Constitutive intracellular expression and activation-induced cell surface up-regulation of CD44v3 in human T lymphocytes
Abstract
The cell adhesion molecule CD44 exists in multiple isoforms generated by alternative RNA splicing. Increased expression of CD44 isoforms containing exon v6 and v9 has been reported to be associated with the activated state of T lymphocytes. Using monoclonal antibodies against variant exon products we studied the expression of another variant exon, v3 on resting and in vitro activated human peripheral blood T cells. We found that CD44v3, in parallel with CD44v6, is up-regulated at the surface of normal T cells stimulated by anti-CD3 antibody or by the phorbol ester PMA, as well as on PMA-stimulated T cell leukemia lines CCRF-CEM and MOLT-4. Beside the cell surface, we demonstrated CD44v3 intracellularly in both resting and activated T cells by flow cytometry and immunomorphology. Reverse transcription-PCR and Western blot analyses confirmed the constitutive expression of CD44v3 in these cells. The increase in the cell surface expression of CD44v3 on stimulated T lymphocytes was inhibited by cycloheximide and brefeldin A, indicating the requirement of de novo protein synthesis and endoplasmic reticulum Golgi transport. Our studies establish CD44v3 as an additional activation marker for human T cells, with a yet unidentified function.
Abbreviations:
-
- CD44v:
-
Variant CD44 isoforms
-
- HB:
-
Heparin binding
1 Introduction
CD44 comprises a widely expressed family of cell surface glycoproteins/proteoglycans involved in cell-matrix and cell-cell interactions, and participating in biological processes such as lymphocyte homing and activation, hematopoiesis, cell migration and tumor progression. The apparent functional diversity of CD44 molecules probably arises from their structural heterogeneity, generated by the alternative splicing of at least ten exons (termed v1-v10) in mice and nine exons (v2-v10) in humans, encoding membrane-proximal domains of the extracellular region 1, 2. Variations in the use of N-linked and O-linked glycosylation sites and glycosaminoglycan (heparan sulfate, chondroitin sulfate, keratan sulfate) assembly sites contribute to structural and functional diversity. The v3 exon product of the CD44 molecule is special in the sense that it is the only exon that has a heparan sulfate assembly site 3–5. V3 exon containing variants carrying heparan sulfate side chains are able to bind and present heparin-binding (HB) growth factors and cytokines, like basic fibroblast growth factor (bFGF), HB-epidermal growth factor (EGF), macrophage inflammatory protein (MIP)-1β and hepatocyte growth factor/scatter factor (HGF/SF), which may have various functional consequences 4, 6, 7.
The 85–95-kDa "standard" or "hematopoietic" form (CD44s/CD44H), which is the principal isoform in hematopoietic cells and lymphocytes, is widely and abundantly expressed. Variant isoforms (CD44v) containing different combinations of alternatively spliced exons are more restricted in their tissue distribution, predominantly expressed in cells and tumors of epithelial origin and subpopulations of stimulated leukocytes.
CD44H has been postulated to act as the principal cell surface receptor for hyaluronan, but it also mediates binding to other extracellular matrix (ECM) components and non-ECM ligands as well (see 8 for review, and 9–12). Besides its role as an adhesion receptor, CD44 also functions as an important signaling molecule in immune reactions, inducing activation of T, B and NK cells, monocytes and dendritic cells 13–20. In T lymphocytes CD44 generally serves as a co-stimulatory molecule, promoting the CD3- or CD2-mediated induction of proliferation and activation 13, 14, 16. The CD44-mediated signaling has been shown to involve a tyrosine kinase-dependent pathway 21, 22.
Many data point to the involvement of variant CD44 molecules in tumor progression, in experimental models as well as in several human tumors, such as lymphomas, colorectal, gastric, renal and cervical carcinomas, and melanomas (see 23, 24 for reviews, and 25–32).
Normal hematopoietic cells and tissues preferentially express the standard type of CD44, while variant isoforms show a highly restricted expression pattern 23, 26, 33. However, during the process of in vitro or in vivo activation, several cell types of the immune system have been reported to express various CD44 epitopes encoded by variant exons. Transient expression of CD44v6 was reported on rat T cells, B cells and macrophages after in vivo antigenic stimulation, and was shown to be important for lymphocyte activation 34. In vitro activation of human T lymphocytes by mitogen or antigen led to transient up-regulation of CD44 domains encoded by exons v6 and v9 26, 35, which were involved in hyaluronate adhesion and in proliferation 36. Activation of B cells induced CD44 isoforms containing the exon products v6, or v6/v7, v8–v10, and v10 27, 37. Monocyte differentiation was accompanied by elevated expression of v6- and v9-containing isoforms 38, while the maturation and activation of dendritic cells up-regulated several CD44 variants (v3, v4, v5, v6 and v9) 20, 39, 40, which played an essential role in their function 40.
Previous analyses of the alterations in CD44 variant isoform expression during activation of normal lymphocytes have focused on isoforms containing certain exon products, mainly v6 and v9 23, 26, 34, 35. Motivated by its unique structural features (described above) enabling the influencing of lymphocyte proliferation and function via the presentation of cytokines, as well as by its association with tissue infiltrating and malignant lymphocytes, we became interested in studying the expression of CD44v3 on resting and in vitro activated T lymphocytes. We provide evidence for the constitutive expression of v3 exon-containing variant(s) in human peripheral blood T lymphocytes and in T cell lines, and for their transient up-regulation at the surface of activated T cells.
2 Results
2.1 Cell surface expression of CD44v3 and CD44v6 on activated human peripheral blood T lymphocytes and T cell lines
Plastic-nonadherent, nylon wool-enriched human pe-ripheral blood lymphocytes were assessed for cell surface expression of the standard CD44 form (CD44H), as well as v3- and v6-containing isoforms by flow cytometry. Cells were counterstained with anti-CD3 antibody to identify T cells. Anti-CD25 (IL-2 receptor) antibody was included as positive control for monitoring T cell activation, and was consistently found up-regulated (Fig. 1).
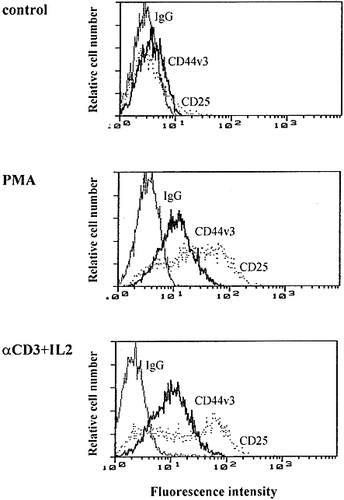
Flow cytometric analysis of the induction of cell surface CD44v3 expression following 24-h PMA or anti-CD3 + IL-2 stimulation of T lymphocytes, gated for CD3 expression. Cells were stained with mAb against CD44v3, CD25 (positive control of activation), and with isotype control Ig. Data are representative of six independent experiments.
As it has been reported by several authors 23, 26, 34, 35, unstimulated T lymphocytes expressed CD44H at the cell surface (not shown), while the expression of the variant isoforms was below the limit detectable by flow cytometry or immunofluorescence analysis (Fig. 1, 2A, and 3A). In vitro activation either with anti-CD3 antibody and IL-2, or with the phorbol ester PMA led to the up-regulation of CD44H (not shown), as well as CD44v3- and CD44v6-encoded epitopes (Fig. 1, 2A, and 3B). Confocal microscopy showed that CD44v3 appeared as random receptor aggregates at the surface of CD3+ cells (Fig. 3C, D). The intensity of staining for CD44v3 at the surface of T lymphocytes 24 h after activation was in a range similar to the expression level of CD44v6 (Fig. 2A), as well as the percentage of positive cells (∼40-70% after PMA activation, and ∼ 20-50% after OKT3 + IL-2).
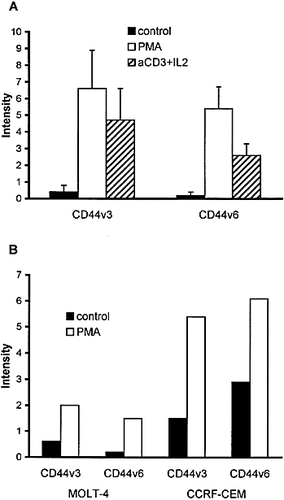
Flow cytometric analysis of cell surface expression of CD44v3 and CD44v6 epitopes on resting and 24-h PMA- or anti-CD3 + IL-2-stimulated human T lymphocytes (A), and unstimulated and 24-h PMA-stimulated T cell leukemia cell lines (B). Results show mean fluorescence intensity of cells stained with anti-CD44v antibodies (isotype controls subtracted). (A) Values represent mean ± SD derived from five experiments using lymphocytes from different donors. (B) Values derived from a representative experiment of two independent experiments.
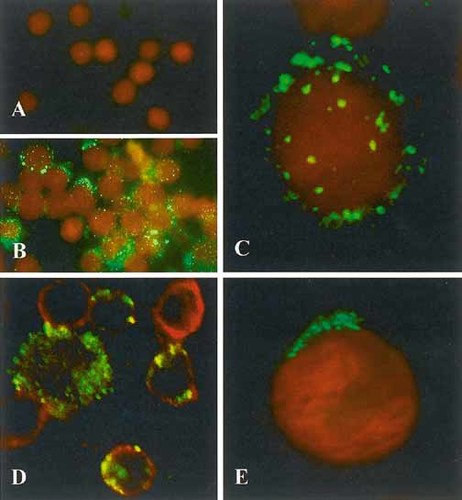
Immunofluorescence localization of CD44v3 in human T cells. (A) Surface labeling of resting peripheral blood T cells for CD44v3. Note the lack of specific staining (green). Red: nuclear staining (propidium iodide); ×700. (B) Surface labeling of PMA-stimulated peripheral blood T cells for CD44v3. The majority of the cells show intense cell surface green fluorescent staining; ×700. (C) Confocal microscopy of PMA-stimulated T lymphocyte following surface labeling for CD44v3. Note the randomly distributed surface fluorescent patches corresponding to CD44v3 epitopes; ×2,800. (D) Confocal microscopy of PMA-stimulated T cells labeled for CD44v3 and CD3. Cells were fixed in PFA before immunostaining. Merged image of green (CD44v3) and red fluorescence (CD3). Note the double-labeled cells, indicating that CD44v3 is present on T cells; ×1,500. (E) Intracellular labeling of a CCRF-CEM cell for CD44v3 following fixation and membrane permeabilization. Confocal microscopy. Note the polarized perinuclear localization of CD44v3-containing tubules and vesicles. Composite image of nuclear staining (red fluorescence) and CD44v3 (green fluorescence); ×2,800.
The effect of PMA was also studied on the human T cell leukemia lines MOLT-4 and CCRF-CEM. Both splice variants were absent from the surface of unstimulated MOLT-4 cells, while low level of expression was detected on CCRF-CEM cells. PMA treatment resulted in an enhanced CD44v3 and v6 expression on both cell lines (Fig. 2B).
The increased expression of CD44v3 by in vitro activated T cells was transient, showing a time kinetics similar to that reported for CD44v6 26, peaking at 24–48 h (Fig. 4). Similar results were obtained in the case of MOLT-4 and CCRF-CEM lines (data not shown).
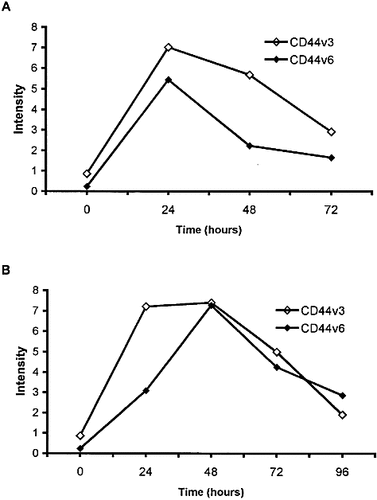
Kinetics of CD44v3 and CD44v6 expression following PMA (A) or anti-CD3 + IL-2 (B) stimulation of human T lymphocytes. Cells were stained and analyzed as in Fig. 2. Data are representative of two independent experiments.
Using CD4- and CD8-specific antibodies to identify T cell subpopulations, we demonstrated that the increase in the overall expression of CD44v3 on T lymphocytes resulted predominantly from an up-regulation of this variant on the CD4+ subset (Fig. 5).
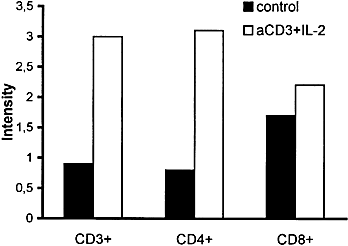
Cell surface expression of CD44v3 on resting and activated T cells and T cell subsets. Unstimulated and anti-CD3 + IL-2 stimulated T cells, gated for CD3, CD4, or CD8 expression, were stained and analyzed as in Fig. 2. Data are representative of three independent experiments.
2.2 Constitutive intracellular expression of CD44v3 in T lymphocytes and T cell lines
To study the intracellular expression of the v3 and v6 exon-encoded epitopes in peripheral blood T cells and T cell subsets, we performed the immune reactions on formaldehyde-fixed and permeabilized cells. Resting T lymphocytes showed intensive intracellular labeling for CD44v3, while they were negative for CD44v6 (Fig. 6A). The intracellular pool of CD44v3 was confined to a tubulovesicular structure in a perinuclear location using confocal microscopy and optical sectioning (Fig. 3E). The analysis of the T cell lines CCRF-CEM and MOLT-4 revealed similar, robust intracellular staining for CD44v3 (Fig. 6B). These results indicate the constitutive expression of v3 exon-containing CD44 variant(s). This was supported by the reverse transcription (RT)-PCR analysis of the two cell lines, showing constitutive mRNA expression of the CD44v3 exon in these cells (Fig. 7A), which was confirmed by the sequence analysis of the PCR product. Beside the ∼ 346-bp major PCR product present in both cell lines (directly spliced v3 exon), a larger product was also detected in CCRF-CEM cells, presumably representing v2-v3 (∼ 475 bp). Western blot analysis of MOLT-4 and CCRF-CEM cell lysates demonstrated a major ∼ 120-kDa and a weaker ∼ 180-kDa band (Fig. 7B).
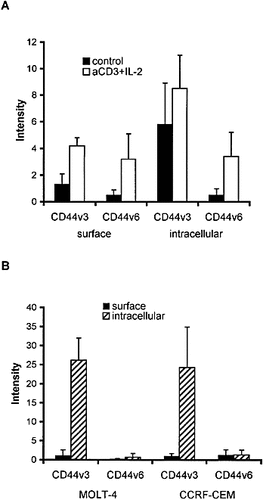
Cell surface vs. intracellular expression of CD44v3 and CD44v6 in resting and anti-CD3 + IL-2 activated T lymphocytes (A) and in T cell lines (B). Unfixed or formaldehyde-fixed and permeabilized cells were labeled and analyzed as in Fig. 2. Values represent mean ± SD derived from three experiments.
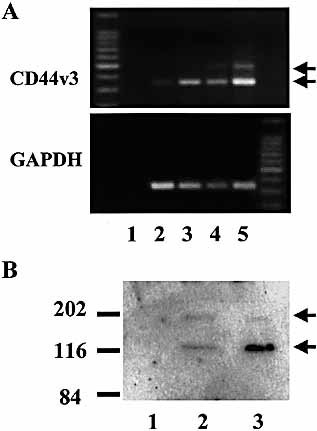
Detection of CD44v3 expression in MOLT-4 and CCRF-CEM cells by RT-PCR (A) and Western blot (B). (A) cDNA samples were amplified with CD44v3-specific (upper panel), or GAPDH-specific primers (control, lower panel). Lane 1, negative control; lane 2, untreated MOLT-4; lane 3, PMA-stimulated MOLT-4; lane 4, untreated CCRF-CEM; lane 5, PMA-stimulated CCRF-CEM. (B) Cell lysates were fractionated on 8% SDS-PAGE, electroblotted, and probed with anti-CD44v3 antibody. Lane 1, negative control; lane 2, MOLT-4; lane 3, CCRF-CEM.
At the same time, in another time course experiment employing shorter incubation periods, we found that treatment of T cells for 15 min with PMA, a direct activator of protein kinase C, did not induce expression of either CD44 variants studied (not shown). This suggested that de novo protein synthesis may be required for cell surface expression of these variants. To support this assumption, we performed 6-h PMA treatment in the presence of the protein synthesis inhibitor cycloheximide. Fig. 8A shows that the induction of cell surface expression of CD44v3 was blocked by the addition of cycloheximide. The same effect was observed on the up-regulation of CD44v6, while the cell surface expression of the constitutively expressed CD44H was not influenced (not shown). Treatment of T cells with brefeldin A, an agent that has been described as a blocker of protein translocation from the endoplasmic reticulum to Golgi 41, 42, also inhibited PMA-induced cell surface up-regulation of CD44v3 (Fig. 8B).
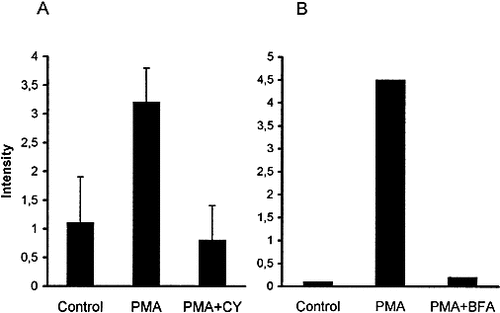
Effect of cycloheximide (A) and brefeldin A (B) on the induction of CD44v3 by PMA treatment of T cells. Cells were treated with PMA for 6 h (A) or 15 h (B) in the absence or presence of cycloheximide (CY) (A) or brefeldin A (BFA) (B), then stained and analyzed as in Fig. 2. Values represent mean ± SD derived from four experiments (A) or are representative of two independent experiments (B).
3 Discussion
Since CD44 isoforms containing variable exon 6 have been directly associated with the lymphatic spread of rat tumor cells 25, CD44v6 gained special interest, prompting investigators to study the role of this variant exon in metastasis formation, as well as in T lymphocyte activation 23, 26, 34. However, the potential involvement of other CD44 variants in these processes should not be overlooked. Indeed, expression of variant exons other than v6 has been described to be important in the malignant progression of different tumor types 23, 24, 31, 32. Also, reactive skin-infiltrating lymphocytes and cutaneous lymphomas were characterized by the absence of CD44v6, and expression of CD44v10 and CD44v3 43, while autoimmune skin infiltrates ex-pressed predominantly CD44v3 44.
In this report we have shown that, beside CD44v6, CD44v3 is also transiently up-regulated at the surface of in vitro activated human peripheral blood T lymphocytes, following a time course similar to that of CD44v6 expression. At the same time, flow cytometric analysis on permealized cells showed the constitutive presence of CD44v3, but not that of CD44v6, in T lymphocytes and T cell lines. Immunocytochemistry and confocal microscopy of the same cells identified a large cytoplasmic tubulovesicular pool of CD44v3. This pool is most probably associated to endoplasmic reticulum-cis Golgi, since a specific inhibitor of this transport, brefeldin A, was able to prevent cell surface up-regulation upon PMA stimulation.
RT-PCR analysis using exon-specific primers supported the presence of CD44v3 in CCRF-CEM and MOLT-4 lines. In accordance with these results, Stauder et al. 30 demonstrated constitutive mRNA expression of v3, v6 and v10 exons by nonstimulated cloned T cells, with an increase in expression and the appearance of larger isoforms containing different exon combinations after PHA stimulation. These findings, however, were not confirmed at protein level. Other studies on murine and human lymphocytes described very low or undetectable amounts of CD44 variant glycoproteins on resting lymphoid cells 23, 26, 34, 35; however, these studies uniformly utilized cell surface labeling.
According to our RT-PCR studies, a smaller portion of the v3 exon-containing CD44 variant(s) in CCRF-CEM cells probably contained the v2 exon as well, while it was not detected in MOLT-4 cells. We have not examined in detail which downstream exon combination(s) are present in these variants. Our preliminary experiments using v3-specific downstream primer and 3prime; constant or v6-specific primers indicated that (i) most isoforms contain v3 exon alone, and (ii) variants where v3 and v6 exons are contained in the same isoform are also present, most of which probably carrying v4 and v5 as well (data not shown). These results are essentially in agreement with earlier findings on a T cell clone and lymph nodes 30. The results of Western blot analysis of MOLT-4 and CCRF-CEM cells also suggested the presence of more than one v3 exon-containing isoforms, although post-translational modifications may also contribute to the diversity at protein level.
The enhanced expression of CD44v3 following stimulation is probably not restricted to T cells, but rather seems to be a general feature of leukocytes. In our preliminary experiments using unseparated PBMC we found that, beside T cells, CD19+ B lymphocytes as well as monocytes also up-regulated CD44v3 expression after stimulation (data not shown). Similarly, CD44v3 expression was previously noted on T cells, B cells and monocytes in autoimmune diseases 44, 45, and on a fraction of normal peripheral blood monocytes 46, while it was reported to be up-regulated on activated monocytes 47 and monocyte-derived dendritic cells 20.
The presence of CD44v3 on multiple types of activated immune cells suggests its potential biological significance. It has repeatedly been proposed that the insertion of variant exons into the CD44 molecule may alter its adhesion to known CD44 ligands, or create new binding specificities 26, 36; in fact, selective binding to certain variant isoforms has been described in the case of osteopontin 48 and glycosaminoglycans 49. This could provide a structural basis for the supposed role of splice variants in tissue-selective lymphocyte homing, indicated by the expression of CD44v3 and v10 in skin-associated lymphocytes 43, 44, and by the bone marrow localization of i.v. injected lymphoma cells transfected with v3-containing variants 50.
Another possible function of v3 exon containing CD44 variants is to bind and present HB growth factors via heparan sulfate side chains. Bennett and colleagues 4, 5 have demonstrated that the v3 exon encodes an SGSG consensus motif that can serve as a heparan sulfate acceptor site, and, consequently, confers the capacity to bind a number of HB growth factors, chemokines and cytokines 4, 5. It needs to be pointed out that, beside the well-known HB growth factors such as bFGF, HB-EGF or HGF, a significant number of other cytokines have been shown to bind heparin and heparan sulfate proteoglycans, including most interleukins 51–53, which are involved in the regulation of growth and functional activity of immune cells. In fact, heparan sulfate-associated IL-2 has been reported to regulate T cell proliferation and apoptosis 54. Taken together, by these mechanisms CD44v3 expressed by lymphocytes and leukocytes may play a role in providing an appropriate cytokine environment for these cells at sites of infiltration, and, thus, may be an important factor of the regulation of immune response.
4 Materials and methods
4.1 Cell separation and culture
Mononuclear cells were separated from peripheral blood of healthy volunteers using Ficoll density gradient centrifugation. Cells were washed in PBS, followed by the removal of adherent cells by incubation in 10% FCS-RPMI 1640 at 37°C in 6-well plastic plates, and T cells were enriched using nylon wool column. The T cell leukemia lines MOLT-4, CCRF-CEM and Jurkat were cultured in RPMI 1640 supplemented with 10% FCS and gentamicin.
For in vitro activation peripheral blood T cells were cultured in 10% FCS-RPMI 1640 either with PMA (10 ng/ml), or in 24-well plates pretreated with OKT3 supernatant (1:400 dilution for 3 h at 37°C, followed by washing three times in PBS), in the presence of IL-2 (100 U/ml; DuPont Med. Prod., Wilmington, DE). CCRF-CEM and MOLT-4 cells were stimulated with PMA (10 ng/ml) for 24 h.
In some experiments T cell stimulation by PMA was carried out in the presence of the protein synthesis inhibitor cycloheximide, or the endoplasmic reticulum to Golgi transport inhibitor brefeldin A 41, 42 (Sigma, St. Louis, MO) at a concentration of 10 μg/ml.
4.2 Antibodies and immunofluorescence
For the detection of cell surface antigen expression, immunofluorescence labeling was performed on unfixed cells at 4°C, or after fixation with 1% buffered paraformaldehyde (PFA). For intracellular antigen detection cells were fixed with PFA for 15 min, followed by permeabilization with 1% saponin for 30 min, and processed at room temperature. Nonspecific antibody binding was blocked using PBS containing 1% BSA. Cells were incubated for 60 min with 10 μg/ml of the primary antibody. Monoclonal anti-CD25 was purchased from DAKO (Glostrup, Denmark); anti-CD44H, anti-CD44v3 and anti-CD44v6 were all from R&D Systems (Abingdon, GB). Murine IgG2b, IgG2a and IgG1 (Sigma) were applied as isotype controls. Cells were washed three times in PBS, then incubated for 60 min with FITC-conjugated goat anti-mouse IgG (1:70; Amersham, Little Chalfont, GB), followed by washing three times in PBS. To identify T lymphocytes or T cell subsets, cells were counterstained with PE-conjugated monoclonal anti-CD3 or anti-CD4 antibodies (Immunotech, Marseille, France) (control: PE-conjugated IgG1, Becton Dickinson, Sunnyvale, CA), or with anti-CD8-Quantum Red (Sigma) for 30 min. Fluorescence was measured by a FACStar flow cytometer (Becton Dickinson).
Surface and cytoplasmic anti-CD44v3-labeled lymphocytes have been also viewed by NIKON Eclipse-E600 epifluorescence microscope (Optoteam, Vienna, Austria) equipped with Spot Junior CCD camera and image analyzing software (Diagnostic Instruments Inc., Sterling Heights, MI). Certain samples were also analyzed by confocal microscopy using LaserSharp software (MRC-1024, Bio-Rad Laboratories GmbH, Munich, Germany). In these cases anti-CD44v3-labeled cells were stained with biotinylated anti-mouse IgG, followed by incubation with streptavidin-FITC (both purchased from Vector Laboratories Inc., Burlingame, CA). Nuclear staining has been performed using propidium iodide (2 μg/ml, 1 min). In the case of double staining, samples were post-labeled with anti-CD3 antibody, followed by incubation with biotinylated anti-mouse IgG and streptavidin-Texas Red (Amersham).
4.3 Western blot analysis
Cells were solubilized in gel sample buffer (5×106/ml), boiled for 5 min, and aliquots of 2.5×105 cell equivalent were separated on 8% SDS-polyacrylamide gels (Mini-Protean II Cell, Bio-Rad Laboratories). Proteins were electrotransferred to PVDF membranes at 200 mA for 130 min, using Mini-Trans-Blot Cell (Bio-Rad Laboratories). Blots were treated with 2% H2O2 in 0.1 M Tris pH 7.5/20% methanol for 30 min, then blocked in 10 mM Tris buffer pH 7.9, containing 150 mM NaCl, 0.1% Tween 20, 5% nonfat dried milk, and 2% BSA, for 1 h. Primary antibodies (anti-CD44v3 or isotype control Ig) were applied overnight at 4°C at a concentration of 1 μg/ml diluted in the same blocking buffer. The blots were then incubated in biotinylated anti-mouse IgG (1:1,500, 1 h), followed by incubation with streptavidin-horseradish peroxidase (1:3,000, 1 h) (both from Vector Laboratories). The membranes were washed four times in TBS after each incubation step. Lysate of equal amount of Jurkat cells served as negative control.
4.4 RNA isolation and RT-PCR analysis
Total RNA was isolated from 107 cells using RNAzolTMB (TEL-TEST, Friendswood, TX), according to the manufacturer's instructions. Of the total RNA 1 μg was used for cDNA synthesis with 0.1 μg oligo(dT)12–18 (Gibco BRL, Life Technologies, Paisley, Scotland), 1 mM dNTP (Gibco BRL) and 50 U MMLV reverse transcriptase (Promega, Madison, WI) for 30 min at 37°C in a total volume of 20 μl; 2.5 μl of each cDNA products were amplified using CD44 standard region 5´ (AGT CAC AGA CCT GCC CAA TGC CTT T) and exon v3 3´ primer (GGT GTC TGT CTC TTT CAT CTT CAT TTT CTT CAT TT) 55, or 5´ (TTC ACC ACC ATG GAG AAG GCT) and 3´ (ACA GCC TTG GCA GCA CCA GT) primers for GAPDH. PCR amplifications were performed in 25-μl reactions, containing 1.5 mM MgCl2, 0.25 mM dNTP (Gibco BRL), 1 U Dupl-A-Taq Taq polymerase (Zenon Biotechological Ltd., Szeged, Hungary), and 1 μM of the forward and the corresponding reverse primers. Reactions were performed in a Crocodile III thermocycler (Appligene/Oncor, Illkirch, France) for 35 cycles (94°C for 30 s, 65°C for 30 s, 72°C for 120 s), with final extension at 72°C for 10 min. For negative controls, reactions were performed in the absence of RNA (to exclude contamination in reaction mixture) or in the absence of reverse transcriptase (to exclude potential cDNA contamination of the RNA preparations). For sequence analysis, PCR products amplified with CD44 primers were pretreated with shrimp alkaline phosphatase/exonuclease I (Amersham) according to the manufacturer's instructions. Fluorescent labeling of the pretreated products was carried out using Big DyeTM Terminator Cycle Sequencing Kit (Perkin-Elmer Biosystems, Foster City, CA). The samples were purified by isopropanol precipitation and run on ABI PRISM 310 Genetic Analyzer (Perkin-Elmer Biosystems).
Acknowledgements
The authors thank Dr. J. Papp for help with DNA sequencing, Dr. K. Nagy for cell lines, and Dr. B. Sarkadi for reagents. This work was supported by the Hungarian Ministry of Health (ETT 082/98 and 444/2000) and by the National Scientific Research Fund (OTKA F026255).
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




