereas the expression of the IL-4-associated CD30 molecule was lower (5 vs. 21 %). In a cohort of 31 XLA patients, plasma levels of soluble (s)LAG-3 and sCD30, chosen as indirect indicators of the Th1 / Th2 activity in vivo, were significantly higher and lower, respectively, than those measured in 31 healthy controls. Likewise, plasma levels of interferon-inducible protein 10 and of macrophage-derived chemokine in XLA patients were significantly higher and lower, respectively, than in healthy controls.
Abbreviations:
-
- XLA:
-
X-linked agammaglobulinemia
-
- TT:
-
Tetanus toxoid
-
- LAG-3:
-
Lymphocyte activation gene-3
-
- IP-10:
-
Interferon-inducible protein 10
-
- MDC:
-
Macrophage-derived chemokine
1 Introduction
X-linked agammaglobulinemia (XLA) is an X chromosomal recessive immunodeficiency characterized by a B cell deficiency with very low serum levels of all immunoglobulin isotypes due to failure of pre-B cells to differentiate into Ig-secreting plasma cells 1. The defective gene responsible for XLA encodes for tyrosine kinase (btk), a cytoplasmic enzyme essential for B cell differentiation 2. Since btk gene is not expressed in mature T lymphocytes, the number of peripheral T cells and T cell subsets is usually normal in XLA patients. Because of thelack of antibody production, affected males undergo severe and recurrent bacterial infections since the first year of life whereas they are able to cope normally with viral infections, suggesting integrity of the mechanisms essential for the generation of efficient T cell priming and cytokine-dependent cell-mediated immunity which are important in conferring protection against viruses 3.
The cytokines produced by effector T cells are crucial for optimal clearance of pathogens. The patterns of released cytokines during specific immune responses are characteristic for distinct subsets of CD4+ T helper (Th) cells, whose polarized forms are Th1 and Th2 4, 5. Th1 cells produce IFN-γ , but not IL-4, express cytotoxicity, providehelp for the production of opsonizing and complement-fixing antibodies by B cells, promote cell-mediated immunity, macrophage activation and phagocytosis. Th2 cells secrete IL-4, IL-5, IL-6, IL-9, IL-10 and IL-13, but not IFN-γ , induce B cell activation, differentiation, and production of high levels of antibodies of all isotypes, recruit eosinophils and mast cells, and tend to inhibit several functions of macrophages. Th1 and Th2, however, are not the only detectable cytokine patterns, rather most of CD4+ Th cells express a mixed pattern, designated as type 0 (Th0), that usually exerts intermediate effects depending on the ratio of cytokines produced and the nature of responding cells. Human Th subsets show preferential expression of some surface molecules and chemokine receptors. Th1 preferentially express lymphocyte activation gene (LAG)-3 6, whereas CD30 is preferentially expressed by Th2 cells 7. Chemokine C receptor (CCR)5 and chemokine CX receptor (CXCR)3 preferentially associate with Th1 cells, whereas CCR3, CCR4, CCR8 and the chemoattractant receptor-homologous molecule CRTH2 are preferentially expressed by Th2 8. The differential expression of chemokine receptors by Th1 or Th2 cells can influence the selective homing in inflammatory sites of each of the two Th subsets, and chemokines, such as IFN-γ inducible protein 10 (IP-10) and macrophage-derived chemokine (MDC), were found to play a role in Th cells migration into tissues 9.
Taking advantage of the preferential expression of certain activation markers and chemokines by Th1 or Th2 cells, we tried to assess whether the Th cell compartment of XLA patients is normally balanced or it is biased toward a predominant Th1 or Th2 pattern.
2 Results
2.1 Predominant Th1 profile of T cell clones derived from peripheral blood T cells of XLA patients
Pheripheral blood T cells from 6 XLA patients (Table 1) and 6 age- and sex-matched healthy controls were cloned by limiting dilution in the presence of PHA and T cell clones obtained were compared for their cytokine secretion profile. As shown in Table 2, in the series of 176 CD4+ PHA-induced T cell clones from XLA patients the proportion of clones with Th1 profile (63 %) was much higher (p < 0.0005) than that found in the parallel series of 192 CD4+ T cell clones from healthy controls (27 %) generated under the same experimental conditions. Conversely, PHA stimulation of peripheral blood T cells of XLA patients resulted in a significantly lower proportions of Th0 (34 %) and Th2 (3 %) clones than those found in control clones (60 and 13 %, respectively).
|
Patients Sex / Age (y) |
btk gene mutation |
IgM |
IgG mg / dl |
IgA |
IgE kU / ml |
Isoagglutinins |
B cells / mm3 |
T cells / mm3 |
|---|---|---|---|---|---|---|---|---|
|
P.M.(M17) |
single base deletion |
< 8 |
63 |
< 6 |
2 |
Absent |
< 10 |
1,480 |
|
F.F.(M11) |
base pair insertion |
38 |
108 |
< 6 |
4 |
Absent |
< 10 |
2,520 |
|
A.M.(M13) |
missense mutation |
< 8 |
161 |
< 6 |
< 2 |
Absent |
< 10 |
1,650 |
|
G.A.(M30) |
missense mutation |
< 8 |
60 |
9 |
< 2 |
Absent |
< 10 |
740 |
|
M.A.(M15) |
missense mutation |
< 8 |
310 |
< 6 |
8 |
Absent |
< 10 |
1,570 |
|
A.A.(M19) |
missense mutation |
< 8 |
290 |
< 6 |
< 2 |
Absent |
< 10 |
1,460 |
|
Patients |
No. of CD4+ T cell clones obtained |
Cytokine profile |
||
|---|---|---|---|---|
|
|
Th1 |
Th0 |
Th2 |
|
|
P.M. |
34 |
20 (59)* |
12 (35) |
2 (6) |
|
F.F. |
24 |
16 (67) |
6 (25) |
2 (8) |
|
A.M. |
24 |
16 (67) |
8 (33) |
0 (−) |
|
G.A. |
20 |
18 (90) |
2 (10) |
0 (−) |
|
M.A. |
38 |
20 (53) |
18 (47) |
0 (−) |
|
A.A. |
36 |
20 (56) |
14 (39) |
2 (5) |
|
All XLA cases |
176 |
110 (63)a |
60 (34)c |
6 (3)e |
|
Controls (n = 6) |
192 |
52 (27)b |
115 (60)d |
25 (13)f |
- Freshly isolated PBMC were cloned under limiting dilution conditions (0.3 cell / well) in microwells containing 105 irradiated autologous PBMC (as feeder cells), PHA (1 % vol / vol) and IL-2 (20 U / ml). T cell blasts (106 / ml) of CD4+ T cell clones obtained were stimulated for 36 h in the presence of PMA (10 ng / ml) plus anti-CD3 mAb (200 ng / ml) and culture supernatants were assayed for IFN-γ and IL-4 content. T cell clones producing IFN-γ , but not IL-4, were coded as Th1; clones producing IL-4, but not IFN-γ , were coded as Th2; clones producing both IFN-γ and IL-4 were coded as Th0.
- * Percent values in parentheses; a vs. b: χ2 46.74, p < 0.0005; c vs. d: χ2 24.51, p < 0.0005; e vs. f: χ2 10.99, p < 0.001.
Since the pattern of cytokines induced by a polyclonal activator, such as PHA, might be different from that induced by antigen stimulation, tetanus toxoid (TT)-specific T cell clones were generated from TT-specific T cell lines derived from peripheral blood T cells of the same XLA patients and controls. Two series of 115 and 136 TT-specific CD4+ T cell clones were derived from XLA patients and controls, respectively. As shown in Table 3, a significantly higher proportion of polarised Th1 (65 vs. 21 %), and a significantly lower proportion of polarized Th2 clones (1 vs. 37 %) were found in XLA patients in comparison with controls. Taken together, these data suggest that, at least under these experimental conditions, the peripheral T-cell compartment of XLA patients tends to express higher potential for IFN-γ , and lower potential for IL-4, production in vitro.
|
Patients |
No. of TT-specific CD4+ T cell clones obtained |
Cytokine profile |
||
|---|---|---|---|---|
|
|
Th1 |
Th0 |
Th2 |
|
|
P.M. |
28 |
22 (79)* |
6 (21) |
0 (−) |
|
F.F. |
26 |
21 (81) |
5 (19) |
0 (−) |
|
A.M. |
19 |
13 (68) |
5 (26) |
1 (6) |
|
G.A. |
14 |
7 (50) |
7 (50) |
0 (−) |
|
M.A. |
11 |
4 (36) |
7 (64) |
0 (−) |
|
A.A. |
17 |
8 (47) |
9 (53) |
0 (−) |
|
All XLA cases |
115 |
75 (65)a |
39 (34) |
1 (1)c |
|
Controls (n = 6) |
136 |
29 (21)b |
57 (42) |
50 (37)d |
- T cell blasts (106 / ml) of TT-specific CD4+ T cell clones obtained were stimulated for 48 h with TT (0.5 μ g / ml) in the presence of irradiated autologous feeder cells and culture supernatants were assayed for cytokine content.
- * Percent values in parentheses; a vs. b: χ2 49.47, p < 0.0005; c vs. d: χ2 49.58, p < 0.0005.
2.2 LAG-3 and CD30 expression by antigen-activated T cells in XLA patients
The preferential expression of Th1 profile by peripheral blood T cells of XLA patients was somehow predictable on the basis of the kinetics of LAG-3 and CD30 membrane expression in their T cell cultures activated with TT. In these experiments, PBMC from 6 XLA patients and 6 control subjects were stimulated with TT and cell cultures were assayed for both LAG-3 and CD30 membrane expression on day 0, 5, and 10. Membrane expression of either LAG-3 or CD30 was virtually absent on day 0 and it remained undetectable on day 5 and 10 in control cultures stimulated with medium alone. However, in TT-stimulated cultures from XLA patients, the mean proportion of T cell blasts expressing membrane LAG-3 increased up to 25 % on day 5 and to 51 % on day 10, whereas in TT-stimulated cultures from healthy controls, the increase of LAG-3+ T cell blasts was substantially (p < 0.005) lower (10.5 % on day 5 and 25 % on day 10, Fig. 1). Conversely, TT-stimulated cultures from XLA patients showed significantly (p < 0.001) lower proportions of CD30+ T cell blasts (3.7 % on day 5 and 5 % on day 10) than TT-stimulated cultures from healthy controls, where CD30+ T cell blasts increased up to 13.4 % on day 5 and 20.7 % on day 10 (Fig. 1). Since the membrane expression of LAG-3 and its secretion in soluble form by activated T cells is associated with IFN-γ secretion 6, whereas CD30 membrane expression and secretion in soluble form is mainly associated with IL-4 production 7, 10, the results of these experiments confirm the observations obtained at clonal level, suggesting that T cell responsiveness in XLA patients is preferentially skewed towards the Th1 profile.
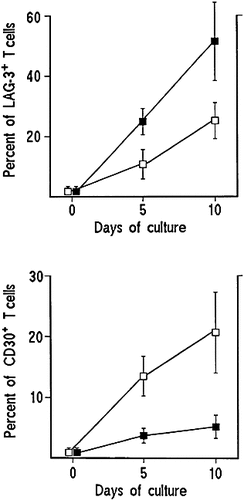
Different membrane expression of LAG-3 and CD30 activation molecules by TT-activated T cell lines generated from PBMC of XLA patients and healthy controls. TT-stimulated cultures were assayed for both LAG-3 and CD30 membrane expression on day 0, 5, and 10 using an FITC-conjugated anti-CD30 mAb, and biotinylated anti-LAG-3 mAb, followed by PE-conjugated streptavidin. Analysis was performed on a FACSCalibur cytofluorimeter. Results represent mean percent values (± SD) of positive cells from the same 6 XLA patients (closed symbols) and 6 healthy controls (open symbols) reported in Tables 2 and 3.
2.3 Serum LAG-3, IP-10, MDC, and CD30 levels in XLA patients
In order to assess the Th1 / Th2 balance in a larger cohort of XLA patients and to provide evidence that the results obtained in vitro reflected a situation also occurring in vivo, soluble LAG-3 (sLAG-3), and CD30 (sCD30) were measured in the plasma of a total number of 31 XLA patients and 31 matched healthy controls. In addition, the levels of IP-10 and MDC, two chemokines whose production has been found to be preferentially associated with the secretion of IFN-γ or IL-4, respectively 11, 12, were measured. In XLA patients, the median of sLAG-3 plasma levels (6.8 kU / ml) was higher than that measured in healthy controls (2.9 kU / ml); conversely the median of sCD30 plasma levels (9.5 U / ml) was lower than that of controls (17.5 U / ml) (Fig. 2), thus indirectly supporting the concept that Th1 activity was higher, and Th2 activity was lower, in XLA patients than in healthy controls. The results of measurement of IP-10 and MDC plasma levels were in agreement with this notion. In fact, in XLA patients the median of IP-10 plasma levels (0.58 vs. 0.27 ng / ml) was higher, whereas the median of MDC plasma levels (0.42 vs. 0,69 ng / ml) was lower than in controls (Fig. 2).
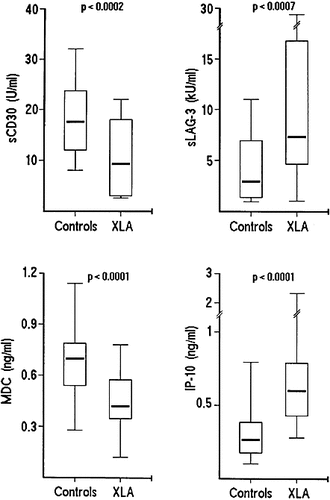
Plasma levels of sLAG-3, sCD30, IP-10 and MDC measured in 31 XLA patients and 31 healthy controls. Median values of sLAG-3, sCD30, IP-10 and MDC are indicated with horizontal bars. The vertical bars indicate the range and horizontal boundaries of the boxes represent the first and third quartiles.
3 Discussion
The results reported in this study indicate that either mitogen or antigen stimulation of peripheral blood T cells from XLA patients result in the activation and clonal expansion of T cells with predominant Th1-type cytokine profile, whereas the same activation signals induce peripheral blood T cells from healthy controls to expand into clonal progenies with predominant Th0 profile, as already reported in earlier studies 13, 14. In agreement with these data are the different kinetics and degree of membrane expression of the IFN-γ -associated LAG-3 6, 15 or the IL-4-related CD30 7, 16 molecules by TT-activated T cells from XLA patients and healthy controls.
IFN-γ and IL-4 would theoretically represent the best tool for tracking Th1 / Th2 responses in vivo. However, since these cytokines are short-range molecules that are rapidly bound by their receptors and / or inactivated by proteases, their levels in the plasma are not reliable to assess the Th1 / Th2 balance in vivo. In order to circumvent this problem and the obvious limits of the clonal analysis, feasible in only a few patients, assays were developed to measure the plasma levels of indirect indicators of preferential Th1 or Th2 activity, such sLAG-3 or sCD30, respectively. Significantly higher levels of sLAG-3 and lower levels of sCD30 were measured in the plasma of 31 XLA patients when compared with an equal cohort of healthy controls. Interestingly, elevated plasma levels of sCD30 were found in Th2-dominated conditions, such as chronic graft versus host disease, and systemic sclerosis, but not in Th1-dominated disorders, such as multiple sclerosis or Crohn′s disease 17.
In this study, plasma levels of two chemokines, such as IP-10 and MDC, were also measured in XLA patients and their healthy controls. MDC and IP-10 can be produced by many different cell typesin different phases of immune and non-immune responses, and therefore their plasma levels may reflect activation of cell types other than those related to the T cell compartment. However, IP-10 is not only induced by IFN-γ , but also acts preferentially on Th1 cells, thus contributing to Th1-related effector functions 11, 18 – 20. MDC exerts a potent chemoattraction for monocytes, monocyte-derived dendritic cells, natural killer cells, and activated T lymphocyte, preferentially those with Th2-type effector functions 12, 21, and high MDC plasma levels have been found in patients suffering from Th2-dominated conditions 12. The observation that higher IP-10 and lower MDC levels were detectable in the plasma of XLA in comparison with healthy individuals, also indirectly supports the notion that T cell responses in XLA patients are preferentially skewed to the Th1 pattern.
The reason why XLA patients are biased to predominant Th1 responses is presently unclear. One possibility is that the Th1 predominancy is dependent on the primary genetic defect at the level of btk gene. In agreement with this hypothesis, mice with mutant nonfunctional btk gene were found to express increased IFN-γ production by immune T cells and increased IL-12 production by macrophages in comparison with wild-type counterparts 22. Another possibility is that the lack antigen presentation by B cells, due to their deficiency, is responsible for poor development of Th2 responses, and hence for hampered down-regulation of the Th1 compartment. This hypothesis may be supported by the observation that the preferential expansion of IL4-producing cells wouldmaximally occur when stimulated with antigens presented by B cells, which are also the principal targets of their cytokines 23. Finally, the possibility also exists that the Th1 bias in XLA patients is secondary to iatrogenic or environmental factors. XLA patients undergo recurrent intravenous immunoglobulin (IVIG) infusions to overcome infections. Indeed, moderate inhibitory effects by IVIG on T cell activation and cytokine production in vitro have been reported 24. However, the study of the effects of IVIG on cytokine regulation in vivo using samples taken before and after replacement-dose IVIG in a group of patients with common variable immunodeficiency or XLA showed that IFN-γ expression was not affected 25. In XLA patients the main critical events are frequent and severe bacterial infections that in pre-IVIG and antibiotic "era" invariantly led those patients to death. The recurrent challenge by bacterial LPS and / or other signals able to trigger IL-12 production by phagocytic and antigen-presenting cells can be the major cause of the preferential Th1 profile shown by most of peripheral T cells of XLA patients. Once well established, Th1 predominancy may self maintain through several mechanisms. IFN-γ and the MHC class II ligand LAG-3 are indeed able to stimulate dendritic cells to produce IL-12, further supporting Th1 development 26.
On possible implication of the results reported in this study may be the explanation for the increased prevalence of autoimmune disorders and the decreased prevalence of allergy in patients with XLA. It has indeed been noticed that Th1-oriented diseases, such as non-septic, rheumatoid-like, arthritis, or type 1 diabetes mellitus, frequently occur in XLA patients 27, 28. In this regard, it has been shown that, in the absence of CD30 signalling, T cells activated, but not yet deleted by the CD95-dependent cross-tolerance mechanism, gain the abilityto proliferate extensively upon secondary encounter with antigen on parenchymal tissues, such as the pancreatic islets. Thus, in a Th1-dominated conditions, where CD30 signalling is hampered, the proliferative potential of autoreactive effector T cells would remain out of control, leading to organ-specific autoimmunity 29 . On the other hand, a Th1 bias in XLA seems to protect from atopy, as suggested by the lack of reports on atopic disorders in XLA patients, in spite of the relatively high frequency of these disorders in the general population 30. The failure to produce immunoglobulins, including IgE, is not a reasonable explanation for this phenomenon, because it has been shown that T cell cytokines secreted in response to allergens play a prominentrole in induction and maintenance of airway allergic inflammation and hyperreactivity, that may occur also in the absence of IgE or B cells 31.
4 Materials and methods
4.1 Patients and healthy controls
Thirty-one male patients (mean age 18 years, range 2 – 37) classified as XLA according to the WHO classification of primary immunodeficiency 1 and 31 age-matched healthy volunteers (mean age 18 years, range 4 – 35) referred to the Pediatric Department of University of Brescia since their infancy were included in this study. All patients and controls received a TT booster within 3 years. Peripheral blood was obtained after informed consent and sample size was kept small according to the guidelines of the ethical committee. No overt infectious disease was present at the time of blood sampling. Six of the XLA patients recruited and six age-matched healthy controls agreed to be enroled also in the in vitro studies of T cell responsiveness. Clinical andlaboratory data of these XLA patients are summarized in Table 1. All the XLA patients suffered from severe recurrent bacterial infections from early infancy, with no clinical evidence of atopic disorder.
4.2 Reagents
TT was kindly provided by Istituto Sieroterapico e Vaccinogeno Sclavo (Siena, Italy). Recombinant human IL-2 was a kind gift from Eurocetus (Milano, Italy). PHA was purchased from Gibco Laboratories (Grand Island, NY). Anti-CD3 (Leu 4), anti-CD4 (Leu 3a), and anti-CD8 (Leu 2a) mAb were purchased from Becton Dickinson (San Jose, CA). The anti-LAG-3 mAb recognizing three different epitopesof LAG-3 molecule (17B4, IgG1; 11E3, IgG1; and 4F4, IgM) were obtained form Ares Serono (Geneva, Switzerland). Anti-CD30 (Ber-H2) mAb was purchased from Dako (Glostrup, Denmark).
4.3 Generation of antigen-specific T cell lines and clones
Freshly isolated PBMC were cloned according to a previously described technique that allows the clonal expansion of virtually every single T cell, regardless of its antigen specificity 32. Briefly, PBMC were seeded under limiting dilution conditions (0.3 cell / well) in round-bottomed microwells containing 105 irradiated autologous PBMC as feeder cells and PHA(1 % v / v) in a final volume of 0.2 ml RPMI 1640 supplemented with IL-2 (20 U / ml) and 10 % FCS (HyClone Laboratories, Logan UT). Growing microcultures were then supplemented at weekly intervals with IL-2 (20 U / ml) and 105 irradiated feeder cells.
TT-specific T-cell lines and clones were generated as described 5. Briefly, 106 PBMC in 2 ml of RPMI 1640 medium supplemented with 2 mM L-glutamine, 2 × 10– 5 M 2-ME and 5 % human serum (complete medium) were stimulated with TT (0.5 μ g / ml) in 24-well flat-bottom plates for 5 days. Human IL-2 (20 U / ml) was then added and cultures were continued for 7 days. Viable T cell blasts were resuspended in complete medium and tested for their TT specificity before cloning. To assess their Ag specificity, 2 × 104 T cell blasts were co-cultured for 48 h with irradiated autologous PBMC (105) in the presence of medium or TT (0.5 μ g / ml). After a 16-h pulse with 0.5 μ Ci [3H] dThd (Amersham International), cultures were harvested and radioactivity was measured by liquid scintillation. To generate TT-specific T cell clones, single T cell blasts obtained from TT-specific lines were seeded under limiting dilution (0.3 cells / well) in round-bottom microwells containing 105 irradiated autologous PBMC as feeder cells and PHA (1 % v / v), as reported 5.
4.4 Cytofluorimetric analysis of cell surface markers and characterization of the cytokine profile of T cell lines and clones
Cell surface marker analysis of T cells was carried out by two- or three-color flow cytometry using fluorochrome-conjugated anti-CD3, anti-CD4, anti-CD8, and anti-CD30 mAb, and biotinylated anti-LAG-3 mAb (17B4), followed by PE-conjugated streptavidin, as reported 6.
To elicit cytokine production by PHA-induced T cell clones, T cell blasts were resuspended at 106 / ml of complete medium and cultured for 36 h in the presence of PMA (10 ng / ml) plus anti-CD3 monoclonal antibody (200 ng / ml). To induce the cytokine production by TT-specific clones, 106 T cell blasts of each clone were co-cultured for 48 h in 1 ml medium with 5 × 105 irradiated autologous PBMC as APC with or without TT (0.5 μ g / ml). Cell-free supernatants were collected and assayed in duplicate for IFN-γ , and IL-4 (BioSource International, Camarillo, CA). Supernatants showing IFN-γ and IL-4 levels 5 SD over the mean levels in control supernatants derived from irradiated feeder cells alone were regarded as positive. T cells clones able to produce IFN-γ , but not IL-4 were categorized as Th1, clones able to produce IL-4 but not IFN-γ , as Th2, and clones producing both IFN-γ and IL-4 as Th0.
4.5 Quantitation of soluble LAG-3, CD30, IP-10 and MDC
Serum soluble(s) LAG-3 was assessed by a in-house ELISA using 11E3 anti-LAG-3 mAb as a capture antibody, and 17B4 biotinylated anti-LAG-3 mAb as a revealing antibody, as previously described 6. LAG-3 levels were indicated in arbitrary units using as a reference reagent a pool of culture supernatants from activated Th1 clones containing high amounts of sLAG-3 (100 arbitrary kU / ml). sCD30 levels were measured by a sandwich enzyme-linked immunoabsorbent assay (Dako, Glostrup, Denmark). IP-10 and MDC levels were measured by commercial assays (Pharmingen, San Diego,CA and R & D Systems, Minneapolis, MN, respectively).
4.6 Statistical analysis
Statistical analyses were done with the χ2 methods or the Wilcoxon signed-rank test and the Mann-Whitney test for comparison on independent groups.
Acknowledgements
This study was supported by grants provided by AIRC, University of Florence, and Istituto Superiore di Sanità.
- WILEY-VCH
- WILEY-VCH




