Smad3 and Smad4 mediate transforming growth factor-β1-induced IgA expression in murine B lymphocytes
Abstract
Transforming growth factor (TGF)-β1 is well established as a critical IgA isotype switching factor and Smad molecules have been reported to act as transducers and transcriptional factors in the expression of TGF-β1-targeted genes. We examined the involvement of Smad proteins in TGF-β1-induced IgA expression. First, we found that TGF-β1 significantly increases endogenous germ-line (GL) α transcripts by LPS-stimulated CH12.LX.4933 (μ+) B lymphoma cells. To investigate its signaling mechanisms, the lymphoma cell line was transfected with pFL3 that contains the TGF-β-responsive element of the GLα promoter, and stimulated with TGF-β1. Similar to endogenous GLα transcripts, TGF-β1 induces GLα promoter activity and overexpression of Smad3 markedly enhances the promoter activity. This activity is further augmented by cotransfected Smad4. On the other hand, Smad7 substantially abrogates the synergistic effect of Smad3/4 onGLα promoter activity. In addition, overexpression of Smad3/4 enhances TGF-β1-induced endogenous GLα transcripts in normal spleen B cells. Finally, in the presence of TGF-β1, overexpression of Smad3/4 selectively increases both surface IgA expression and IgA production. The results from the present study indicate that Smad3, Smad4, and Smad7, at least in part, serve as mediators linking TGF-β1 to transcriptional regulation of IgA switching related gene and regulation of IgA class switching.
Abbreviations:
-
- GLα transcripts:
-
Germ-line transcripts of IgA constant gene
-
- Cα:
-
Constant region of IgA
1 Introduction
It is now well established that TGF-β1 induces IgA isotype switching in vitro. Thus, TGF-β1 increases IgA synthesis by LPS-stimulated murine B cells 1–3. We have previously reported that TGF-β1 increases the precursor frequency of IgA-producing cells at the clonal level 4. In addition, TGF-β1 was shown to increase the half-life of productive α mRNA 5. Ig isotype switching occurs as a recombination event that results in juxtaposition of the rearranged VHDHJH gene upstream of a new CH gene 6. TGF-β1 increases switch recombination to Cα in the presence of LPS 7, 8. On the other hand, transcription of the corresponding unrearranged CH gene to produce germ-line (GL) transcripts, precedes transcription of productive Ig mRNA. This event is known to be regulated by cytokines 9. TGF-β1 increases GLα transcripts in murine, human and rabbit B cells 10–16. It became evident that expression of GL transcripts is aprerequisite for subsequent switch recombination 9, 17.
TGF-β signals from the membrane to the nucleus through TGF-β receptors and their downstream effectors, termed Smad proteins 18, 19. Upon stimulationby TGF-β1, Smad2 and Smad3 become phosphorylated by the activated TGF-β receptors and form homodimeric and heterodimeric complexes with Smad4 20–23. These Smad complexes translocate into the nucleus where they bind specific DNA sequences in target promoters, thereby activating transcription. One of the genes induced by TGF-β1 is the GLα gene, which must be transcribed to obtain class switch recombination to IgA.
It was reported that TGF-β1 treatment of I.29 μ lymphoma cells and splenic B cells induces synthesis of core binding factor (CBF)α3/PEBP2αC/AML2 and increases the binding of CBFα 3 to the mouse GLα promoter 24. Recently, it has been shown that Smad3/4 and CBFα 3 cooperate to mediate TGF-β1-induced mouse and human GLα promoter activity 25, 26, suggesting that Smad3/4 are important molecules in the induction of IgA isotype switching.
In the present study, we asked whether Smad proteins can mediate TGF-β1-induced IgA expression by examining their effects on levels of endogenous GLα transcripts and on IgA expression at the surface of B cells. We find that overexpressed Smad3 and 4 synergistically increase TGF-β1-induced GLα promoter activity, whereas Smad7 inhibits this synergy. Furthermore, Smad3/4 augments TGF-β1-induced expression of endogenous GLα transcripts in mouse spleen cells. Finally, Smad3/4 up-regulates the expression of surface IgA and the synthesis of IgA by LPS-stimulated spleen B cells in the presence of TGF-β1.
2 Results
2.1 Involvement of TGF-β1 and Smad proteins in GLα transcription
TGF-β1 increases IgA class switching in cultured cells 1–3. In the present study, we examined the mechanisms by which TGF-β1 induces IgA expression in the sIgM+ mouse B cell line, CH12.LX.4933 and in normal mouse spleen B cells. First, we determined whether TGF-β1 increases the expression of GLα transcripts in CH12.LX B lymphoma cell lines, CH12.LX.4933 (sIgM+) and CH12.LX.4F10 (sIgA+). Measurable amounts of endogenous GLα transcripts were detected in LPS-stimulated CH12.LX.4933 in the absence of TGF-β1 and the level was substantially increased in the presence of TGF-β1. In contrast, no GLα transcripts were detected in LPS-stimulated CH12.LX.4F10 (α+) cells which have already switched to IgA expression (Fig. 1A). To further understand how TGF-β1 induces GLα transcription, CH12.LX.4933 lymphomas were transfected with pFL3, a plasmid reporter containing a portion of GLα promoter 12, and stimulated with TGF-β1. TGF-β1 stimulated reporter gene activity by approximately 2.5-fold (Fig. 1B) and, further, it increased the reporter activity in a dose-dependent manner (data not shown). These results strongly confirm previous results suggesting that TGF-β1 induces endogenous GLα transcription through the GLα promoter 11–14. Next, we examined whether Smad proteins play any role in signaling of TGF-β1-induced GLα promoter activity. Smad3 was overexpressed with pFL3 in CH12.LX.4933 lymphomas. As shown in Fig. 1B, Smad3 caused 2-fold increase in GLα promoter activity. To verify that transfected Smad3 gene is expressed at the protein level, we performed immunoprecipitation with anti-FLAG antibody and Western blotting with anti-Smad3 antibody (Fig. 1C).
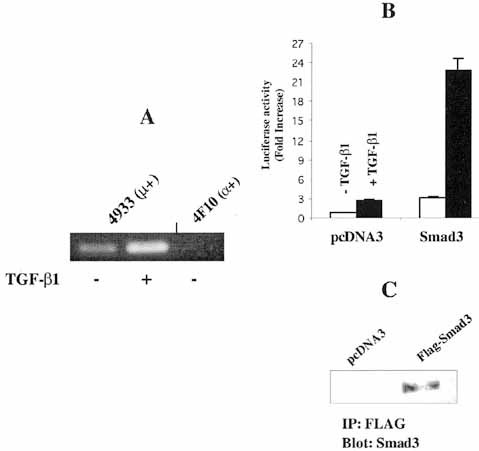
Smad3 mediates TGF-β1-induced GLα transcriptional activity. (A) Effect of TGF-β1 on the expression of endogenous GLα transcripts. CH12.LX.4933 cells (sIgM+) and CH12.LX.4F10 (sIgA+) were stimulated with LPS (12.5 μg/ml) and TGF-β1 (2 ng/ml), and incubated for 60 h. GLα transcriptional level was determined by RT-PCR. (B) Involvement of Smad3 in promoter activity of GLα reporter gene. Flag-Smad3 plasmid (30 μg) or empty vector (pcDNA3, 30 μg), and reporter plasmid, pFL3 (30 μg) were co-transfected into CH12.LX.4933 B lymphoma cells. TGF-β1 (2 ng/ml) was added to culture and luciferase activity was determined following 24 h incubation. Data represent the average luciferase activity from four independent transfections ± SEM. (C) Immunoprecipitation of transfected Smad3. N-terminally FLAG-tagged Smad3 constructs was transfected into CH12.LX.4933 B lymphoma cells and its expression was detected by immunoprecipitation with the anti-FLAG antibody followed by immunoblotting using anti-Smad3 antibody.
Subsequently, we examined whether Smad4 and Smad7 regulate the activity of the GLα promoter (Fig. 2). Smad4 or Smad7 alone had little effect on the activity of pFL3. However, overexpressed Smad4 synergized with Smad3 leading to a 16-fold increased induction of the GLα promoter. These results are consistent with previous reports indicating that Smad3 and Smad4 cooperatively induce TGF-β1-dependent heteromeric complex for transcriptional activation 20, 21, 27. On the other hand, overexpression of Smad7, a TGF-β-inducible antagonist of TGF-β signaling 28, led to 76% reduction in the promoter activity in the Smad3/4-mediated promoter activity (Fig. 2).
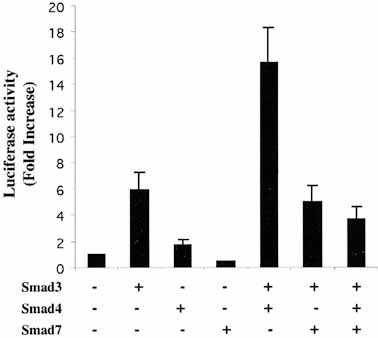
Involvement of overexpressed Smad3, Smad4 and Smad7 on TGF-β1-induced transcriptional activity of GLα reporter gene. Various combinations of Smad molecules were cotransfected with pFL3 reporter plasmid into CH12.LX.4933 B lymphoma cells. An identical amount of Smad was used for each transfection, i.e. single transfection, 30 μg; double transfection, 15 μg; triple transfection, 10 μg. As a negative control, pcDNA3 (30 μg) was transfected (first lane). TGF-β1 (1 ng/ml) was added to all cultures. Data represent the average luciferase activity from three independent transfections ± SEM.
Since phosphorylated Smad2 and Smad3 are known to form heterodimeric complexes with Smad4, the involvement of Smad2 in GLα promoter activity was also examined. Transfected Smad2 alone or Smad2/4 in combination did not increase the promoter activity (data not shown). Similar to our results, Smad2 has also been found to be unable to activate a reporter plasmid driven by an intact GLα promoter, although Smad3 in combination with Smad4 activate it well 25.
2.2 Effect of Smad3 and 4 on IgA expression in B lymphoma cells
Since overexpressed Smad3 and 4 markedly increased GLα promoter activity, it was important to ask next whether Smad3 and 4 are involved in inducing IgA switching events. I.29μ B lymphoma cells were transfected with Smad3 and 4 and their effects on IgA expression was assessed by measuring the degree of surface IgA expression and IgA secretion. As shown in Table 1, TGF-β1 significantly increased surface IgA expression and this was further augmented by overexpressed Smad3/4. In parallel, Smad3/4 further enhanced the total IgA secretion which was mediated by TGF-β1. Thus, these data suggest that Smad3 and Smad4 mediate TGF-β1-induced IgA isotype synthesis in the tested B lymphomas.
|
TGF-β1 |
Smad3 / 4 |
Surface IgAb) (%) |
Fold increase |
IgA secretionc) (ng / ml) |
|---|---|---|---|---|
|
− |
− |
2.09 ± 0.10 |
1.0 |
105 ± 4.0 |
|
+ |
− |
4.53 ± 0.25 |
2.2 |
126 ± 7.6 |
|
− |
+ |
2.54 ± 0.27 |
1.2 |
101 ± 8.2 |
|
+ |
+ |
5.87 ± 0.69 |
2.8 |
184 ± 7.9 |
- a) I.29 μ B lymphoma cells (sIgM+) were transfected with 15 μg of each Smad3 and Smad4 or empty vector (pcDNA3, 30 μg) and stimulated with LPS (50 μg / ml), nicotinamide (10 mM) and TGF-β1 (2 ng / ml) in 20 % FBS complete medium 55.
- b) Following 48-h incubation, cells were stained with PE-labeled anti-mouse IgA and analyzed by FACScan. Data are means ± SEM of six independent experiments.
- c) Culture supernatants were harvested following incubation for 5 days and level of IgA were determined by ELISA. Data are means ± SEM of three cultures in two separate experiments.
2.3 Smad3 and 4 mediate TGF-β1-induced endogenous GLα transcription
To obtain the direct evidence that Smad molecules are implicated in IgA regulation in normal B cell population, we determined the effect of transfected Smad3/4 on endogenous GLα transcripts in LPS-activated spleen cells. Since it is generally believed to be difficult to transfect the primary cell population, transfection efficiency of expression vectors was demonstrated using pGFP-C1, a plasmid expressing green fluorescent protein (GFP), in normal spleen cells. At 2 days following electroporation, up to 46% of the normal spleen cells expressed GFP, depending on the electroporation condition (Fig. 3). These conditions were adopted for further transfections. As shown in Fig. 4, in the absence of TGF-β1, endogenous GLα transcripts are not expressed by LPS-activated spleen cells, unlike CH12.LX B lymphoma cells (Fig. 1A). TGF-β1 increases expression of endogenous GLα transcripts and this increase is further augmented by overexpressed Smad3/4. In contrast, expression of endogenous GLγ1 transcripts is diminished by transfected Smad3/4 in the presence of TGF-β1 (Fig. 4). Similarly, transfected Smad3/4 also decreased the expression of GLγ3 transcripts (data not shown). Taken together, our results suggest that Smad3 and Smad4 are specific transducing molecules in TGF-β1-induced GLα transcription in normal spleen B cells.
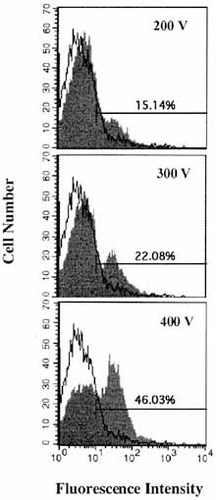
Flow cytometric analysis of the cells transfected with pGFP-C1 plasmids. pGFP-C1 (30 μg) was transfected into 2×107 normal spleen cells by electroporation. Electric shocks used in the transfections were 200 V/0.4 cm, 300 V/0.4 cm, and 400 V/0.4 cm. After 48 h incubation, fluorescence intensity was measured by FACScan.
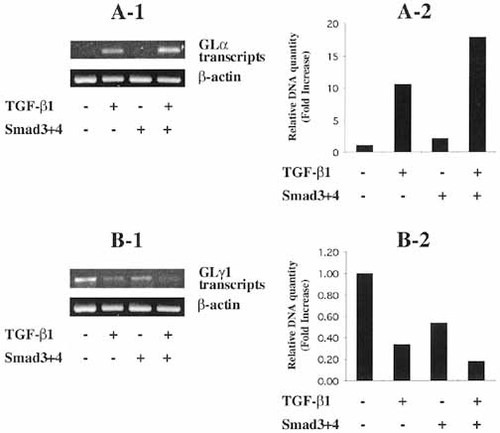
Effects of overexpressed Smad3 and Smad4 on the activity of endogenous GL transcripts. Normal spleen cells were transfected with Smad3 (10 μg) and Smad4 (10 μg) or pcDNA3 (20 μg), and cells were incubated with LPS (12.5 μg/ml) and TGF-β1 (1 ng/ml) for 48 h. Levels of endogenous GLα transcripts were measured by RT-PCR. Relative GLα DNA quantity following normalization using the expression of β-actin gene by 1D Main program (Bioneer Corp.); (A-1) GLα transcripts; (A-2) relative GLα DNA quantity; (B-1) GLγ1 transcripts; (B-2) relative GLγ1 DNA quantity. Densitometrical data were obtained from two independent experiments.
2.4 Effect of Smad3/4 on Ig synthesis in normal spleen B cells
We and others have previously shown that IgA isotype expression is selectively induced by TGF-β1 and is further augmented by the addition of either IL-2 or IL-5 to cultures of LPS-activated murine spleen B cells 1–3 or to PWM or CD40L (or anti-CD40) -activated human tonsillar B cells 29. In contrast, TGF-β1 suppresses the production of IgM and IgG1 isotypes by LPS-activated murine spleen B cells. It was important to ask, therefore, whether overexpressed Smad3/4 could augment TGF-β1-induced IgA expression in LPS-activated murine spleen cells. TGF-β1 treatment induces surface IgA expression by approximately twofold, and this is further increased when Smad3/4 is overexpressed (approximately fourfold) in whole spleen cells (Fig. 5A). Furthermore, under the same conditions, a similar pattern of surface IgA expression was observed using a purified B cell population (Fig. 5B).
To determine if these B cells could proceed to secretion of IgA, we measured total IgA and IgG1 secretion. IgA secretion is slightly increased by TGF-β1 and this is further augmented by overexpression of Smad3/4 in the culture of whole spleen cells (Table 2). In contrast, total IgG1 production was decreased by TGF-β1 and further diminished by overexpressed Smad3/4. Subsequently, we determined the effect of Smad3/4 on Ig secretion by fractionated spleen B cells. As shown in Table 2, the effect of TGF-β1 and Smad3/4 on IgA secretion was more striking than that by unfractionated whole spleen cells. Thus, overexpressed Smad3/4 in the presence of TGF-β1 increased IgA secretion by LPS-activated spleen B cells by four- to tenfold. In addition, This Smad3/4-mediated TGF-β1-stimulatory effect on IgA secretion was further augmented by IL-5. In contrast, TGF-β1 consistently decreased IgG1 secretion under any conditions, and overexpressed Smad3/4 resulted in little to marginal effect on IgG1 secretion.
These data suggest that the ability of Smad3 and 4 to mediate TGF-β1 induction of GLα transcripts results in an increase in IgA surface expression and secretion by mouse B cells. Furthermore, TGF-β1 inhibits GLγ1 transcripts and IgG1 secretion in these same cultures.
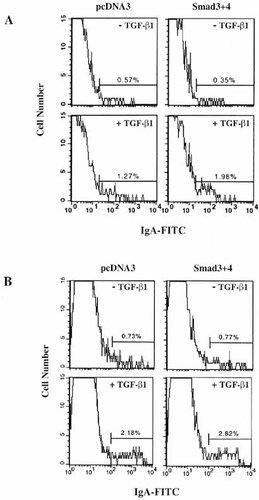
Flow cytometric analysis of normal spleen B cells transfected with Smad3/4. (A) Murine spleen whole cells prestimulated with LPS were transfected with Smad3 (10 μg) and Smad4 (10 μg) or pcDNA3 (20 μg) by electroporation. Cells were then cultured with LPS (12.5 μg/ml) and TGF-β1 (1 ng/ml) for 72 h, and cells were stained with FITC-labeled anti-mouse IgA. (B) Purified spleen B cells prestimulated with LPS were transfected with Smad3 (15 μg) and Smad4 (15 μg) or pcDNA3 (30 μg) by electroporation. Cells were then cultured with LPS (25 μg/ml) and TGF-β1 (0.2 ng/ml) for 72 h, and cells were stained with PE-labeled anti-mouse IgA.
|
Cells |
TGF-β1 |
Smad3 / 4 |
IL-5 |
IgA ng / ml |
Fold increase |
IgG1 ng / ml |
Fold increase |
|---|---|---|---|---|---|---|---|
|
Whole spleen cells |
− |
− |
− |
3,390 ± 155 |
1.0 |
9,572 ± 1391 |
1.0 |
|
|
+ |
− |
− |
3,915 ± 133 |
1.2 |
6,425 ± 776 |
0.67 |
|
|
− |
+ |
− |
3,168 ± 167 |
0.9 |
9,388 ± 545 |
0.98 |
|
|
+ |
+ |
− |
5,713 ± 342 |
1.7 |
4,414 ± 962 |
0.46 |
|
Spleen B cells |
− |
− |
− |
114 ± 10 |
1.0 |
N. D. |
|
|
|
+ |
− |
− |
742 ± 72 |
6.5 |
N. D. |
|
|
|
|
− |
+ |
− |
70 ± 11 |
0.6 |
N. D. |
|
|
|
+ |
+ |
− |
1,360 ± 71 |
10.2 |
N. D. |
|
Spleen B cells |
− |
− |
− |
19 ± 0.2 |
1.0 |
2,013 ± 116 |
1.0 |
|
|
+ |
− |
− |
62 ± 2.3 |
3.3 |
36 ± 2 |
0.02 |
|
|
− |
+ |
− |
30 ± 0.9 |
1.6 |
3,100 ± 519 |
1.5 |
|
|
− |
− |
+ |
33 ± 4.1 |
1.7 |
3,140 ± 587 |
1.6 |
|
|
+ |
+ |
− |
73 ± 1.2 |
3.8 |
92 ± 12 |
0.05 |
|
|
+ |
− |
+ |
143 ± 18 |
7.5 |
420 ± 80 |
0.2 |
|
|
− |
+ |
+ |
55 ± 11 |
2.9 |
2,820 ± 540 |
1.4 |
|
|
+ |
+ |
+ |
216 ± 16 |
11.4 |
166 ± 14 |
0.1 |
- a) Whole spleen cells or spleen B cells preactivated with LPS were transfected with Smad3 / 4 and cultured with LPS, TGF-β1, and IL-5. Following 7 days of culture, supernatants were harvestedand levels of IgA and IgG1 were determined by ELISA. Data are means ± SEM of triplicate cultures. N. D.: Not determined.
3 Discussion
The present studies demonstrate that Smad3 and 4 act as mediators in TGF-β1-induced IgA isotype expression in mouse B lymphocytes. Overexpression of Smad3/4 increases TGF-β1-induced expression of a reporter plasmid driven by the TGF-β-responsive region of the Ig GLα DNA in the B lymphoma cell line, CH12.LX.4933 and increases endogenous GLα transcripts in LPS-activated, TGF-β1-stimulated normal spleen B cells. Furthermore, transiently overexpressed Smad3 and 4 enhances surface IgA expression and total IgA secretion by LPS-activated, TGF-β1-stimulated spleen B cells. In contrast, total IgG1 secretion diminished under the same conditions, These results suggest that TGF-β1 selectively induces IgA isotype switching through Smad3 and Smad4 molecules.The findings herein extend recent studies on the involvement of Smad3/4 in TGF-β1-induced Ig GL α transcription 25, 26. It has been shown that Smad3 forms a complex with PEBP2αC and stimulates transcription of the GL Ig Cα promoter, and that overexpressed Smad4 further increases the promoter activity 25. Furthermore, a dominant negative Smad3 protein inhibited transcription induced by the activated TGF-β1 receptor I, suggesting that transcription of the reporter GL Ig Cα is induced by the endogenous Smad3 activated by TGF-β1 25. Our results together with the studies demonstrating a good correlation between the specificity of GL transcription and the specificity of isotype switching event 11–15, 24 indicate that TGF-β1 causes IgA isotype switching at least in part through Smad3/4-mediated GLα transcription.
The activated TGF-β1 receptor induces phosphorylation of two proteins, Smad2 and Smad3 23, 27, 30–33, which form heterodimeric complexes with Smad4 that translocate to the nucleus, where they regulate transcriptional responses 20–23. It has been shown that the Smad3/4 complex binds CAGA sequences, termed Smad-binding elements (SBE) 12, 34–38. The GLα (pFL3) reporter used in this study contains SBE. Thus, reporter activity is increased by overexpressed Smad3, and in particular by overexpressed Smad3/4. However, the reporter activity is not increased by either overexpressed Smad2 or overexpressed Smad2/4. Althoughboth activated Smad2 and Smad3 can interact with Smad4, Smad2/4 and Smad3/4 complex bind different DNA elements. It has been shown that Smad2, complexed with Smad4, interacts with the forkhead protein FAST-1 39, 40, which together binds the FAST-1 DNA motif, AAT(A/C)(C/A)ACA within the activin response element (ARE) 23, 27, 31, 39–41. The FAST-1 binding motif is not found in the promoter sequence of the GLα (pFL3) reporter. Presumably, the lack of a FAST-1 binding motif within pFL3 promoter may explain why Smad2 does not affect the reporter activity in the present study. Smad2 has also been found to be unable to activate a reporter plasmid driven by an intact GLαpromoter 25.
One of the inhibitory Smad, Smad7, which is induced by TGF-β1 42, can interact with an activated TGF-β1 receptor, resulting in blocking of phosphorylation of Smad2 and Smad3 28, 43–45. We found that transfected Smad7 substantially inhibits Smad3/4-mediated GLα transcription in CH12.LX.4933 cells (Fig. 3) and in normal spleen cells (data not shown). This observation appears to be meaningful in the context of IgA regulation. It has been recently shown that IFN-γ signaling through the Jak1/Stat1 pathway rapidly increases the expression of Smad7 causing the inhibition of TGF-β1-mediated Smad3 phosphorylation and an attendant loss of TGF-β1 signaling to the nucleus43. In addition, it has been shown that IFN-γ reduces the level of endogenous GLα transcripts and IgA switching 11. Taken together, one can postulate that TGF-β1-induced Smad3-mediated IgA synthesis is down-regulated by Smad7-mediated IFN-γ signaling.
In conclusion, TGF-β1 is not formally established as a physiological mediator for IgA isotype switching in vivo, although there is much in vitro evidence for its importance for IgA switching 1–3, 29, 46. Nevertheless, TGF-β1 has been considered such a mediator since TGF-β1 stimulates GLα promoter activity [11–13, 24 and deletion of TGF-β1 gene or TGF-β receptor type II gene results in severe loss of IgA expression 47, 48. Based on all studies and the present reports herein, we conclude that Smad3 and Smad4 are key molecules in TGF-β1 signaling towards IgA switching event. Along with Smad3/4, recent studies showed that PEBP2/CBF/AML 24, 25, Ets family proteins 49, and CREB/ATF 50 are involved in the transcriptional activity of TGF-β1 inducible GLα promoter. Thus, it will be interesting to investigate the cooperative effect of PEBP2, Ets, and CREB with Smad in IgA expression.
4 Materials and methods
4.1 Animals
BALB/c mice were purchased from B&K Universal Co. (Fermont, CA) and maintained ad libitum on Purina Laboratory Rodent Chow 5001 (Ralston Purina Co., Richmond, IN) in an animal environmental control chamber (Myung-Jin Inst. Co., Seoul). Eight- to twelve-week-old mice were used in this study.
4.2 Cell lines and cell culture
Murine B cell lymphoma lines, CH12.LX.4933 (surface μ+δ+) and CH12.LX.4F10 (surface α+) were generously provided by Dr. Geoffrey Haughton (University of North Carolina, Chapel Hill, NC). These cells were cultured at 37°C in a humidified atmosphere containing 5% CO2 in RPMI 1640 medium (Sigma) supplemented with 10% FBS, 50 μM 2-ME, 5 mM HEPES, penicillin (100 U/ml)/ streptomycin (100 μg/ml). I.29μ (subclone, 22D; sIgM+ B cell lines) cells were provided by Dr. Janet Stavnezer (University of Massachusetts Medical School, MA). I.29μ were cultured in 8% CO2, and the medium was supplemented with 0.1 U/ml of purified human insulin (Novo Nordisk, Princeton, NJ), and 20% FBS.
Mouse spleen cell suspensions were prepared as described before 51. Spleens removed from mice were smashed on sterile metal gauze in 0.01 M PBS. Cell suspensions were briefly washed in PBS and treated with 0.83% ammonium chloride to lyse RBC. Cells were then washed with HBSS two times and suspended in complete medium. Whole spleen cells (1×106 cells/ml) were cultured with addition of LPS (12.5 μg/ml, E. coli 0127:B8, Sigma) and TGF-β1 (1 ng/ml). Spleen B cells were purified by depletion of T cells, using rat mAb for Thy1.2 (HO13.4),Thy1 (Jij.10), CD4 (GK1.5), and CD8 (3.168) followed by addition of anti-rat κ-chain mAb (MAR 18.5) and guinea pig complement. Purified spleen B cells were cultured with addition of LPS (25 μg/ml) and TGF-β1 (0.2 ng/ml).
4.3 Expression plasmids
Mammalian expression vectors, Smad2 32, Smad3 27, Smad4 52 and Smad7 28, which were subcloned into N-terminal Flag tagged-pcDNA3 44, were generously provided by Dr. Masahiro Kawabata (Department of Biochemistry, The Cancer Institute, Tokyo, Japan). A reporter plasmid, pFL3 containing the luciferase gene driven by TGF-β-responsive element of the GLα promoter 12, was provided by Dr. Janet Stavnezer (University of Massachusetts Medical School, Worcester, MA).
4.4 Transfection and luciferase assays
A total of 1×107 cells for CH12.LX.4933 or 5×107 cells for I.29μ or 2×107–3×107 cells for normal spleen cells were suspended in 800 μl RPMI 1640 (serum and antibiotics free) and transferred to a 1.5-ml microtube. For luciferase assays, 30 μg of each expression vector and reporter along with 10 μg pCMV5β gal (Stratagene) were transfected to CH12.LX. 4933. The final volume of the mixture was adjusted to 800 μl in a cuvette (Gap: 0.4 cm). The cells were exposed to a single pulse at 950 μF and 300–400 Vusing a Gene Pulser II (Bio-Rad, Hercules, CA). After transfection, cells were rested at room temperature for 10 min and then diluted into culture medium (RPMI 1640) including 10% FBS, LPS and TGF-β1. Cells were incubated at 37°C in a CO2 incubator. For the luciferase and β-gal assays, cells were harvested following 24 h of culture, and lysed in Triton X-100 buffer (0.1% Triton X-100, 1 M Tris-HCl, pH 8.0). Cell debris was removed by centrifugation at 12,000 rpm for 15 min, and then the extracts were stored at –70°C until used. Samples with an equal amount of proteins were mixed with luciferin substrate solution and level of luciferase activity was determined by a luminometer (Berthold Multi-Biolumat LB9505C, Laboratorium, Germany). The β-gal assay was also performed toassess the activity of pCMV5βgal 53. Values of luciferase activity were normalized by β-gal activity.
4.5 Immunoprecipitation and Western blot
CH12.LX.4933 cells were transfected with 50 μg pcDNA3-Flag-Smad3 or pcDNA3 plasmid. At 72 h post-transfection, cells were washed and solubilized in a buffer containing 50 mM Tris, 150 mM NaCl, 1% Triton X-100, 0.5% NP40, 1 mM Na3VO4 plus protease inhibitors. The cell lysates were pelleted by centrifugation and the supernatants were incubated with anti-Flag M2 antibodies (Sigma) for 2 h, followed by incubation with protein A-Sepharose beads (Pharmacia biotech, Piscataway, NJ) for 1 h at 4°C. The beads were washed twice with the solubilizing buffer. The immune complexes were then eluted by boiling for 5 min in SDS sample buffer (50 mM Tris-HCl, pH 6.8, 0.1% bromophenol blue, 10% glycerol, 2% SDS) containing 100 mM DTT and separated by SDS-8% polyacrylamidegel electrophoresis. Proteins were electrotransferred to nitrocellulose membrane and the membrane was incubated with rabbit anti-Smad3 antibody (Zymed, San Francisco, CA) for 1.5 h at room temperature. Subsequently, horseradish peroxidase-conjugated goat anti-rabbit antibody (Sigma) was added and incubated for 1.5 h at room temperature. The membrane was developed using 3 mg/ml chloronaphthol in 0.01 M PBS and 0.03 M H2O2.
4.6 RNA preparation and RT-PCR
Total cellular RNA were extracted from cultured cells using TRIzol (Gibco BRL, Grand Island, NY) according to manufacturer's instructions. In brief, cells were lysed in 1 ml of TRIzol solution. After adding 0.2 ml of chloroform and mixing completely, the mixture was incubated at room temperature for 3 min. After centrifugation, the aqueous phase was transferred to a new tube and precipitated by addition to an equal volume of isopropanol, followed by incubation at room temperature for 10 min. RNA was recovered by centrifugation at 4oC for 10 min. The RNA pellet was dissolved in DEPC-treated H2O and quantified by UV scanning.
Reverse transcription and PCR were performed as follows. Briefly, 1 μg RNA was reverse-transcribed in the presence of 2.5 μM oligo d(T)16 as primers and 50 U Moloney murine leukemia virus reverse transcriptase in a buffer containing 10 mM Tris-HCl (pH 8.3), 50 mM MgCl2, 20 U RNase inhibitor, and 1 mM of the four deoxyribonucleotide triphosphate (dNTP), in a total volume of 20 μl. The mixture was incubated at 37°C for 1 h for cDNA synthesis and further incubated at 95°C for 10 min for heat inactivation, and stored at 4°C until used. For cDNA amplification, 40 μl of PCR master mixed containing 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 2 mM MgCl2, 200 μM dNTP, 1 U Taq DNA polymerase and 50 pmol each of "downstream" and "upstream" primers were added to each sample. The primers were synthesized by Bioneer Corp. (Seoul, Korea), i.e. GLα sense, CTACCATAGGGGAAGATAGCCT and antisense, TAATCGTGAATCAGGCAG (amplified product, 225bp) 54; GLγ1 sense, CAGCCTGGTGTCAACTAG and antisense, CTGTACATATGCAAGGCT (amplified product, 532 bp) 54; β-actin sense, CATGTTTGAGACCTTCAACACCCC and antisense, GCCATCTCCTGCTCGAAGTCTAG. All reagents except primers were purchased from Promega Corp. (Madison, WI). The number of PCR cycles performed to yield an optimal signal was established experimentallyas 38 cycles for GLα and γ1 transcripts and 30 cycles for β-actin. Hot start PCR was used to increase specificity of the amplification. Each cycle consisted of 45 s at 95°C for DNA denaturation, 45 s at 55°C for primer annealing, and 2 min at 72°C for primer extension. The PCR reaction for GLα transcripts and GLγ1 transcripts was performed in parallel with β-actin to normalize the cDNA concentrations within each set of samples. After the cycles, aliquots of PCR products were resolved by electrophoresis in a 2% agarose gel.
4.7 Flow cytometry analysis
Cultured cells were washed with HBSS and resuspended in DMEM, 5% FBS, 0.1% NaN3 at a density of 1×106 cells/ml. FITC-conjugated goat anti-mouse IgA (Becton Dickinson, San Jose, CA) was added to the cell suspension and placed at 4°C for 30 min. Cells were washed with HBSS three times and resuspended in PBS-1% formalin. Cytofluorometric analysis was carried out usinga FACScan (Becton Dickinson, Mountain View, CA).
4.8 Isotype-specific ELISA
Produced antibodies were detected using a modified ELISA 50. Affinity-purified anti-isotype-specific antibody was added at 1.2 μg/ml in 0.05 M bicarbonate buffer (pH 9.3) to 96-well U-bottom polyvinyl microplates (Falcon, Becton Dickinson & Co., Oxnard, CA). Plates were washed with PBS containing 0.05% Tween-20 followed by overnight incubation at 4°C, and blocked for 1 h with 1% gelatin solution. After washing, 50 μl of standard myeloma proteins and culture supernatants were added to each well and incubated for 1 h at 37°C. Horseradish-peroxidase conjugated anti-isotype-specific antibody (Southern Biotechnology, Birmingham, AL) were added to each well and incubated for 1 h. Plates were then washed and 0.2 mM 2,2′-azino-bis (3-ethylbenzthiazoline)-6-sulfonic acid (ABTS, Sigma) was added. After 15-min incubation, colorimetric reaction was measured at 405 nm with an automatic microtiter reader (450; Bio-Rad).
Acknowledgements
The authors thank Dr. Janet Stavnezer for critical reading of the manuscript, and C. E. Schrader and J. Vardo for their helpful advice in animal study. Thiswork was accomplished with Research Fund provided by Korea Research Foundation, Support for Faculty Research Abroad (to P.-H. Kim) and by the NIH grant AI42108 (to J. Stavnezer).
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




