Changing responsiveness to chemokines allows medullary plasmablasts to leave lymph nodes
Abstract
During T cell-dependent antibody responses lymph node B cells differentiate either to plasmablasts that grow in the medullary cords, or to blasts that proliferate in follicles forming germinal centers. Many plasmablasts differentiate to plasma cells locally, but some leave the medullary cords and migrate to downstream lymph nodes. To assess the basis for this migration, changes in the responsiveness of B cells to a range of chemokines have been studied as they differentiate. Naive B cells express high levels of CCR6, CCR7, CXCR4 and CXCR5. When activated B cells grow in follicles the expression of these chemokine receptors and the responsiveness to the respective chemokines is retained. During the extrafollicular response, plasmablast expression of CXCR5 and responsiveness to B-lymphocyte chemoattractant (CXCR5) as well as to secondary lymphoid tissue chemokine (CCR7) and stromal cell-derived factor (SDF)-1 (CXCR4) are lost while a weak response towards the CCR6 chemokine LARC is maintained. Despite losing responsiveness to SDF-1, extrafollicular plasmablasts still express high levels of CXCR4 on the cell surface. These results suggest that the combined loss of chemokine receptor expression and of chemokine responsiveness may be a necessary prerequisite for cells to migrate to the medullary cords and subsequently enter the efferent lymph.
Abbreviations:
-
- SLC:
-
Secondary lymphoid tissue chemokine
-
- SDF:
-
Stromal cell-derived factor
-
- ELC:
-
EBI 1-ligand chemokine
-
- BLC:
-
B-lymphocyte chemoattractant
-
- MMTV:
-
Mouse mammary tumor virus
-
- BrdU:
-
5-bromo-2′-deoxyuridine
-
- CGG:
-
Chicken gammaglobulin
-
- RANTES:
-
Regulated upon activation, normal T cell expressed and secreted
-
- LARC:
-
Liver and activation-regulated chemokine
1 Introduction
Chemokines are important factors responsible for guiding leukocytes to specific localizations of the body 1 – 4. Naive B and T cells enter the lymph node in the paracortex through high endothelial venules 5 – 7. Chemokines such as secondary lymphoid tissue chemokine (SLC) and stromal cell-derived factor (SDF)-1 are responsible for induction of firm arrest of the rolling lymphocytes 8 – 11. After diapedesis naive lymphocytes are attracted via SLC / EBI 1-ligand chemokine (ELC) and SDF-1 to the lymph node paracortex 2. Activated skin Langerhans cells enter the lymph node via ELC / SLC-CCR7 interactions 2, 12, 13. Once arrived in the strategically important positions to prime T cell responses, they recruit naive T and B cells and induce T cell priming when they make contact with antigen-specific T cells 3, 14 – 17. Deleting the CCR7 gene by homologous recombination leads to lack of efficient antigen presentation in the draining lymph node. In addition, infiltration of lymph nodes with naive T cells is strongly reduced 13. Similarly the plt mutation leads to absence of SLC mRNA and hence markedly reduced numbers of T cells in the lymph nodes 18. Both recirculating B cells and T cells enter the lymph node through high endothelial venules, but B cells are less dependent on expression of CCR7 for this transmigration than T cells suggesting alternative receptors taking part in lymph node B cell homing 13, 19, 20. Once T cells, B cells and dendritic cells enter the lymph nodes, chemokine gradients provide key factors for attracting these cells to specific localizations within the lymph node during homeostasis and immune response 2, 3, 13, 21. Naive B cells express CXCR5 and are attracted to the continuous chemokine gradient that originates from the follicles 3, 22. In the spleen the chemokine receptor CXCR5 is responsible for their migration in direction of B-lymphocyte chemoattractant (BLC) in the B zones 20, 22, 23. In lymph nodes there is a likely role for CXCR5, but even in the absence of this receptor B cells are found in follicles. After T cells are primed they move to the edge of the T zone and follicles where they interface with the intra-nodal lymphatics. In this site they make cognate interaction with B cells that have specifically bound and processed antigen 24, 25. Upon activation and differentiation leukocytes change their pattern of responsiveness towards chemokines, a mechanism leading to specific migration programs to new sets of chemokines 1, 12, 26.
Whereas the chemokine receptor expression and response profiles of naive and germinal center B cells are well described, little is known about these in plasmablasts. They leave the follicular and paracortical regions of the lymph node and localize in medullary cords. In the order of 107 plasmablasts emigrate from the lymph node between days 5 – 7 after antigen priming (27; Finke et al., submitted). Here we analyzed the expression of chemokine receptors in B cells isolated from follicular as well as extrafollicular regions using RNase protection assays. The expression was compared with the capacity of the different B cell populations to migrate ex vivo in response to various chemokines. Maturation in germinal centers did not lead to a detectable reduction of chemokine receptor expression (apart from a reduction in the weakly expressed CCR5). Conversely, extrafollicular syndecan-1high B cells lost expression of most chemokine receptors. In addition, the only remaining chemokine receptor, which is known to attract B cells to the lymph node – CXCR4 – did not induce migration. These data suggest that the loss of responsiveness of medullary plasmablasts to a range of chemokines may be necessary for the emigration of these cells from lymph nodes at the height of the extrafollicular response.
2 Results and discussion
2.1 Isolation of the differentiating B cell subsets during lymph node responses to MMTV or NP-CGG / alum
After s. c. injection of mouse mammary tumor virus (MMTV) or NP-chicken gammaglobulin (CGG) / alum, dendritic cells in the paracortex of the draining lymph node prime the T cell response 16, 28, 29. The interaction of B cells presenting antigen with primed T cells occurs in the outer T zone and the adjacent regions of the B cell follicles 24, 25. Activated B cells either migrate to follicles, where they form germinal centers and undergo affinity maturation 30, or to medullary cords where they differentiate to plasmablasts and then plasma cells. The medullary cord plasmablasts to not undergo Ig V-region gene mutation 24, 28. In response to NP-CGG / alum, germinal centers are already apparent by 5 days and reach peak size around day 8 and last for approximately a month. Critically, after MMTV injection germinal centers appear later, i. e. day 8 – 10 28, so that the peak of plasmablast growth in the medullary cords (see below) is reached well before germinal centers are seen. The germinal centers in the MMTV response are still present after 120 days, presumably reflecting the continued presence of infected B cells in this site 31.
Extrafolicular plasmablasts growing in medullary cords reach peak numbers between days 5 – 7 after immunization with either NP-CGG or MMTV 28. A proportion of the plasmablasts growing in the medullary cords secrete switched Ig isotypes; the switching process is triggered by the cognate interaction with primed T cells in the outer T zone 32. Many of the plasma cells generated in medullary cords die in situ, but in the order of 107 plasmablasts emigrate from the draining lymph node and settle in different lymphoid and non-lymphoid organs including the bone marrow (33, 34; Finke et al., submitted). This emigration peaks at day 7 and so precedes the formation of germinal centers. A second wave of plasmablast emigration from the nodes peaks during the third week after immunization, reflecting output from the germinal centers (Finke et al., submitted).
The differentiating B cell populations are characterized by specific surface markers 28, 34, 35. Germinal center B cells are stained strongly with PNA, are GL-7+ and mainly IgD–, and B220+. Extrafollicular plasmablasts are B220low, MHC class IIint and syndecan-1high.
The different B cell populations were isolated as follows using FACS sortring (Table 1 and Fig. 1): (1) Small IgD+ B cells were isolated from the lymph nodes of non-immunized mice as forward scatter (FSC)low, IgD+, B220+ GL-7– cells. (2) Follicular IgD+ B blasts were isolated as IgD+, B220+, GL-7+ cells from draining lymph nodes 12 days after NP-CGG / alum immunization. (3) Germinal center B cells were also isolated from lymph nodes 12 days after NP-CGG / alum immunization as IgD–, B220+, GL-7+ cells. (4) Syndecan-1int B cells were isolated from day 6 MMTV-infected lymph nodes as syndecan-1int, B220high, MHC class IIhigh cells. At this time few if any PNA+ cells are seen in follicles 28. This syndecan-1int population made up about one third to one tenth of the syndecan-1-expressing B cells (see Fig. 1). (5) Syndecan-1high plasmablasts and plasma cells were isolated from draining lymph nodes 6 days after infection with MMTV as FSChigh, syndecan-1high, B220low, MHC class IIint cells.
|
Type of cell |
Surface markers for sorting |
Purity after sorting |
|---|---|---|
|
Small IgD+ cells |
FSClow, IgD+, B220+, GL-7– |
> 99.5 % |
|
IgD+ blasts |
IgD+, B220+, GL-7+ |
> 98 % |
|
IgD– GL-7+ GC B cells |
IgD–, B220+, GL-7+ |
> 98 % |
|
Syndecanint B cells |
Syndecan-1low, B220high MHC class IIhigh |
> 97 % |
|
Syndecanhigh plasmablasts and plasma cells |
FSChigh, syndecan-1high, B220low MHC class IIint |
> 99 % |
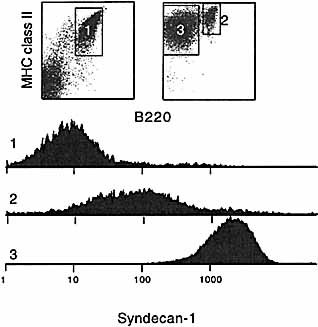
FACS profiles of syndecan-1-enriched B cells. FACS profile after MACS enrichment of syndecan-1+ cells. In the left dotplot the flow-through of the MACS column is shown representing syndecan-1– cells (1), on the right side the retained fraction using biotinylated anti-syndecan-1 antibody followed by streptavidin-labeled microbeads. The B220+, MHC class II+ syndecan-1int B cells represent 21 % of the enriched population (2), the syndecan-1high plasmablasts (B220low, class IIhigh) 78 % (3). The syndecan-1 expression levels of the three B cell populations are shown in histograms.
2.2 Localizing B cell subsets in the lymph node as they differentiate in response to MMTV
Fig. 2 shows a lymph node section on day 5 after injection of MMTV into the footpad, stained for syndecan-1 in blue, IgD in brown, and 5-bromo-2′-deoxyuridine (BrdU) in red. Small IgD+ B cells are found in the cortex, and plasmablasts are largely confined to the medullary cords where they are abundant 5 – 6 days after MMTV injection and quickly decrease in numbers on days 7 – 8. Practically no superantigen-reactive T cells are detectable in this site 28, 34. Some syndecan-1+ cells are also present in the follicles and the T zone (Fig. 2). The syndecan-1 staining on these cells appears weaker than on the medullary plasmablasts; they might belong to the syndecan-1int population identified by flow cytometry (Fig. 1, Table 1). GL-7+ B cells are found almost exclusively in germinal centers 36, 37, suggesting that the IgD+ GL-7+ blast population identified by flow cytometry is located in germinal centers.
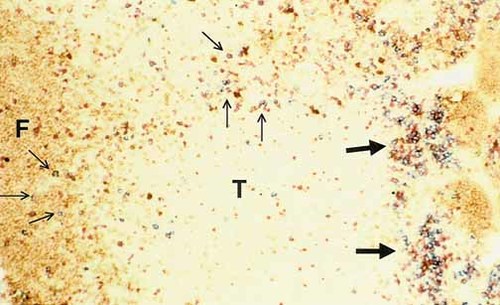
Overview of MMTV-primed lymph node 6 days after injection. Blue: syndecan-1, brown: IgD, red: BrdU. T: T zone, F: follicle. Bold arrows indicate the localization of the medullary cords with late plasma blasts. Scattered through the follicles and the paracortex are early activated B cells (light arrows).
2.3 Chemokine receptor expression during follicular and extrafollicular B cell differentiation
Chemokine receptor mRNA expression was quantified in freshly isolated B cell populations by RNase protection assays (Fig. 3 A – D). Small IgD+ B cells were found to express high levels of CXCR4 and CXCR5 mRNA as previously described 22, 38. CXCR4 mRNA remained high in all the five purified B cell populations. CXCR5 mRNA was high in all the cortical B cell populations – small IgD+ B cells, IgD+ B blasts, and GL7+, IgD– germinal center B cells. In contrast it had almost disappeared in both the syndecan-1int and syndecan-1high populations. We confirmed these observations by antibody staining and could show that CXCR4 expression on the cell surface was maintained in syndecan-1high plasmablasts localized in medullary cords (R. Förster, M. Lipp and H.A.-O., unpublished observations). CXCR5 has been shown to be responsible for follicular localization of naive B cells as well as activated B cells and subpopulations of activated T cells in the follicles 20, 22, 23, 39. There is evidence that BLC is produced by follicular dendritic cells. Nevertheless, in rats B cells have been shown to migrate to follicles deficient in follicular dendritic cells 40, 41. Naive B cells express high levels of CCR7 27, 38. CCR7 is constitutively expressed on recirculating B cells, memory B cells as well as their immediate bone marrow precursors, but is lost on a majority of cells after activation 42 – 44. CCR7 is down-regulated after B cell activation to form germinal centers 45, but a recent study showed maintenance of migration towards SLC 27.
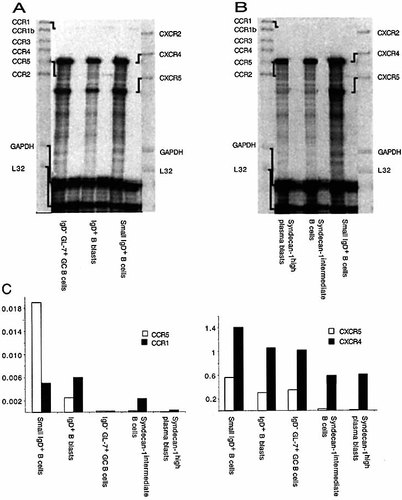
Chemokine receptor expression by RNase protection assay. (A) Follicular B cell differentiation after immunization with NP-CGG in alum, day 14. Naive follicular as well as IgD+ blasts and germinal center B cells express comparable levels of CXCR4 and CXCR5. (B) Extrafollicular B cell differentiation after MMTV(SW) injection, day 6. Before appearance of germinal centers extrafollicular plasmablasts appear in the medullary cords. The syndecan-1int B cell population is still localized to follicles and paracortex. (C) Quantitation of CCR expression in (A) and (B) using GAPDH or L32 as internal standards. (D) Quantitation of CXCR expression in (A) and (B) using GAPDH or L32 as internal standards.
Other CC chemokine receptors were expressed only very weakly (CCR1, CCR5), or were undetectable (CCR1b, CCR2, CCR3, CCR4) in naive and differentiated B cells (Fig. 3 A, B). Small IgD+ B cells, germinal center cells and IgD+ B blasts expressed low levels of mRNA for CCR1. Strong CCR5 mRNA expression was only found on small IgD+ follicular B cells; it was weakly expressed by the IgD+ GL7+ early germinal center B cells. We cannot exclude contaminating dendritic cells or other types of cells as a source for this mRNA although the high purity of our cells reduces this possibility.
2.4 Changes in chemotactic responses ex vivo associated with B cell maturation
The migratory behavior in vitro of naive, syndecan-1high and syndecan-1int B cells was compared. For this purpose the migration in vitro of freshly isolated syndecan-1+ cells towards various murine chemokines was measured in Boyden chambers (Fig. 4). The chemokines tested were regulated upon activation, normal T cell expressed and secreted (RANTES; binding to CCR1, CCR3, and CCR5), SDF-1 (binding to CXCR4), BLC (binding to CXCR5), liver and activation-regulated chemokine (LARC) (binding to CCR6) and SLC (binding to CCR7). Naive B cells migrated towards SDF-1, BLC and SLC as exprected from the RNase protection assays and previous publications 27. No significant migration was seen towards RANTES, but there was weak reproducible migration towards LARC. Similar responses to LARC were obtaind with human blood and tonsil B cell subsets (Legler, unpublished observation) as well as with naive mouse B cells 27. All these cell types express CCR6 mRNA and protein on the surface 27, 46. The migration of the syndecan-1int cells in response to these chemokines was comparble to those shown by naive B cells. Further differentiated high-level syndecan-1-expressing cells lost the migration towards ELC, SDF-1 and BLC, despite the continued expression of CXCR4 (Fig. 4 and for summary of expression and migration data see Fig. 5). It is likely that the reduced expression of CXCR5 mRNA in the intermediate extrafollicular population did not yet result in sufficient modulation of the receptor. This hypothesis can be addressed when antibodies become available. It has been observed previously that human tonsillar germinal center B cells still express CXCR4, but have lost migration capabilities towards SDF-1 38. Germinal center cells in mice have retained both expression and migration properties 27. This might represent differences between mice and humans or alternatively differences between chemokine receptor expression and responsiveness in chronically activated tonsils and primary immune responses in popliteal lymph nodes.
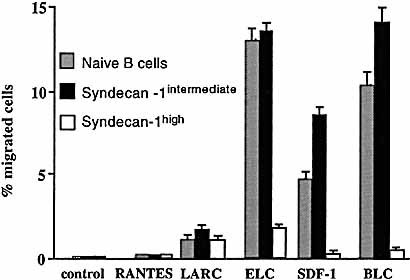
Migration of naive, syndecan-1int and extrafollicular B cells. The cells were sorted on day 6 after MMTV injection and analyzed in Boyden chambers with titrated amounts of the indicated chemokines. The results shown were the chemokine concentrations giving maximal migration (LARC 10– 6 M, ELC 10– 7 M, SDF-1 10– 8 M, BLC 10– 6 M, RANTES 10– 9 M). Grey bars naive B cells, black bars syndecan-1int, white bars syndecan-1high plasmablasts.
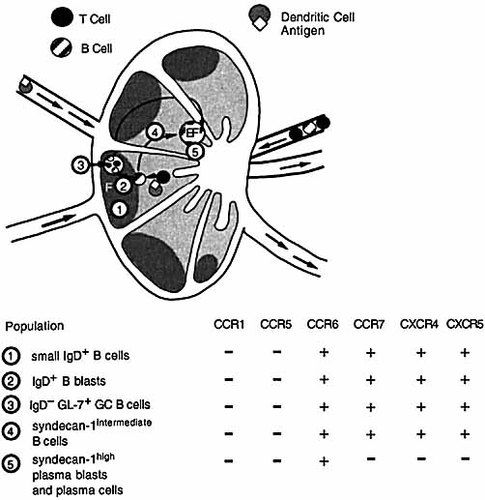
Model summarizing expression and migration data. In the upper part the localization of the different B cell populations is shown, in the lower part their chemokine expression and migration properties to the relevant chemokines. Naive small IgD+ B cells (1) become activated by antigen and primed T cells to become IgD+ blasts (2). They either further differentiate into IgD–, GL7+ germinal center B cells (3) or directly into syndecan-1int B cells (4) and syndecan-1high plasmablasts and plasma cells (5). Their chemokine receptor expression and responsiveness is summarized below the figure. F: follicle; EF: extrafollicular plasmablast.
Retained CXCR4 expression during maturation of syndecan-1+ plasmablasts, but loss of the capacity to migrate towards SDF-1 may allow emigration first from the T zone to the medulla and subsequently from the lymph node. CXCR4-SDF-1 interactions have been strongly implicated in bone marrow retention and migration of differentiating lymphocytes during bone marrow differentiation. In later stages of bone marrow differentiation lymphocytes respond to SLC / ELC, BLC and LARC 27. It will be interesting to see whether the retained CXCR4 will regain access to the signaling pathways that allow cells emigrating from the lymph node to be attracted to and / or retained in the bone marrow 9, 38, 47, 48. Preliminary results indicate that emigrating plasmablasts express the activated form of α4β1 integrin which might allow chemokine-independent immigration to target tissues via vascular cell adhesion molecule (VCAM)-1 (Finke et al., submitted). Alternatively other chemokine receptors not included in the study might be implicated in this migration.
The results presented in this report show the correlation between loss of chemokine responsiveness and / or chemokine receptor expression in plasmablasts when many of them leaving the lymph node. It is plausible that the loss of CXCR5, CXCR4 and CCR7 expression or responsiveness is a necessary prerequisite for cells to leave the chemokine gradient, which is continuously present within the lymph node. Integrin expression, the re-expression of chemokine receptors as well as the reactivation of CXCR4 might allow them to relocate to other target organs such as bone marrow.
3 Concluding remarks
The results presented in this report show that there is a correlation between loss of chemokine responsiveness and emigration of plasma blasts from the lymph node. This responsiveness does not always correlate with surface chemokine receptor expression since CXCR4 expression on the surface is maintained in these cells despite the loss of responsiveness to SDF-1. It is plausible that the loss of CXCR5, CXCR4 and CCR7 expression or responsiveness is a necessary prerequisite for cells to leave the chemokine gradient, which is continuously present within the lymph node. Integrin expression, the re-expression of chemokine receptors, expression of unknown chemokine receptors as well as the reactivation of CXCR4 might allow them to relocate to other target organs such as bone marrow.
4 Materials and methods
4.1 Mice
Female BALB / c mice were obtained from Harlan / Olac Ltd (Bicester, GB) and were used at 6 – 8 weeks of age.
4.2 Antibodies
The following antibodies were used: biotinylated syndecan-1 and anti-IgD, PE-labeled anti-MHC class II, FITC-labeled GL-7 (Pharmingen, San Diego, CA), FITC- or PE-labeled anti-B220, and streptavidin-CyChrome (TAGO, Burlingame, CA). Sheep anti-IgD (Binding-site Ltd, Birmingham, GB), mouse anti-BrdU (Dako Ltd, High Wycombe, GB) were used as purified IgG. In immunohistology sheep anti-mouse IgD was detected with peroxidase-labeled donkey anti-sheep Ig (Binding Site Ltd); mouse anti-BrdU was detected with goat anti-mouse IgG (Dako Ltd). Biotinylated anti-syndecan-1 was detected with StreptABCcomplex alkaline phosphatase (Binding-site Ltd.).
4.3 Chemokines
The murine chemokines RANTES, LARC, SDF-1, SLC and BLC were from R & D systems.
4.4 Immunohistology
Immunohistological reagents and staining was done exactly as described in 28. Briefly, lymph nodes from mice injected with MMTV into the footpad 5 days earlier were frozen in liquid nitrogen. The thymidine analogoue BrdU was administered at 2 mg / mouse in saline i. p. 2 h before the mice were killed. Frozen lymph node sections were prepared and stained with syndecan-1 antibodies followed by alkaline phosphatase-coupled secondary antibody and FastBlue in blue, for IgD with anti-IgD antiserum and peroxidase-coupled secondary antiserum and DAB in brown, and for BrdU with alkaline phosphatase-coupled secondary antibody and FastRed in red.
4.5 Purification of B cell subpopulations
Extrafollicular syndecan-1high plasmablasts and syndecan-1int B cells were enriched by MACS using anti-syndecan-1 biotin labeling followed by incubation with streptavidin MACS beads according to the manufacturer's descriptions. Similarly, follicular B cells were enriched by GL-7FITC labeling followed by anti-FITC beads (Miltenyi Biotech, Bergisch Gladbach, Germany). Thereafter the cells were sorted to > 99 % purity by FACS sorting using the markers described in Table 1. Dendritic cells and macrophages were excluded using anti-CD11c and Mac-1 antibodies (Pharmingen).
4.6 RNase protection
RNase protection was performed using the RiboQuant multi-probe RPA system with the mouse chemokine / chemokine receptor multi-probe template sets mCR-5 and mCR-6 following the manufacturer's description (Pharmingen). RNA isolated from 8 × 106 – 10 × 106 cells was used per lane.
4.7 Migration assay
Cell migration was assessed in 48-well Boyden chambers (Neuro Probe, Cabin John, MD) using collagen IV-precoated polyvinylpyrrolidone-free polycarbonate membranes (Nucleopore) with 3-μm pores as described 23 with minor adaptions for murine cells. Briefly, FACS-sorted cells were incubated for 1 h at 37 °C prior to the chemotaxis assay. DMEM supplemented with 10 mM Hepes pH 7.4, 0.05 mM 2-ME and 1 % BSA was used to dissolve the chemokines and to suspend the cells (105 cells / upper well). Following incubation for 120 min at 37 °C under CO2-buffered conditions, migrated cells in triplicate wells were counted and expressed as percent of the total. Optimal chemokine concentrations for migration were: LARC 10– 6 M, ELC 10– 7 M, SDF-1 10– 8 M, BLC 10– 6 M, RANTES 10– 9 M.
Acknowledgements
We would like to thank P. Zaech for cell sorting. D.F.L. and H.A.O. are supported by the Giorgi-Cavalieri Foundation. HAO (grants no. 31 – 32271.94 and 31 – 59165.99) as well as P.L. and M.B. (31 – 55997.98) are supported by the Swiss National Science Foundation. K.M.T. and I.C.M.M. are supported by a British Medical Research Council programme grant.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




