Control of T cell hyperactivation in IL-2-deficient mice by CD4+CD25– and CD4+CD25+ T cells: evidence for two distinct regulatory mechanisms
Abstract
In IL-2-deficient mice, antigen-activated CD4 T cells accumulate and cause lethal immune pathology. Wild-type cells of hematopoietic origin present in the same animal are able to prevent this hyperactivation of T cells, but the mechanisms and cells controlling the IL-2-deficient cells are unknown. Here we show that IL-2– CD4 cells with an ovalbumin-specific transgenic TCR (IL-2– OVAtg) undergo both clonal expansion and clonal contraction when transferred to euthymic recipients and challenged with antigen, but continuously expand in athymic hosts. Cotransfer of wild-type CD4 T cells prevents the accumulation of IL-2-deficient cells. On the residual IL-2– TCRtg cells CD69 and CD25 are up-regulated, suggesting that activation per se is not suppressed and that the cells had received an IL-2 signal. Since IL-2 is able to restore the defective antigen-induced cell death (AICD) of IL-2-deficient T cells in vitro, paracrine IL-2 provided by the wild-type CD4 cells may thus be able to allow clonal contraction of IL-2-deficient cells also in vivo. Interestingly however, regulatory CD4+CD25+ cells also efficiently contain the clone size of antigen-stimulated IL-2-deficient T cells. Since CD4+CD25+ cells do not produce IL-2, this suggests a mechanism of suppression distinct from paracrine IL-2 delivery. In keeping with this, the residual IL-2– TCRtg cells recovered after cotransfer of regulatory CD4+CD25+ cells do not show increased CD25 or CD69 expression, suggesting that they had not received paracrine IL-2 and that clonal containment occurred at the level of initial activation rather than clonal contraction by AICD. IL-2 deficiency therefore mayupset T cell homeostasis by two distinct mechanisms: the failure to program expanding T cells for apoptosis, and the failure to generate functional CD4+CD25+ regulatory cells.
Abbreviations:
-
- AICD:
-
Antigen-induced cell death
-
- IBD:
-
Inflammatory bowel disease
1 Introduction
IL-2 was originally identified as a potent T cell growth factor 1. Subsequently, the spectrum of target cells addressed and of biological responses elicited by IL-2 was found to be much broader than originally thought. Nevertheless, promotion of T cell proliferation is the key activity attributed to this cytokine, and the rationale for pharmacological intervention withIL-2 signaling has remained the suppression of T cell activation in vivo.
Surprisingly, inactivation of the IL-2 gene by gene targeting revealed that in mice, IL-2 is not essential for the generation, clonal expansion or differentiation of lymphocytes to effector cells but rather has a unique role in preventing the accumulation of activated CD4 T cells 2–4. In the absence of IL-2 or a functional IL-2 receptor, an immunopathological condition develops 5–7 that we have called the IL-2-deficiency syndrome 8. Its key features are splenomegaly and lymphadenopathy with apredominant contribution of activated CD4 T cells, inflammatory bowel disease (IBD), multi-organ lymphocytic infiltrates, autoantibody formation and anemia. The severity of this syndrome and hence the importance of IL-2 for negative regulation of the immune system are illustrated by the drastic reduction in life span of IL-2-deficient animals which, depending on the genetic background studied, can be as short as a few weeks 6. Similar, although not entirely overlapping lymphoproliferative syndromes have been reported for IL-2Rα 9 and IL-2Rβ-deficientmice 10.
Previous studies from our group have formally shown that the development of immune pathology is T cell dependent and requires antigenic stimulation. Thus, athymic IL-2-deficient mice remain healthy 11, and maintenance of IL-2– mice with an intact thymus in a germ-free environment delays the onset of disease 5. Furthermore, we 12, 13 and others 14, 15 have restricted the repertoire of IL-2– mice by introducing a transgenic TCR specific for a model antigen normally not present as an environmental or autoantigen. Again, this greatly reduces the hyperactivation of the T cell compartment and the ensuing pathology characteristic of the IL-2-deficiency syndrome. Finally, a comparison of superantigen-induced clonal expansion and subsequent clonal "contraction" directly demonstrated a defect in the removal of antigen-activated T cells in the absence of IL-2 in vivo 16.
The mechanisms by which clonal expansion and execution of effector functions of CD4 T cells are counter-regulated in vivo are only incompletely understood. Quite clearly, the drastic defect in the control of CD4 T cell activation found in IL-2-deficient mice, points to a central role of this cytokine as a negative regulator of the immune response. To date, two main pathways have been explored to discover the underlying molecular and cellular mechanisms:
First, a direct effect of IL-2 signaling on clonal contraction of IL-2-deficient T cells is suggested by the following findings: In vitro activation of wild-type T cells under conditions which prevent IL-2 signaling results in protection from antigen-induced cell death (AICD) in response to religation of the TCR 17, 18. Furthermore, activated IL-2-deficient T cells are resistant to CD95-triggered apoptosis in spite of normal or even enhanced levels of CD95 expression and resistance is reversed by exogenous IL-2 15, 16.
Secondly, it was investigated whether wild-type hematopoietic cells could control the hyperactivation of IL-2-deficient T cells in vivo. In mixed bone marrow radiation chimeras, we could show that 30% of wild-type contribution to the hematopoietic system suffices to prevent development of splenomegaly, lymphadenopathy and bone marrow infiltration, indicating that control of IL-2– by IL-2+ cells is indeed possible 11. More recently, Suzuki et al. 19 demonstrated that the same holds true for the containment of T cells deficient in IL-2Rβ, indicating that wild-type cells do not necessarily exert their regulatory function via paracrine IL-2 delivery. Similarly, Malek et al. 20 reported that IL-2Rβ expression during T cell development suffices to prevent disease in otherwise IL-2Rβ-deficient mice. These results suggest a regulatory effect by a T cell that depends on IL-2 for its generation. One such candidate population was identified by Suzuki et al. 19 as α/βTCR expressing CD8 T cells that eliminate IL-2Rβ-deficient activated T cells by cell-mediated cytotoxicity. However, CD4 T cells also had regulatory potential in this system, although their mechanism of action was not further addressed.
An attractive candidate for a cell type within the CD4 subset which may counteract the IL-2-deficiency syndrome are CD4+CD25+ T cells, a population of regulatory cells currently under intense investigation 21. CD4+CD25+ T cells are thymus-derived 22 suppressors of autoimmune and inflammatory T cell responses in vivo 23–26. There is compelling evidence that the lack of these regulatory cells results in autoimmunity when experimental procedures such as postnatal thymectomy are employed to prevent a complete development of all T cell subsets (reviewed in 21). The specificity of CD4+CD25+ T cells is unknown, but there is some evidence for reactivity to tissue-specific self antigens, and autoreactivity is further supported by their selection by endogenous retroviral superantigens in the thymus 22.
CD4+CD25+ T cells are also potent suppressors of T cell proliferation in vitro 27, 28. While some discrepancies exist with regard to their reported mechanism(s) of action in vitro vs. in in vivo, in particular with regard to the importance of the anti-inflammatory cytokines IL-10 and TGF-β which they produce, there is consensus that CD4+CD25+ T cells inhibit immune responses at a very early stage, either by inactivating APC or through direct T-T interactions 21.
Importantly, CD4+CD25+ T cells are absent in IL-2-deficient mice 22, although this conclusion has the caveat that for full up-regulation of CD25, IL-2 itself is required. In the present report, we have used IL-2-deficient, OVA plus IAd-specific TCR transgenic CD4 T cells in an adoptive transfer system to search for wild-type T cell populations that are able to contain antigen-driven hyperactivation upon cotransfer. The results suggest that CD4 T cells from wild-type mice are effective regulators of IL-2-deficient T cells, and that their major, CD25–, and their minor, CD25+ subset restrict their clone size by two distinct mechanisms.
2 Results and discussion
2.1 Amelioration of the IL-2-deficiency syndrome by restriction of the TCR repertoire
BALB/c mice are particularly sensitive to the immunopathology caused by a genetic deficiency in IL-2. As has been described in detail earlier 6, IL-2– BALB/c mice do not survive beyond 5 weeks of age and die with the typical symptoms of the IL-2-deficiency syndrome. Since the ameliorating effect of a germ-free environment on the development of immune pathology had suggested that the accumulation of activated CD4 T cells in these animals is an antigen-driven event 5, we restricted the TCR repertoire of IL-2-deficient BALB/c mice by introducing the OVA plus IAd-specific TCR DO.11.10 13. As expected, these TCR-transgenic IL-2-deficient BALB/c mice showed an increased life span with a mean survival time of 4 months (data not shown). This is, however, still well below the average life expectancy of wild-type BALB/c mice, indicating that the DO.11.10 transgene provided incomplete protection from T cell hyperactivation. Indeed, penetrance of the TCR transgene as assessed by expression of the KJ1-26 idiotypic determinant was incomplete (between 70 and 95%), and activated T cells accumulated with time, presumably as a result of endogenous TCR α-chain expression (data not shown). Nevertheless, young adult DO.11.10 IL-2– BALB/c mice provided a reproducible source of antigen-responsive CD4 T cells 13 which were employed for the following experiments.
2.2 Clonal expansion and contraction of adoptively transferred DO.11.10 IL-2+ or IL-2– CD4 T cells
Following the procedure established by Jenkins and colleagues for the evaluation of clonal dynamics of T lymphocytes in vivo 29, we transferred 2×106 Id+ CD4 T cells from IL-2-deficient or IL-2 wild-type BALB/c mice into syngeneic BALB/c or athymic BALB/c nu/nu recipients. After subcutaneous immunization with the cognate OVA peptide 323–339 in IFA, absolute numbers of Id+ T cells in the draining lymph nodes were followed over time. As can be seen in Fig. 1, both IL-2 wild-type and IL-2-deficient Id+ CD4 T cells were increased in number over unstimulated controls 1 week after cell transfer into BALB/c mice (clonal expansion), as has recently been reported by others 14. Regardless of the presence of an intact IL-2 gene in the Id+ cells transferred, this was followed by a decrease to baseline levels over the next 2 weeks (clonal contraction). In contrast, when DO.11.10 transgenic CD4 T cells were transferred into athymic recipients, the kinetics of IL-2+ and IL-2– Id+ cells recovered were dramatically different: While TCR transgenic cells with an intact IL-2 gene contracted normally, those from IL-2-deficient donors continued to expand throughout the observation period of 3 weeks. Thus this system recapitulates in a well-controlled fashion the accumulation of antigen-expanded T cells previously observed in IL-2 deficient animals 5, 8, 16. Moreover, the results show that T cells with an intact IL-2 gene, either present in the TCR-transgenic inoculum or in the recipient are able to counteract the accumulation of clonally expanded, activated CD4 T cells which is the hallmark of the IL-2-deficiency syndrome. This is in keeping with our earlier findings on mixed bone marrow chimeras in which a fraction of wild-type hematopoietic cells effectively prevented the IL-2-deficient T cells present in the same animal from causing immune pathology 11.
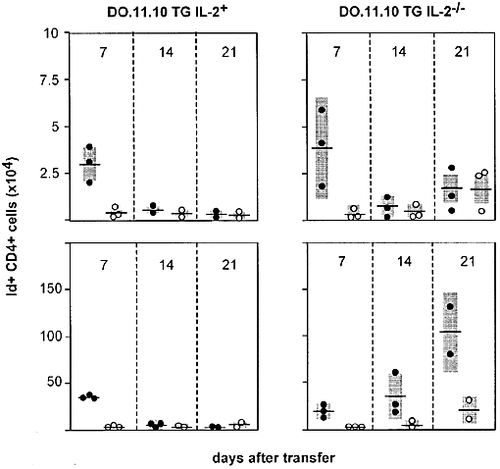
Uncontrolled antigen-driven expansion of IL-2-deficient TCR-transgenic CD4 T cells in athymic hosts. IL-2 wild-type or IL-2-deficient CD4 cells (2×106) expressing the DO11.10 OVA-specific TCR were transferred into normal (top panels) or athymic (bottom panels) BALB/c and challenged with the OVA323–339 peptide in IFA (filled circles) or with IFA alone (open circles) 1 day later. At the times indicated, draining lymph nodes were harvested and the absolute number of Id+ cells was determined. Each dot represents an individual animal, mean values are indicated by horizontal bars and SD by shaded areas.
2.3 Restoration of clonal contraction of IL-2-deficient CD4 T cells by cotransferred T cells and T cell subsets
To test whether IL-2 sufficient T cells are able to control the abnormal accumulation of IL-2-deficient CD4 cells, T cells from normal BALB/c mice were cotransferred with IL-2-deficient DO.11.10 transgenic CD4 cells into athymic BALB/c recipients. As shown in Fig. 2A, the antigen-induced accumulation of Id+ IL-2-deficient CD4 cells observed three weeks later was completely abolished by the cotransfer of a fivefold excess of wild-type T cells. This suppression appeared to act mostly at the level of clonal contraction because only a small reduction in cell number was observed 1 week after cell transfer and antigenic stimulation but was complete 2 weeks later.
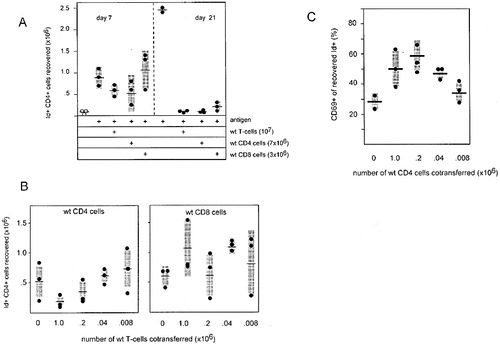
IL-2-sufficient T cells prevent the abnormal accumulation of antigen-stimulated IL-2-deficient CD4 cells. (A) Suppression is more pronounced at late versus early time points after stimulation. (B) Under limiting conditions, CD4, but not CD8 cells prevent accumulation of IL-2-deficient cells. (C) Expression of the early activation marker CD69 is not suppressed but increased on residual IL-2-deficient cells after suppression by CD4 cells. Cell transfer and analysis were as given in Fig. 1 except that purified T cells or T cell subsets were cotransferred as indicated. Analysis in (B) and (C) was performed on day 21 after transfer. Each dot represents an individual animal, mean values are indicated by horizontal bars and SD by shaded areas.
When the CD4 and CD8 subsets present in the wild-type T cell population were administered separately, both exhibited regulatory activity. However, since the CD4 fraction appeared slightly more effective than the CD8 fraction, and because suppression appeared to be in saturation, wild-type CD4 and CD8 T cells were titrated in another experiment at lower numbers to test for their suppressive potential. As shown in Fig. 2B, CD4 T cells inhibited the accumulation of IL-2-deficient TCR transgenic CD4 T cells much more efficiently than CD8 T cells, suggesting that CD4 cells are mainly responsible for the correction of IL-2-deficient CD4 T cell accumulation in an environment containing wild-type T cells. This result is in seeming contrast to the recent findings of Suzuki et al. 19 who found that CD8 cells are superior to CD4 cells in counteracting the accumulation of T cells lacking the IL-2Rβ chain. In this system, however, clonal contraction via paracrine provision of IL-2, expected to be primarily produced by CD4 T cells, cannot play a role. The ability of IL-2 to sensitize IL-2-deficient (but not IL-2Rβ-deficient) T cells for AICD 15, 16 thus provides an explanation for this discrepancy.
Interestingly, the residual IL-2-deficient Id+ cells recovered after cotransfer with wild-type CD4 T cells contained a higher fraction of cells expressing the activation marker CD69 than controls without regulatory cells (Fig. 2C). This supports the notion that these cells acted mainly at the level of clonal contraction rather than by inhibiting initial activation of the IL-2-deficient OVA-specific T cells.
2.4 CD4+CD25+ cells effectively counteract accumulation of antigen-activated IL-2-deficient CD4 cells
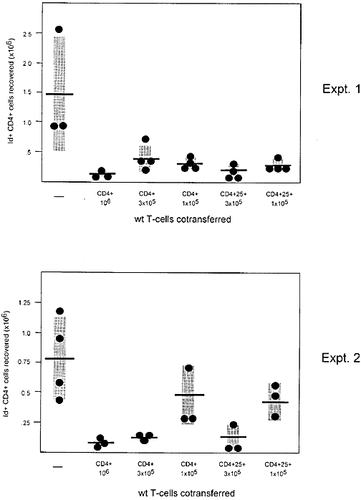
There are two obvious possible mechanisms through which wild-type CD4 T cells may correct the defective control of clone size in IL-2-deficient T cells: provision of paracrine IL-2 which may restore sensitivity to AICD, and counter-regulation of T cell activation by a regulatory T cell population. A good candidate for such a CD4 T cell subset are the CD4+CD25+ cells producing IL-10 and TGF-β but not IL-2 (reviewed in 21). These thymus-derived cells have previously been shown to suppress autoimmunity and inflammation in a variety of systems 23–26. To test whether CD4+CD25+ T cells from wild-type BALB/c mice were able to suppress the accumulation of IL-2-deficient antigen activated CD4 T cells, we initially sorted BALB/c lymph node CD4 T cells into the CD25+ and CD25– subsets and tested both populations in the adoptive cotransfer system employed in the previous experiments. Analysis of recipients of CD25-depleted CD4 T cells was, however, confounded by the occurrence of diarrhea and wasting (data not shown). This is in line with the recent identification of CD4+CD25+ cells as suppressors of IBD and wasting induced by CD45RBhigh CD4 T cells in response to microbial and dietary antigens of the gut 21, 23, 25, 26. Accordingly, unseparated CD4 T cells were compared with the CD4+CD25+ subset for their regulatory capacity upon cotransfer with IL-2-deficient TCR transgenic CD4 cells. As shown in Fig. 3, CD4+CD25+ cells were at least as potent as unseparated CD4 cells, demonstrating the efficacy of this subset in controlling hyperactivation of IL-2-deficient T cells.
2.5 Control of T cell expansion by CD4+CD25+ cells is independent of paracrine IL-2 delivery
CD4+25+ cells do not produce IL-2 when isolated ex vivo 22, suggesting that paracrine IL-2 delivery is not essential for preventing the accumulation of IL-2-deficient cells. To test whether the injected regulatory cells remain stable with regard to this phenotype, we tested the capacity of Id– cells recovered 3 weeks after transfer to produce IL-2. As shown in Fig. 4A, there was a low background of CD4 cells capable of producing IL-2 in Id– CD4 cells recovered from athymic mice that had not received regulatory cells. These cells are derived from the endogenous extrathymically matured T cell population found in athymic mice which is largely immuno-incompetent because of its oligoclonal repertoire 30. Upon transfer of unseparated CD4 T cells, the frequency of recovered Id– CD4 cells able to produce IL-2 rose to about 30%. In contrast, no increase above background was detected upon cotransfer of CD4+CD25+ T cells, indicating that they had not acquired the capacity to produce IL-2 during the course of the adoptive transfer experiment.
To test for the persistence of CD4+CD25+ regulatory cells and for the impact of paracrine IL-2 on the TCR-transgenic IL-2 deficient indicator cells, the expression of CD25 itself was investigated on both populations. For comparison, mice that had received unseparated CD4 regulatory cells were included. As shown in Fig. 5B, CD25 expression was twice as frequent among Id– cells recovered from mice which had received CD4+CD25+ regulators as compared to those that had received unseparated CD4 cells. In contrast, CD25 expression on the Id+ IL-2-deficient indicator cells followed an inverse pattern: cotransfer of unseparated CD4 T cells, but not of purified CD4+CD25+ cells had resulted in up-regulation of CD25 on the residual recovered TCR-transgenic cells. Since IL-2 is known to up-regulate CD25, the α-chain of its receptor 31, 32, this result suggests that unseparated CD4 T cells actually had provided IL-2 to the IL-2-deficient indicator cells whereas CD4+CD25+ cells had not.
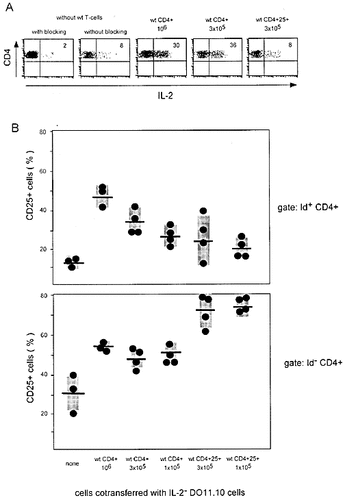
Expression of IL-2 and CD25 by cells recovered after cotransfer of IL-2-deficient indicator and IL-2-sufficient regulator cells. (A) Id– CD4 cells recovered after cotransfer of unseparated CD4 cells, but not their CD25+ subset, are able to produce IL-2. Intracellular cytokine staining was performed on cells recovered on day 21 after transfer, gated to exclude Id+ cells. (B) CD4+CD25+ regulatory cells remain CD25+ during transfer but fail to induce CD25 on IL-2-deficient Id+ cells. Draining lymph node cells were analyzed on day 21 after cotransfer of the populations indicated and antigenic stimulation. Frequencies of CD25+ cells among gated Id– and Id+ CD4 cells are shown. Each dot represents an individual animal, mean values are indicated by horizontal bars and SD by shaded areas.
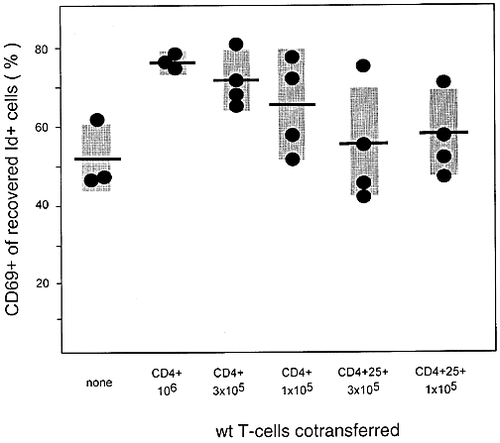
Enhanced frequency of IL-2-deficient CD4 cells expressing the early activation marker CD69 after clonal control by unseparated CD4 cells but not by purified CD4+CD25+ regulatory cells. Cells were analyzed by three-color flow cytometry on day 21 after cotransfer and antigenic stimulation. Frequencies of CD69+ cells among the Id+ population are shown. Each dot represents an individual animal, mean values are indicated by horizontal bars and SD by shaded areas.
Taken together, these results indicate that CD4+CD25+ cells do not down-regulate the accumulation of IL-2-deficient cells via paracrine provision of IL-2. In keeping with this finding, defective clonal contraction of T cells from IL-2Rβ-deficient mice that are unable to receive an IL-2 signal is also controlled by wild-type T cells 19. Although CD8 cells were found superior to CD4 cells in this system, the inhibitory component contained in the latter may have been mediated by their CD4+CD25+ subset.
2.6 Two mechanisms of clonal control?
Although the above results illustrate the efficacy of CD4+CD25+ cells as down-regulators of T cell hyperactivation caused by IL-2 deficiency, we also found that unseparated C4 cells of which only about 5–10% are CD25+ are similarly potent suppressors. This suggested that the dominant mechanisms of suppression mediated by unseparated versus purified CD4+CD25+ cells may have been different. Indeed, the increased expression of CD25 on residual IL-2 deficient TCR transgenic cells after stimulation in the presence of unseparated CD4 but not of CD4+CD25+ cells indicated that different signals had been received by the IL-2-deficient cells from the two populations of regulatory cells, and that sensitization for AICD by IL-2 may have played a role in the former but not in the latter setting. If so, initial activation would have proceeded unimpaired when the majority of cotransferred regulatory cells were CD25– but able to produce IL-2 (whole CD4 cells). In contrast, our current knowledge of immune regulation by CD4+CD25+ cells indicates that they suppress clonal expansion of CD4+CD25– T cells at the level of initial expansion 21.
To obtain further information on the activation status of IL-2-deficient TCR transgenic T cells recovered after stimulation in the presence of unseparated versus CD25+ wild-type CD4 T cells, the early activation marker CD69 was investigated. As shown in Fig. 2C and 7, the frequency of CD69 expressing cells within the Id+ population recovered 3 weeks after cell transfer was strongly increased when unseparated CD4 cells were used to prevent accumulation of clonally expanded cells, while cotransfer of CD4+CD25+ regulatory cells did not lead to increased CD69 expression. Thus, in spite of an equally effective containment of antigen-driven clonal expansion of IL-2-deficient CD4 cells in both settings, their activation status was actually enhanced in the presence of unseparated wild-type cells capable of producing IL-2 while this was not the case when CD4+CD25+ regulatory cells were employed which are incapable of producing IL-2.
3 Concluding remarks
The present results suggest that CD4 T cells can restrict the clone size of antigen-activated IL-2-deficient CD4 cells in at least two ways: Their CD25+ subset blocks activation at the level of initial triggering, whereas their CD25– subset provides paracrine IL-2, thereby allowing clonal contraction via AICD.
At present, neither the mechanism by which IL-2 sensitizes activated T cells for AICD nor the mode by which CD4+CD25+ cells prevent T cell activation are fully understood. With regard to the regulation of the apoptotic machinery by IL-2, both CD95 and TCR-induced AICD are affected. In molecular terms, a requirement of IL-2 for the down-regulation of the anti-apoptotic factor cFLIP has been described 15. Moreover, our own unpublished results indicate that IL-2 positively regulates A1, another anti-apoptotic protein acting further downstream in the death signaling cascade.
It is intriguing that in addition to the para- and autocrine effects of IL-2 on controlling the clone size of IL-2 responsive T cells, this cytokine may also be essential for the generation ofthe immunosuppressive CD4+CD25+ subset which prevents T cell activation at an early stage 22. In this context, it is noteworthy that (retrovirally encoded) selfantigens do not only induce IL-2Rα chain expression during the development of CD4+CD25+ regulatory cells in the thymus 22, but that self-recognition during thymocyte development also induces the IL-2Rβ chain which is essential for the perception of an IL-2 signal 33. While the relationship between self-reactive IL-2Rβ+ thymocytes and CD4+CD25+ regulatory cells is at present unclear, a recent report shows that IL-2Rβ+ expression during thymic development suffices to prevent lymphoproliferative disease in otherwise IL-2Rβ deficient mice 20, underlining the importance of IL-2 signaling during T cell development for the maintenance of peripheral T cell homeostasis. It is therefore likely that the initial conclusion obtained in IL-2-deficient mice that IL-2 plays no role in T cell development 2 needs to be revised with respect to this very small but functionally very important subset.
4 Materials and methods
4.1 Mice
BALB/c mice were bred at the Institute's barrier-contained facilities. BALB/c nu/nu mice were obtained from Harlan Winkelmann, Borchen, Germany, and Charles River, Sulzfeld Germany. Unirradiated male animals around 8 weeks of age were used as recipients for adoptive transfer. IL-2-deficient BALB/c mice expressing the DO11.10 TCR transgene were generated by backcrossing as described 13 from IL-2-deficient 2 and DO.11.10 transgenic 34 BALB/c mice. The colony was maintained with IL-2+/– DO11.10 transgenic breeding pairs, and the IL-2 genotype offspring was identified by genomic PCR 2. IL-2-deficient (–/–) and -sufficient (+/+ or +/–) DO11.10 transgenic mice were used at 12 weeks or younger. These animals as well as all recipients of transferred cells were kept in sterilized individually ventilated cages throughout the experiment.
4.2 Cell purification and transfer
Lymph node cells were first enriched for T cells by nylon-wool passage followed by purification (>95%) of T cells or their CD4 or CD8 subsets using negative selection on Immunocolumns (Cedarlane Laboratories Ltd., Hornby, Ontario, Canada) according to the manufacturer's instructions. CD4+CD25+ cells were further purified (98%) by positive enrichment using a MiniMACS magnetic separator (Miltenyi Biotec, Bergisch-Gladbach, Germany) and electronic cell sorting (FACS Vantage®, BD Lifesciences, Mountain View, CA). The adoptive transfer, immunization and monitoring of draining lymph nodes followed the procedure of Kearny et al. 29. Purified CD4+ lymph node T cells form DO11.10 TCR transgenic mice containing 2×106 Id+ cells and, as given, wild-type T cells, were i.v. injected in.2 ml BSS. OVA323–339 peptide (20 μg) were emulsified in IFA and injected s.c. at the scruff of the neck 1 day later. At the time points indicated, animals were sacrificed and axillary lymph nodes prepared for further analysis.
4.3 Antibodies
mAb to CD4 (clone GK1.5), CD8α (53–6.7), CD8β (53–5.8), CD16/32 (2.4G2), CD25 (7D4), CD69 (H1.2F3), IL-2 (JES5–16E3) were obtained unconjugated and FITC-, Cychrome- or PE-conjugated from PharMingen-BD Lifesciences. Anti-DO.11.10 idiotype (KJ1–26, 35) was prepared from a cell line kindly donated by Drs. Haskins, Kappler and Marrack, and FITC conjugated using standard procedures. Staining was performed on 1–3×105 cells in PBS/ 0.1% BSA/0.02% NaN3 on ice following standard procedures. For cell surface staining, Fc-receptors were blocked using anti-CD16/32, followed by incubation with fluorochrome-conjugated mAb. For intracellular cytokine staining, a modification of a previously described protocol 36 was used. Cells were stimulated for 4 h with PMA (Sigma, St. Louis, MO) at 2 ng/ml and ionomycin (Sigma) at 500 ng/ml. Brefeldin A (Sigma) was added at 1 μg/ml for the last 2 h. Cells were harvested, washed and stained for cell surface antigens. Fixation was performed with 4% formaldehyde for 20 min at room temperature, followed by permeabilization with 0.5% saponin in PBS/BSA/Azide for 10 min. Nonspecificbinding was blocked with normal rat Ig (10 μg/ml) and cells were stained with directly conjugated anti-IL-2 mAb or isotype control for 20 min at room temperature. Blocking by pretreatment with unlabeled anti-IL-2 mAb in a control sample was used to ensure specificity. Flow cytometric analyses was performed with a FACScan® flow cytometer (Becton Dickinson). Living cells (10,000) were computed and analyzed using the Cell Quest software and are shown as dot plots using a logarithmic scale. Isotype-matched negative controls were used to set background levels to the first logarithmic decade. CD4+DO11.10 transgenic cells were identified by double staining with GK1.5 and CD4 and KJ1–26, and absolute numbers of recovered Id+ cells were calculated from their fraction among trypan-blue excluding total draining (axillary) lymph node cells.
Acknowledgements
We thank Drs. Haskins, Marrack and Kappler for providing the KJ1–26 Hybridoma, and Dr. Ken Murphy for DO.11.10 TCR transgenic mice. Supported by the German Ministry for Education and Research through IZKF Würzburg (01 KS 903), and by Fonds der Chemischen Industrie e.V.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




