Structural and functional dissection of the cytoplasmic domain of the transmembrane adaptor protein SIT (SHP2-interacting transmembrane adaptor protein)
The first two authors contributed equally to this work.
Abstract
SIT (SHP2-interacting transmembrane adaptor protein) is a recently identified transmembrane adaptor protein, which is expressed in lymphocytes. Its structural properties, in particular the presence of five potential tyrosine phosphorylation sites, suggest involvement of SIT in TCR-mediated recruitment of SH2 domain-containing intracellular signaling molecules to the plasma membrane. Indeed, it has recently been demonstrated that SIT inducibly interacts with the SH2-containing protein tyrosine phosphatase 2 (SHP2) via an immunoreceptor tyrosine-based inhibition motif (ITIM). Moreover, SIT is capable to inhibit TCR-mediated signals proximal of activation of protein kinase C. However, inhibition of T cell activation by SIT occurs independently of SHP2 binding. The present study was performed to further characterize the molecular interaction between SIT and intracellular effector molecules and to identify the protein(s) mediating its inhibitory function. We demonstrate that SIT not only interacts with SHP2 but also with the adaptor protein Grb2 via two consensus YxN motifs. However, mutation of both Grb2-binding sites also does not influence the inhibitory function of SIT. In contrast, mutation of the tyrosine-based signaling motif Y168 ASV completely abrogates the ability of SIT to inhibit T cell activation. Co-precipitation experiments revealed that the tyrosine kinase p50csk could represent the negative regulatory effector molecule that binds to this motif.
Abbreviations:
-
- Csk:
-
C terminal src kinase
-
- Grb2:
-
Growth factor receptor binding protein 2
-
- LAT:
-
Linker for activation of T cells
-
- NF-AT:
-
Nuclear factor of activated T cells
-
- PAG:
-
Phosphoprotein associated with GEM
-
- PAG:
-
Phosphoprotein associated with GEM
-
- PI3-K:
-
Phosphatidylinositol 3-kinase
-
- PLC:
-
Phospholipase C
-
- PTK:
-
Protein tyrosine kinase
-
- SH:
-
Src-homology
-
- SHP:
-
SH2-containing protein tyrosine phosphatase
-
- SHIP:
-
SH2-domain containing inositol phosphatase
-
- SIT:
-
SHP2-interacting transmembrane adaptor protein
-
- SLP:
-
SH2 domain containing leukocyte phosphoprotein
1 Introduction
Among the earliest intracellular events that occur after engagement of the T cell receptor (TCR) complex are tyrosine phosphorylations of numerous proteins which result from activation of protein tyrosine kinases (PTK) of the Src (e. g. Lck, Fyn), Syk (ZAP-70, Syk) and Tec (e. g. ltk) family (for recent reviews see 1 – 4). It is now well established that these initial phosphorylations are a pre-requisite to allow coupling of the TCR to intracellular signaling pathways such as the Ras / Raf / MAPK pathway or the calcineurin /NF-AT pathway. Moreover, it is well documented that TCR-mediated tyrosine phosphorylations are accompanied by and are required for the translocation of intracellular signaling molecules (e. g. Grb2, SHP2, SLP-76 and others) from the cytosol to the inner leaflet of the plasma membrane. The precise molecular mechanisms whereby these cytoplasmic effector and adaptor molecules are targeted to the plasma membrane after TCR engagement were until recently only poorly understood. Major progress in solving this puzzle has been achieved through the identification of a novel group of proteins that has been termed transmembrane adaptor proteins. These polypeptides are characterized by very short extracellular domains and comparatively long cytoplasmic tails, which contain several tyrosine-based signaling motifs (between five and ten). Upon phosphorylation by Src and / or Syk-PTK the tyrosine-based signaling motifs become rapidly phosphorylated and then serve as docking sites for SH2 domain-containing intracellular signaling molecules (reviewed in 4, 5).
The so far best characterized transmembrane adaptor protein is LAT (linker for activation of T cells) 6, 7. LAT is a type III transmembrane protein that carries 10 tyrosine residues in its cytoplasmic tail, among which 6 represent potential tyrosine-based signaling motifs. LAT becomes rapidly tyrosine phosphorylated after T cell activation by ZAP-70 and then directly interacts with the SH2-domains of Grb2, Gads and PLCγ 1 and indirectly with other intracellular signaling molecules including PI3-kinase, SLP-76 and Cbl 6, 7. Compelling evidence that LAT is required for the propagation of TCR-mediated signals was provided by the analysis of LAT-deficient Jurkat variants. These cells exhibit severe defects in TCR-mediated phosphorylation and activation of PLCγ 1, generation of calcium fluxes, activation of the Ras pathway and IL2-gene expression 8, 9. Moreover, LAT-deficient mice show an arrest in thymic development at the CD4–CD8– stage that results in a loss of mature peripheral blood T-cells 10. All these data suggest that LAT is indispensable for T cell activation and T cell development.
Shortly after the identification of LAT, the molecular cloning of three additional transmembrane adaptor proteins, TRIM (T cell receptor interacting molecule) 11, SIT (SHP2-interacting transmembrane adaptor protein) 12 and PAG / Cbp (protein associated with GEMs / Csk binding protein) 13, 14 has been reported.
TRIM has been initially indentified as a T cell-specific disulfide-linked homodimer which associates and co-modulates with the TCR / CD3 / ζ complex 15, 16. TRIM becomes tyrosine phosphorylates by Src kinases and then associates with the 85-kDa subunit of PI3-kinase via a typical consensus YxxM motif and possibly via its YGNL and / or YASL motifs with two additional so far non-identified polypeptides of 43 and 50 kDa. However, the function of TRIM remains elusive.
The most recently identified transmembrane adaptor protein PAG / Cbp is a type III transmembrane protein with an extracellular domain of 16 amino acids (aa) and a 397 aa cytoplasmic tail 13, 14. In contrast to the other transmembrane adaptor proteins known so far, PAG is ubiquitously expressed. In its cytoplasmic tail it carries a total of 10 potential tyrosine-based signaling motifs among which at least one (Y317 in human PAG) mediates an association with the PTK Csk, the major negative regulator of Src-family kinases. Thus, PAG / Cbp could be involved in negative regulation of T cell activation by recruiting Csk to the plasma membrane followed by down-regulation of T cell activation. Accordingly, overexpression of PAG in COS or 293T cells attenuates the kinase activity of Src and Fyn via a mechanism that involves binding of Csk 13, 14. In resting peripheral blood T cells PAG is expressed as a constitutively tyrosine-phosphorylated polypeptide that recruits Csk to the membrane. Perhaps more importantly, immediately after T cell activation PAG becomes dephosphorylated by an unknown protein tyrosine phosphatase and releases Csk 13. These data suggest that one function of PAG might be to down-regulate the enzymatic activities of Src kinases in resting T cells by recruiting Csk and thus to participate in keeping the cells in a quiescent state.
SIT is a disulfide-linked homodimeric polypeptide which is exclusively expressed in lymphocytes. In contrast to the other transmembrane adaptor proteins known so far, SIT is a heavily glycosylated polypeptide 12. This could indicate that SIT possesses an external ligand that modulates its function. The cytoplasmic domain of SIT contains five potential tyrosine phosphorylation sites among which one is an ITIM. This motif mediates an inducible interaction with the tyrosine phosphatase SHP2. Importantly, overexpression of SIT in Jurkat T cells inhibits TCR-mediated activation of the nuclear factor of T cells (NF-AT) via a mechanism that is likely located upstream of the activation of phospholipase C (PLC) and protein kinase C (PKC). However, mutation of the tyrosine residue within the ITIM does not prevent SIT-mediated inhibition of NF-AT activity although it almost completely abrogates the interaction between SIT and SHP2 12. These data suggest that SIT exerts its inhibitory function via a mechanism that does not involve SHP2 or the ITIM. The present study was conducted to better understand the molecular mechanisms leading to tyrosine phosphorylation of SIT and to elucidate the structural basis for its negative regulatory function during T cell activation.
2 Results
2.1 Identification of the sites of tyrosine phosphorylation within the cytoplasmic domain of SIT
The cytoplasmic domain of SIT carries a total of five potential sites of tyrosine phosphorylation. These are Y90GNL, Y128TSL, VKY148SEV (ITIM), Y168ASV and Y188ANS. To assess which of these five tyrosines represent in vivo phosphorylation sites, we generated a series of chimeric SIT molecules in which the cytoplasmic tail of SIT was fused to the transmembrane and the extracellular domains of CD8. The cytoplasmic domains of the individual CD8 / CD8 / SIT chimeras corresponded either to wild-type SIT or carried mutations of four of the five potential tyrosine phosphorylation sites. A scheme depicting the various CD8 / CD8 / 4F chimeras that we used in these experiments is shown in Fig. 1 B.
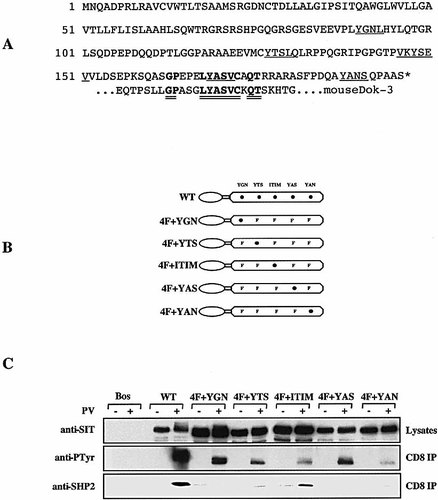
Identification of the tyrosine phosphorylation sites within in the cytoplasmic domain of SIT. (A) Amino acid sequence of human SIT. The five tyrosine-based signaling motifs are underlined. The amino acids that are conserved between human SIT and Dok-3 (see Sect. 3) are double underlined. (B) A cDNA construct coding for a chimeric molecule encompassing the extracellular and transmembrane domain of human CD8 fused to the cytoplasmic portion of SIT was generated and cloned into the pEF-Bos eukaryotic expression vector. The wild-type CD8 / CD8 / SIT construct was then used as a template to generate the depicted variants in which individual tyrosine residues (•) were replaced by phenylalanines (F). (C) cDNA constructs encoding the SIT chimeras depicted in (B) were transiently expressed in Jurkat T cells by electroporation. Following overnight incubation, the cells were activated for 2 min with pervanadate (PV). Subsequently cells were lysed in NP 40-containing buffer and subjected to CD8 immunoprecipitation. Following SDS-PAGE, proteins were subjected to sequential anti-PTyr anti-SHP2 and anti-SIT Western blotting.
The individual cDNA contructs were transfected into Jurkat T cells by electroporation. Following overnight incubation the expression of the chimeras was assessed by FACS analysis (see e. g. Fig. 4) and by anti-SIT Western blotting (Fig. 1 C). Subsequently, the transfectants were either left untreated or were briefly treated with pervanadate, lysed in NP40-containing buffer, subjected to CD8 immunoprecipitation followed by anti-phosphotyrosine (PTyr) Western-blotting. Fig. 1 C demonstrates that under these experimental conditions the CD8 / CD8 / SIT, the CD8 / CD8 / 4F + YGN and the CD8 / CD8 / 4F + YAS chimeras became strongly tyrosine phosphorylated, whereas the phosphorylation of the CD8 / CD8 / 4F + YTS, the CD8 / CD8 / 4F + ITIM and the CD8 / CD8 / 4F + YAN chimeras was much weaker. The overall level of tyrosine phosphorylations was YGNL ≥ YASV > YTSL > ITIM > YANS. The data indicate that the major sites of SIT tyrosine phosphorylation are the YGNL and the YASV motifs. However, the YTSL, the ITIM and the YANS motifs likely also become tyrosine phosphorylated after T cell activation. Indeed, when we analyzed the individual CD8 immunoprecipitates shown in Fig. 1 C for co-precipitation of SHP-2, we observed that SHP2 was recruited by the wild-type CD8 / CD8 / SIT chimera and the CD8 / CD8 / 4F + ITIM chimera but not by any of the other mutants tested (Fig. 1 C, middle panel). This confirms our previous data which had suggested that the ITIM represents the in vivo phosphorylation site mediating the association between SIT and SHP2 (12 and see also below).
2.2 SIT associates with SHP2 and Grb2 after T cell activation
The YGNL motif of SIT represents a potential binding site for the SH2 domain of the cytosolic adaptor protein Grb2 17. Given the above finding that this motif likely represents a major site of tyrosine phosphorylation we next assessed whether SIT, besides recruiting SHP2, would also associated with Grb2 after T cell activation. To this end Jurkat cells were stimulated for increasing periods of time with the clonotypic anti-TCR mAb C305 and then lysed in NP40-containing buffer. Subsequently, anti-SIT immunoprecipitates were prepared and analyzed for SIT tyrosine phosphorylation and co-precipitation of SHP2 and Grb2 by Western blotting. The upper panel of Fig. 2 A demonstrates that an increase in SIT phosphotyrosine content is already detectable after 1 min of TCR-mediated activation of Jurkat T cells and that it remains detectable for at least 1 h. As previously reported 12, the rapid increase in SIT phosphorylation is accompanied by an association between SIT and SHP2 (Fig. 2 A, middle panel). Perhaps more importantly, a time-dependent interaction of SIT with Grb2 was also detectable (Fig. 2 A, lower panel). Densitometric analysis of the blots revealed that the interaction between SIT and SHP2 or Grb2 was peaking at approximately 5 min after TCR engagement, and then slowly declined (Fig. 2 B). These results demonstrate that SIT not only associates with SHP2 but also with Grb2 upon T cell activation.
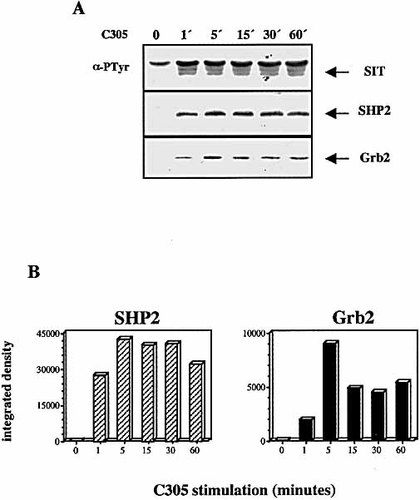
TCR stimulation of Jurkat cells results in the recruitment of Grb2 and SHP2 to SIT. (A) Jurkat cells (4 × 107) were stimulated with C305 mAb for the indicated periods of time at 37 °C. Lysates were subjected to immunoprecipitation using anti-SIT antibodies, separated on a SDS-10 % PAGE, and immunoblotted with the anti-PTyr mAb 4G10 (upper panel). The membrane was subsequently stripped and reprobed with anti-SHP2 (middle panel) and anti-Grb2 (lower panel) mAbs. (B) Densitometric analysis of the bands corresponding to SHP2 and Grb2 shown in (A).
2.3 Grb2 binds to the YGNL and YANS motifs of SIT
To assess whether the inducible association between SIT and Grb2 is mediated via the YGNL motif we overexpressed CD8 / CD8 / SIT chimeras in Jurkat T-cells in which single tyrosine residues were mutated to phenylalanine (see Fig. 3 A for a schematic representation of the individual cDNA constructs). Again, the transfectants were left unstimulated or were treated with pervanadate. Subsequently, NP40 lysates were prepared and subjected to anti-CD8 immunoprecipitation. Immunoprecipitates were then analyzed for the presence of SHP2 and Grb2 by Western blotting (Fig. 3 B). These experiments revealed that the YTSL, the ITIM and the YASV motif are dispensable for the association between SIT and Grb2 since almost identical amounts of Grb2 were detectable in CD8 precipitates prepared from transfectants expressing these three mutants compared to cells expressing wild-type SIT (Fig. 3 B).
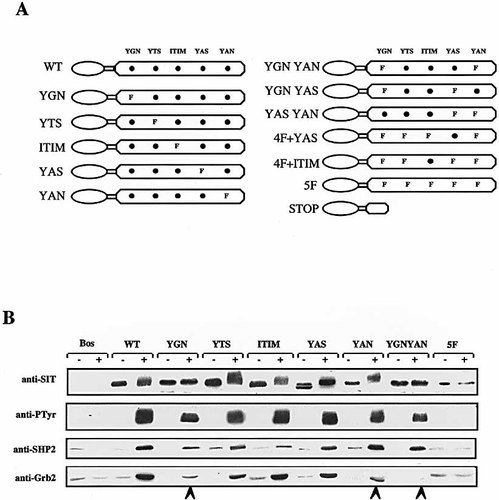
The YANS and YGNL motifs of SIT mediate the association with Grb2. (A) Schematic representation of the cDNA constructs used for the experiments shown in this figure as well as those shown in Fig. 4 (see legend of Fig. 1 for nomenclature). (B) Jurkat cells were transfected with empty pEF-Bos vector (Bos) or with the indicated constructs. After 18 h, 2 × 106 cells were left unstimulated (−) or treated with pervanadate (+) for 2 min. NP40 lysates were subjected to anti-CD8 immunoprecipitation followed by anti-pTyr Western blot analysis. The blot was then stripped and re-probed with anti-SIT, anti-SHP2 or anti-Grb2 antibodies, as indicated.
In marked contrast, mutation of the YGNL motif resulted in an impaired ability of SIT to recruit Grb2 following T cell activation (arrow in Fig. 3 B), indicating that this motif represents a Grb2 binding site. However, we observed that the CD8 / CD8 / YAN chimera also had a reduced ability to associate with Grb2 despite the above observation that this motif apparently does not represent a major site of tyrosine phosphorylation. Moreover, concomitant mutation of the YGNL and the YANS motif (CD8 / CD8 / YGN + YAN chimera) resulted in a complete loss of Grb2 binding while the association with SHP2 was unaffected. These data strongly suggest that the association between SIT and Grb2 occurs on two different sites, namely YGNL and YANS (with YGNL being the major binding site).
2.4 Inhibition of T cell activation by SIT requires an intact YASV motif
Previous data had suggested that SIT might exert an inhibitory function during T cell activation. This hypothesis was based on the finding that overexpression of wild-type SIT or a CD8 / CD8 / SIT chimera in Jurkat T cells strongly impaired induction of the transcriptional activity of NF-AT after recruitment of the TCR 12. However, inhibition of T cell activation by SIT is apparently not mediated via SHP2 since a SIT-ITIM mutant or a CD8 / CD8 / ITIM chimera which had largely lost their ability to bind to SHP2 still inhibited TCR-mediated induction of NF-AT activity 12.
To examine further the molecular mechanisms underlying the inhibitory function of SIT, the wild-type CD8 / CD8 / SIT chimera or tyrosine mutants thereof (see Fig. 3 A for details) were transiently overexpressed in Jurkat cells together with a luciferase reporter gene construct that is driven by a triplicated NF-AT binding site of the IL-2 promoter. A truncated version of the CD8 / CD8 / SIT chimera lacking the entire cytolasmic tail of SIT (CD8 / CD8 / STOP), a CD8 / CD8 / SIT chimera in which all five tyrosine phosphorylation sites were mutated to phenlyalanine (CD8 / CD8 / 5F) as well as a chimeric TRIM molecule consisting of the extracellular domain of human HLA-A2 fused to a full-length TRIM molecule (Ch_F), served as negative controls. Following transfection, cells were allowed to rest for 18 h and were then stimulated for 6 h using either the anti-TCR mAb C305 or the CD3ϵ mAb OKT3. Subsequently, the TCR-mediated induction of NF-AT activity was determined.
As shown in Fig. 4 A, overexpression of the CD8 / CD8 / SIT chimera induced the previously described decrease in TCR- and CD3ϵ-mediated activation of NF-AT. Deletion of the entire intracellular domain of SIT (CD8 / CD8 / STOP) or mutation of all five tyrosine residues (CD8 / CD8 / 5F) abrogated the ability of the chimera to inhibit NF-AT activation. In addition, overexpression of Ch_F did not influence TCR-mediated activation of NF-AT, as previously reported 15. These data demonstrate that i) inhibition of T cell activation is a specific property of SIT, ii) SIT-mediated inhibition of T cell activation requires an intact cytoplasmic domain and iii) it involves one or more of the five cytoplasmic tyrosine residues of SIT.
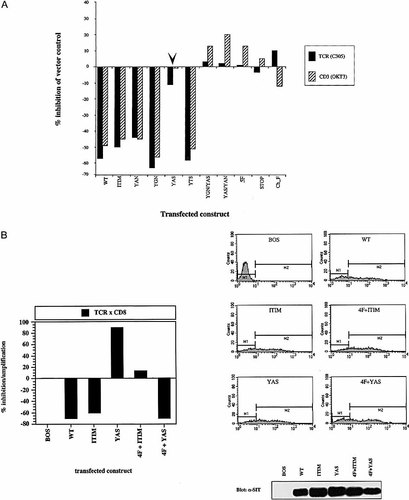
The YASV-motif is responsible for SIT-mediated inhibition of TCR / CD3-induced NF-AT activation. (A) Jurkat cells were co-transfected with empty vector (pEF-Bos), a cDNA encoding the CD8 / CD8 / SIT chimera (WT) or the indicated SIT-variants, together with a NF-AT driven luciferase reporter construct. Eighteen hours later, cells were stimulated with crosslinked anti-TCR (C305) or CD3ϵ (OKT3) mAb for 6 h and then analyzed for luciferase activity as described in Sect. 4. The luciferase units obtained after stimulation of cells transfected with the empty vector were set as 100 %. Shown is the relative inhibition of the response of each transfectant in comparison to the response of the vector transfectant. Equal levels of expression of the individual chimeras were verified by anti-SIT Western blotting and by FACS analysis using an anti-CD8 mAb (not shown but see e. g. Fig. 4 B). (B) Cells were transfected with the indicated constructs as described in (A). Transfectants were subsequently stimulated with crosslinked anti-TCR mAb C305 alone or with a combination of crosslinked C305 and CD8 mAb (MEM-31). The luciferase units after stimulation with crosslinked C305 alone were set as 100 %. Shown is the relative inhibition / amplification of this response following co-crosslinking of the CD8-chimera with the TCR. The following equation was used: normalized luciferase units after TCR × CD8 co-stimulation divided by normalized luciferase units after TCR-stimulation alone. The expression of the constructs was verified by anti-SIT immunoblotting (lower panel) and by indirect immunofluorescence using mAb MEM-31.
As reported previously, overexpression of the CD8 / CD8 / ITIM-chimera, which is strongly impaired in its ability to bind SHP2 (see e. g. Fig. 3 B and 12) still inhibited T cell activation. The same was true for CD8 / CD8 / SIT chimeras in which the two Grb2-binding sites (CD8 / CD8 / YGN or CD8 / CD8 / YAN chimera) were mutated individually or concomitantly, as well as for the CD8 / CD8 / YTS chimera. These findings suggested that the YGNL, YTSL, ITIM or YANS motifs do not mediate the inhibitory signal exerted by SIT. In marked contrast, all CD8 / CD8 / SIT chimeras in which the YASV motif was mutated either alone (arrow) or in combination with other tyrosine residues were strongly impaired in their ability to inhibit TCR responses.
Similar data as shown in Fig. 4 A were obtained when the individual CD8 / CD8 / SIT chimeras were co-ligated with the TCR (Fig. 4 B). Under these experimental conditions the wild-type CD8 / CD8 / SIT and the CD8 / CD8 / ITIM chimera induced an approximately 60 – 70 % inhibition of NF-AT activity. Perhaps more importantly, an identical down-regulation of NF-AT activity was produced by the CD8 / CD8 / 4F + YAS chimera (in which only the YASV motif is intact), whereas co-crosslinking of the CD8 / CD8 / 4F + ITIM chimera did not influence induction of NF-AT activity. From this experiment as well as from the data shown in Fig. 4 A we conclude that the inhibitory function of SIT is exclusively mediated via the YASV motif.
Surprisingly, we observed that the CD8 / CD8 / YAS chimera even up-regulated NF-AT activity when it was co-ligated with the TCR. This suggested that in the absence of a functional YASV motif, SIT can provide a positive – rather than a negative – regulatory signal for T cell activation rather (Fig. 4 B). To investigate, which of the remaining four tyrosine residues that are present in the CD8 / CD8 / YAS chimera is responsible for up-regulation of T cell activation in the absence of YASV, we overexpressed the individual CD8 / CD8 / 4F chimeras in Jurkat T cells and assessed their influence on induction of NF-AT activity after co-crosslinking with the TCR. As shown in Fig. 5 A the CD8 / CD8 / 4F + YTS, the CD8 / CD8 / 4F + ITIM and the CD8 / CD8 / 4F + YAN chimeras had no major impact on NF-AT activity after co-ligation with the TCR. In marked contrast, co-engagement of the CD8 / CD8 / 4F + YGN chimera strongly up-regulated the response under conditions where the CD8 / CD8 / SIT and the CD8 / CD8 / 4F + YAS chimeras inhibited NF-AT activity. Thus, in the absence of a phosphorylatable YASV motif SIT can exert a positive regulatory function for T cell activation which is mediated via the YGNL motif.
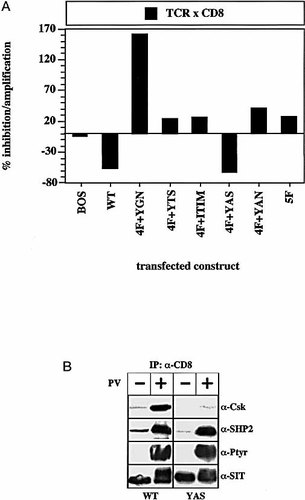
The YASV – motif is responsible for SIT-mediated inhibition of TCR / CD3-induced NF-AT activation. (A) The same experiment as shown in Fig. 4 B using the CD8 / CD8 / 4F-chimeras. (B) Jurkat cells stably expressing the CD8 / CD8 / SIT or the CD8/CD8/YAS chimeras were briefly treated with pervanadate. Subsequently, cells were lysed in NP40 containing buffer and subjected to CD8 immunoprecipitation. The tyrosine phosphorylation status of the chimeras as well as the presence of Csk and SHP2 in the immunoprecipitates were assessed by sequential Western -blotting (anti-Ptyr → anti-Csk → anti-SHP-2 → anti-SIT).
2.5 The PTK Csk binds to the YASV motif
Given the above data indicating a critical role for the YASV motif for SIT-mediated inhibition of T cell activation, we were interested to identify the molecule that binds to this motif. To this end we established Jurkat variants stably expressing the CD8 / CD8 / SIT or the CD8 / CD8 / YAS chimera, respectively. The individual transfectants as well as the parental (CD8–) Jurkat cell line were metabolically labeled with a mixture of 35S-cysteine and 35S-methionine and subjected to brief pervanadate treatment. Subsequently, CD8 immunoprecipitates were prepared from postnuclear lysates, subjected to two-dimensional gel electrophoresis and analyzed by autoradiography. By using this approach we repeatedly observed a 45 – 50-kDa polypeptide (p45) with an estimated isoelectric point of 7.0 co-precipitating with the CD8 / CD8 / SIT chimera but not with the CD8 / CD8 / ITIM chimera (not shown).
Both the molecular weight of p45 as well as its isoelectric point are similar to those of the PTK p50csk, the major negative regulator of src PTK. Moreover, the YASV motif of SIT shows striking similarities to the tyrosine-based signaling motif Y314SSV which has recently been shown to represent the major bindig site for the SH2 domain of Csk within the cytoplasmic domain of the transmembrane adapter protein PAG / Cbp 13, 14. Therefore we argued that p45 is identical to Csk and that SIT might recruit Csk via the YASV motif. To assess this question we performed anti-Csk Western blot analysis of CD8 immunoprecipitates prepared from the above Jurkat variants after treatment with pervanadate. As shown in Fig. 5 B large amounts of Csk co-precipitated with the CD8 / CD8 / SIT but not with the CD8 / CD8 / YASV chimera under these experimental conditions. Thus, Csk is the primary candidate for being the negative regulatory molecule that binds to YASV and mediates the inhibition of T cell activation by SIT.
3 Discussion
During the last years major efforts have been made to understand better the molecular mechanisms controlling the quality of an immune response after engagement of the TCR. The recent identification of a novel group of integral membrane proteins that carry multiple tyrosine-based signaling motifs in their cytoplasmic domains has offered new perspectives to explain how TCR-mediated signals are coupled to intracellular signaling pathways to yield an appropriate cellular response. This group of signaling molecules has collectively been termed transmembrane adaptor proteins and so far comprises LAT, TRIM, PAG / Cbp and SIT.
While it is meanwhile well established that the expression and tyrosine phosphorylation of LAT is essential for T cell activation and T cell development, the cellular functions of TRIM, PAG / Cbp and SIT are less well defined. We thought that one way to better understand the roles of these novel adaptor proteins during T cell activation might be to identify their sites of tyrosine phosphorylation as well as the signaling proteins that inducibly bind to their cytoplasmic domains. In this study we have performed a structure-function analysis of SIT.
The cytoplasmic domain of SIT contains five potential sites of tyrosine phosphorylation. These are Y90GNL, Y128TSL, Y148SEV, Y168ASV and Y188ANS. Overexpression of CD8 / CD8 / SIT chimeras in Jurkat T cells in which four of these five sites were mutated to phenylalanine (CD8 / CD8 / 4F chimeras) revealed that under optimal conditions (pervanadate stimulation) all five tyrosines can serve as substrates for protein tyrosine kinases. However, the levels of tyrosine phosphorylation of the individual 4F chimeras were quite different. Thus, the YGNL and the YASV motifs clearly seem to represent the preferred phosphorylation sites whereas YTSL, ITIM and YANS become phosphorylated to a much lesser extent (the overall order seems to be: YGNL ≥ YASV > YTSL > ITIM > YANS). At present we do not know whether these differences in tyrosine phosphorylation indeed reflect the in vivo situation or whether the lower levels of tyrosine phosphorylation of the YTSL, the ITIM and the YANS motifs are due to the fact that their full phosphorylation can only be achieved after YGNL and YASV have been phosphorylated. This possibility cannot be excluded because our previous experiments had suggested that, although the Syk-PTK ZAP-70 and Syk alone cannot induce detectable tyrosine phosphorylation of SIT in a COS cell system, optimal tyrosine phosphorylation of SIT apparently requires co-expression of both Src and Syk PTK 12. Moreover, our previous data as well as the experiments described in this report clearly demonstrate that, besides YGNL and YASV, at least the ITIM and the YANS motifs can bind intracellular effector molecules (SHP2 and Grb2) after T cell activation (Fig. 3).
Based on the studies performed by Songyang et al. 17, we argued that the YGNL motif could mediate an interaction between SIT and the cytoplasmic adaptor molecule Grb2. Indeed, an analysis of SIT immunoprecipitates prepared from TCR-stimulated Jurkat T cells not only corroborated an activation-induced association between SIT and SHP2 but also revealed an interaction between SIT and Grb2 (Fig. 2). By using chimeric CD8 / CD8 / SIT constructs in which individual tyrosine residues were mutated to phenylalanine we confirmed that Y188GNL represents a Grb2 binding site (Fig. 3 B). However, we also observed that mutation of this site only partially abolished the association between SIT and Grb2. This suggested the existence of at least one additional Grb2 binding site within the cytoplasmic domain of SIT, which we identified as the Y188 ANS motif. Thus, simultaneous mutation of both YGNL and YANS motifs completely ablated the ability of SIT to recruit Grb2 whereas single mutation of either motif only partially interfered with the association between the two molecules (Fig. 3 B). These data suggest that after T cell activation Grb2 can interact with SIT via two different sites, namely Y90 GNL and Y188 ANS.
Previous studies had indicated that overexpression of SIT in Jurkat cells leads to down-regulation of the TCR / CD3-induced activation of NF-AT and that this inhibitory function is dependent on the presence of its cytoplasmic domain. We here extended these initial observations by demonstrating that the inhibitory function of SIT is dependent on tyrosine phosphorylation. Thus, a CD8 / CD8/ SIT chimera in which all five tyrosines were mutated to phenylalanine (CD8 / CD8 / 5F chimera) had completely lost its capability to inhibit T cell activation (Fig. 4 A).
By mutational analysis we identified the Y168 ASV motif as the phosphorylation site that seems to be responsible for SIT mediated inhibition of T cell activation. Overexpression of a CD8 / CD8 / SIT chimera in which only the YASV motif was mutated (CD8 / CD8 / YAS chimera) fully rescued the NF-AT response following T cell activation, whereas chimeras carrying mutations in the YGNL, the ITIM, the YTSL or the YANS motifs had no influence on the ability of SIT to inhibit T cell responses (Fig. 4 A). Moreover, co-ligation of a CD8 / CD8 / SIT chimera, which only contained the YASV motif (CD8 / CD8 / 4F + YAS) with the TCR-inhibited activation of NF-AT in an identical fashion as the wild-type protein (Fig. 4 B). All these data suggest that the inhibitory function of SIT is exclusively mediated via the YASV motif.
However, mutation of the YASV motif has no obvious impact on the binding of SHP2 and / or Grb2 to SIT (Fig. 3 B). Therefore we proposed the existence of a SH2 domain containing negative regulatory effector molecule that selectively binds to the phosphorylated YASV motif and mediates the inhibitory effect on T cell activation. Two-dimensional analysis of CD8 immunoprecipitates prepared from metabolically labeled Jurkat T cells stably expressing either the CD8 / CD8 / SIT or the CD8 / CD8 / YAS chimera resulted in the identification of a 45-kDa polypeptide(p45) with an estimated isoelectric point of about 7.0 that specifically co-precipitated with the wild-type chimera but not with the YASV mutant. We argued that p45 may be the tyrosine kinase Csk, which possesses the appropriate molecular weight and isoelectric point. In addition, Csk has recently been demonstrated to bind via a YSSV motif (which is very similar to the YASV-motif of SIT) to human and rat homologoues of the transmembrane adapter protein PAG / Cbp 13, 14. Based on these molecular properties of Csk it was not surprising that we identified endogenous Csk in CD8 immunoprecipitates prepared from the CD8 / CD8 / SIT transfectants but not in those obtained from Jurkat T cells expressing the CD8 / CD8 / YAS mutant (Fig. 5 B). Collectively, these findings suggest that Csk could represent the negative regulatory protein that binds to the YASV motif and mediates the negative regulatory effect exerted by SIT on TCR-induced NF-AT activity.
The obvious question emerging from the finding that Csk is capable to bind to SIT is which TCR-mediated signaling pathway(s) leading to activation of NF-AT is / are controlled by this interaction. In this regard it is important to remember that overexpression of SIT in the Jurkat variant J.HM1.2.2 (which co-expresses a TCR and the human muscarinic receptor type 1) does not inhibit induction of NF-AT activity after triggering of G-protein coupled receptors 12. This suggests that SIT controls signaling processes mediated via ITAM-containing immunoreceptors. Moreover, we had demonstrated that the inhibitory effect of SIT on TCR-mediated induction of NF-AT activity can be bypassed by treatment of SIT transfectants with a combination of a phorbol ester and ionomycin (which indicates that SIT likely exerts its function upstream of activation of PKC).
However, so far our attempts to show inhibition of (a) proximal tyrosine phosphorylation event(s) as an indicator of Csk-mediated inhibition of Src kinase activity in cells overexpressing SIT have not been successful. For example we did not observe significant alterations of the global TCR-mediated tyrosine phosphorylation of cellular proteins after overexpression of SIT (either transiently or stably). In addition, tyrosine phosphorylation of TCRζ, LAT, PLCγ and also phosphorylation of Erk after TCR triggering seem to occur normally in cells overexpressing SIT. Collectively, these findings suggest that at least the initial activation of Src kinases occurs normally in cells overexpressing SIT. Therefore, further studies are required to elucidate the question which TCR-mediated signaling pathway(s) is / are controlled by SIT.
It is important to note that the functional activities of SIT show striking similarities to those that have recently been described for the negative regulatory adaptor protein Dok-3 18. Like SIT Dok-3 inducibly associates with Csk and down-regulates BCR-mediated activation of NF-AT via an unknown mechanism. The inhibitory effect of Dok-3 depends on the presence of the C-terminal tail of the molecule which contains four potential tyrosine-based signaling motifs among which one is likely responsible for recruitment of Csk 18. Interestingly, the same YASV motif that mediates the association between SIT and Csk is also expressed in Dok-3 and the amino acids flanking both motifs are well conserved between the two molecules (see Fig. 1 A). It is therefore tempting to speculate that the YASV motif of Dok-3 also mediates its association with Csk and that this motif is involved in the inhibition of BCR-mediated signaling. Furthermore, the overall functional similarities between the two molecules might suggest that they control the same pathways of immunoreceptor-mediated signaling in different types of cells.
Previously we have demonstrated that the cytoplasmic tyrosine phosphatase SHP2 binds to the ITIM within the cytoplasmic domain of SIT. In T cells it is believed that SHP2 is involved in regulation of the MAPK pathway leading to activation of the transcription factor AP-1 19. The NF-AT reporter construct that we used in this study contains an AP-1 binding site and therefore we thought that, if SIT-associated SHP2 regulated AP-1 activity, then the CD8 / CD8 / 4F-ITIM chimera should be capable to upregulate NF-AT when overexpressed in T cells. However, this is clearly not the case. Even co-ligation of the CD8 / CD8 / 4F + ITIM chimera with the TCR failed to exert any measurable effect on the activity of the NF-AT reporter construct, although the chimera was capable to bind SHP2 (Fig. 1 C). From these data we conclude that the SIT-associated fraction of SHP2 regulates an (unknown) pathway of T cell activation that does not result in activation of AP-1.
An interesting observation during this study was that the functional effect of the individual CD8 / CD8 / SIT chimeras could be best demonstrated when the chimeras were co-crosslinked with theTCR using a CD8 antibody (Figs. 4 B and 5 A). As already mentioned above SIT represents a heavily glycosylated molecule that could possess an external ligand. One possibility to explain our results would be that the CD8 antibody mimics the function of this putative ligand. In this scenario engagement of SIT by its ligand would bring SIT in close proximity of the TCR, thus allowing the down-regulation of TCR-mediated signals. However, whether SIT indeed has an external ligand and whether this putative ligand regulates its function requires further investigations.
We also would like to point out that, although our previous studies as well as the experiments described in this report, support the view that SIT represents a negative regulatory adaptor protein, the possibility cannot be excluded that under particular conditions of T cell activation SIT exerts a positive rather than a negative regulatory function.
As shown in Figs. 2 and 3, SIT is capable of recruiting Grb2 via the YGNL motif following T cell triggering. In addition, it seems that YGNL represents a preferred tyrosine phosphorylation site (Figs. 1 and 3). Finally, our experiments using the CD8 / CD8 / YASV and the CD8 / CD8 / 4F + YGN chimera indicate that in the absence of phosphorylatable YASV, SIT enhances TCR-mediated activation of NF-AT (Figs. 4 B and 5 A). This effect is mediated via the YGNL motif and thus likely involves a Grb2-controlled signaling pathway (Fig. 5 A).
One could imagine a situation in which a preferential (or even exclusive) phosphorylation of YGNL occurs in vivo, for example after TCR triggering by a weak agonist. Thus, depending on its state of phosphorylation SIT (as well as other transmembrane and cytosolic adaptor proteins) could serve as a multifunctional protein that helps to integrate the quality of an externally applied signal into an appropriate cellular response. For the further undertanding of SIT function it will be important to elucidate the question whether the phosphorylation status of SIT as well as its association with cytosolic effector molecules is influenced by the quality of an externally applied signal. Moreover, it will be important to identify the molecule(s) that bind to the SIT / Grb2 complex. Potential candidates are SOS 20, 21, SLP-76 22, Dynamin 20, TNF-R1 23 and SHIP 24 which all have been demonstrated to bind one or two of the Grb2-SH3 domains. Experiments are underway to assess these questions.
4 Materials and methods
4.1 Cells and antibodies
Jurkat cells were maintained in RPMI 1640 supplement-ed with 10 % FCS, 2 % glutamin and 1 % penicillin-streptomycin (Gibco BRL, Germany). The Jurkat variants stably expressing the CD8 / CD8 / SIT and the CD8 / CD8 / YAS chimeras were generated and propagated as previously described 11. The anti-SIT polyclonal antiserum was raised by immunizing rabbits with a KLH-coupled synthetic peptide corresponding to amino acids 96 – 114 12. After affinity purification on peptide-coupled protein A-Sepharose beads, antibodies were used at 2 μg / mlfor Western blotting. Antibodies against SHP2, phosphotyrosine (4G10) (Upstate Biotechnology Inc.), Grb2 (Santa Cruz) and Csk (Santa Cruz) were diluted at 1 μg / ml for immunoblotting. For immunoprecipitation experiments, protein A-Sepharose-purified anti-CD8 mAb AICD8.1 or MEM-31 mAb were covalently coupled to CNBr-activated Sepharose beads (6 mg mAb / ml of packed beads).
4.2 Immunoprecipitation and Western blotting
Untransfected Jurkat T cells (4 × 107 for the experiment shown in Fig. 2) or Jurkat cells (2 × 106) that had been transiently transfected with cDNA encoding the various CD8 / CD8 / SIT chimeras (see below) were stimulated with culture supernatant of C305 mAb (provided by Dr. A. Weiss, University of California, San Francisco, CA) for the indicated period of time, or with a mixture of 0.1 mM vanadate plus 1 mM H2O2 for 2 min, at 37 °C. Immunoprecipitations were performed using the crude polyclonal rabbit antiserum directed at SIT (1 : 100 v / v) followed by collection of the immune complexes using protein A-Sepharose or by using 15 μl of packed beads coupled with protein A-purified CD8 mAb. After several washes, the precipitates were subjected to SDS-PAGE and further processed for Western blot analysis using standard procedures, as described elsewhere 12, 16.
4.3 cDNA constructs and transfection
Generation of the chimeric CD8 / CD8 / SIT molecule and its cloning into the eukaryotic pEF-Bos expression vector have been previously described 12. Point mutation of the tyrosine residues (replaced by phenylalanine) within selected signaling motif(s) has been performed using the Quick Change site-directed Mutagenesis kit from Stratagene according to the manufacturer'srecommendations.
4.4 Luciferase reporter gene assay
Jurkat cells were transiently transfected by electroporation using 30 μg of the indicated cDNA construct together with 10 μg of the NF-AT reporter construct and 1 μg of a rennilla luciferase reporter gene. Cells were allowed to recover for 18 h and incubated in duplicates (7 × 104 cells / well) with medium, immobilized anti-TCR (C305) or anti-CD3 (OKT3) mAb, or PMA (10– 8 M) plus ionomycin (1 μg / ml) in a 96-well plate (U96 Maxisorp, Nunc). After 6 h of stimulation, cells were harvested, washed twice in PBS and lysed for 15 min at room temperature. The normalized luciferase activity of the NF-AT reporter gene was measured in each supernatant using the dual luciferase assay system from Promega.




