Induction of neonatal lupus in pups of mice immunized with synthetic peptides derived from amino acid sequences of the serotoninergic 5-HT4 receptor
Abstract
We have previously suggested that the recognition of a cross-reactive epitope on the 5-HT4 receptor and the 52-kDa SSA / Ro protein by serotonin-antagonizing autoantibodies could explain the electrophysiological symptoms of congenital heart block in neonatal lupus. To confirm this hypothesis, we immunized female mice with four synthetic peptides corresponding to the recognized epitopes. All mice developed anti-peptide antibodies, which cross-reacted with the Ro52 and 5-HT4 receptor peptides and recognized both cognate proteins. Peptide-immune mice were mated. The pups from mice immunized with the Ro52 peptides had no symptoms of neonatal lupus apart from bradycardia. However, pups from mice immunized with the 5-HT4 receptor peptides and bradycardia, atrioventricular block of type I or II, longer QT intervals, skin rashes and neuromotoric problems. The 5-HT4 receptor was detectable in the different fetal tissues affected (heart, skin and brain) by immunohistochemistry. Hearts from diseased pups were less developed and showed disorganized myocardial hyperplasia, compared to the normal littermates. These results demonstrate that the serotoninergic 5-HT4 receptor is the antigenic target of physiopathological autoantibodies in neonatal lupus.
1 Introduction
The 48-kDa SSB / La (La), the 52-kDa SSA / Ro (Ro52) and the 60-kDa SSA / Ro (Ro60) ribonucleoproteins are antigenic targets strongly associated with the autoimmune response in mothers wholse children have neonatal lupus and isolated congenital heart block (CHB) 1, 2. The atrioventricular block can be of various degrees 1, 3 and has been ascribed to the effect of the autoantibodies passively transferred across the placenta, which presumably injure the heart of the developing fetus. Despite the presence of circulating autoantibodies, the maternal hearts are never affected. We have previously shown that there is a cross-reactive B cell epitope between the Ro52 protein and the human 5-HT4 serotoninergic receptor, and that cross-reactive autoantibodies have an inhibitory effect on serotonin activation by the receptor 4. In order to prove the pathogenic role of these autoantibodies in the development of CHB, we immunized female BALB / c mice with synthetic peptides encompassing the cross-reactive epitope (Table 1). Peptide-immune mice were mated in order to study the offspring for electrocardiographic abnormalities as has been previously shown in pups from mice immunized with the Ro52 protein 5 or mice that had received IgG fractions from mothers whose children had neonatal lupus 6.
|
Peptide derived from the human serotoninergic 5-HT4-receptor |
|
|---|---|
|
Human G21V (165 – 185) 7 |
G I I D L I E K R K F N Q N S N S T Y C V |
|
C 15 Q (164 – 177) |
(C) I G I I D L I E K R K F N Q |
|
Mouse (165 – 185) 14 |
G I V D V I E K R K F S H N S N S T W C V |
|
Peptides derived from Ro52 |
|
|
R18L (365 – 382) 15 |
R K G H F L L S S K S G F W T I W L |
|
C15T (366 – 379) |
(C) K G H F L L S S K S G F W T |
|
Mouse (369 – 386) 11 |
R K G Q F S L S P E N G F W T |
- a) Bold underlined amino acids are the same in the two proteins, bold amino acids are similar. For comparative reasons the regions corresponding to the mouse 5-HT4 receptor and the mouse52-kDa protein are added.
2 Results
2.1 Immunochemical analysis of anti-peptide antibodies
All mice immunized with the four peptides G21V, C15Q, R18L and C15T (Table 1) developed anti-peptide anti-bodies. Peptides G21V and C15Q derived from the 5-HT4 receptor induced higher titers (1 / 500 – 1 / 16,000) than those elicited by the Ro52-derived peptides R18L and C15T (1 / 10 – 1 / 3,200). The antibodies were predominantly of the IgG1 isotype. Cross-reactivity was demonstrable among the different peptides, with the exception of the anti-R18L antibodies which did not cross-react with the C15Q 5-HT4 receptor-derived peptide. Anti-peptide antibodies were tested for receptor recognition by immunoblotting on membrane proteins from CHO cells transfected with the 5-HT4 receptor 7. A 60-kDa protein corresponding to the glycosylated form of the receptor was recognized by the anti-peptide sera (Fig. 1 A). Antibodies raised against the G21V, C15Q, R18L and C15T peptides cross-reacted with the rRo52 protein as assessed by a competition immunoassay (Fig. 1 B).
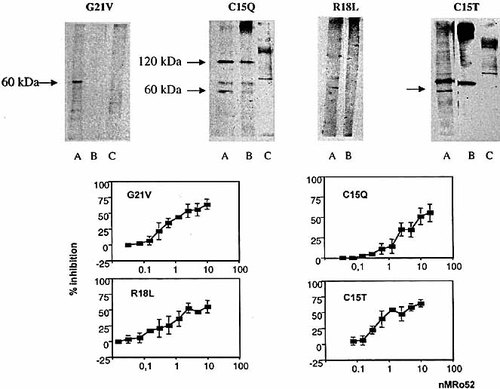
Immunochemical characterization of the antibodies induced in mice by immunization with peptides derived from the amino acid sequence of the 5-HT4 receptor. (A) The anti-peptide antibodies recognize the 5-HT4 receptor in immunoblot. Lane A: the receptor protein (60 kDa), expressed on membranes of transfected CHO cells, is recognized by the mouse sera. Lane B: the recognition is abolished after preincubation with 50 μg / ml of the peptide used as immunogen. Lane C: no protein band is recognized on untransfected CHO cells. Only the anti-R18L antibodies showed an absence of recognition. Anti-peptide antibodies are labeled as in Table 1. (B) The anti-peptide antibodies recognized rRo52. Recognition of the peptide used as immunogen was inhibited after preincubation with rRo52. Mean inhibition percentage and SD are shown for four individual mouse sera. Anti-peptide antibodies are labeled as in Table 1.
2.2 Pathogenicity of the anti-peptide antibodies
Peptide-immune mice were mated and the pups were monitored 2 days after birth. In the group immunized with the G21V peptide, the fertility was significantly lower than in the other groups and could be correlated with antibody titers (Table 2). The number of pups from mothers immunized with the other peptides was comparable to those of the control mothers.
Mice immunized with the 5-HT4-derived peptides (G21V and C15Q) produced seven stillborn pups which were autopsied. Of the living pups, 44 % showed skin rash at birth which disappeared 2 weeks later (Fig. 2 A) whereas 67 % showed neuromotoric problems and tremors, suggesting central nervous system abnormalities. Analysis of the electrocardiograms from 2-day-old pups revealed sinus bradycardia and an increased QT inverval in 28 % and 27 % of the animals, respectively. Thirteen percent of the pups showed atrioventricular block of type I or II (Fig. 2 B, Table 2). PQ and QRS intervals were normal (Table 3).
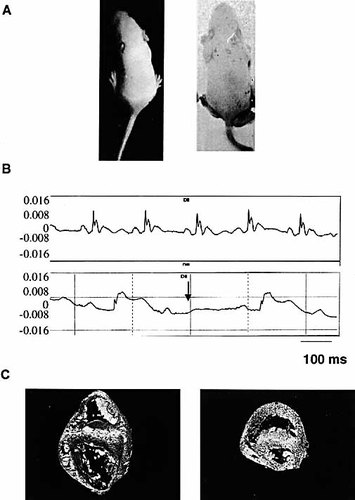
Pathology of the pups from mothers immunized with the 5-HT4 receptor-derived peptide. (A) A 7-day-old pup (right) from a immunized mother compared to a 7-day-old pup of a control mother (left). Note skin rash, clearly visible due to the lack of hair growth. (B) ECG of a 2-day-old pup from a control mother (upper) and a 2-day-old pup from an immunized mother (lower). Bradycardia in this pup and the atrio-ventricular block are indicated by an arrow. (C) Comparison of a heart from a normal and a stillborn pup from the same litter. Transversal cut through the heart in phase contrast showing a decrease in heart size and hyperplasia in the myocardium (right) compared to the control.
|
Immunized |
Mothers |
Litters |
Pups alive |
Dead at birth |
Skin rash |
Neuromotor problemsa) |
AVBb) |
|---|---|---|---|---|---|---|---|
|
Controls |
14 |
10 |
32 |
2 |
0 |
0 |
0 |
|
G21V |
16 |
7c) |
29 |
5 |
14 |
24 |
3 |
|
C15Q |
4 |
4 |
24 |
2 |
6 |
6 |
3 |
|
R18L |
5 |
5 |
20 |
0 |
0 |
0 |
0 |
|
C15T |
4 |
5 |
19 |
0 |
0 |
0 |
0 |
- a) Pups had tremors and lack of coordination in their movements.
- b) Two of the three pups of G21V-immunized mothers had atrioventricular blocks (AVB) of type I, the third one of type II. The three pups of C15Q-immunized mothers had AVB of type II.
- c) Fertility of G21V-immunized mice was significantly lower than that of the controls and was correlated with the antibody titer of the individual mice.
|
Peptide |
Heart rate (beats / min) |
PQ(s) |
QRS(s) |
QT(s) |
|---|---|---|---|---|
|
Controls |
332 ± 41 |
0.113 ± 0.015 |
0.028 ± 0.003 |
0.144 ± 0.025 |
|
G21V |
265 ± 31 (p = 0.0013)a) (24 %)b) |
0.119 ± 0.015 |
0.027 ± 0.002 |
0.157 ± 0.032 (24 %) |
|
C15Q |
282 ± 62 (p = 0.0152) (33 %) |
0.125 ± 0.015 (4 %) |
0.026 ± 0.003 |
0.171 ± 0.034 (p = 0.0022) (31 %) |
|
R18L |
324 ± 80 (17 %) |
0.115 ± 0.013 |
0.026 ± 0.003 |
0.154 ± 0.05 |
|
C15T |
336 ± 36 |
0.117 ± 0.012 |
0.026 ± 0.004 |
0.146 ± 0.031 |
- a) Probabilities (p) are indicated where significant differences from the controls were found.
- b) (n %) corresponds to the % of pups exceeding the limit values of the control mice.
On the contrary, mice immunized with the Ro52 peptides did not show any skin rash, neuromotoric disorders or atrioventricular block. This was not due to the lower antibody titers, since G21V peptide-immune mice having the highest anti-body titers were infertile, and the diseased pups were delivered from mothers with antibody titers equivalent to those of mice immune to Ro52 peptides. Analysis of the electrocardiograms showed no significant differences to those from the control group although 17 % of pups from the R18L immune mice had a slight bradycardia.
2.3 Histology of the target organs
To verify that the pathological symptoms of the pups were really due to the interference of antibodies with 5-HT4 receptor function, the presence of the target receptor was determined in the heart, brain and skin of fetuses by immunohistochemistry. In all three tissues the presence of the 5-HT4 receptor was demonstrable (Fig. 3). It is interesting to note that the cardiac 5-HT4 receptor was not detectable 5 days after birth.
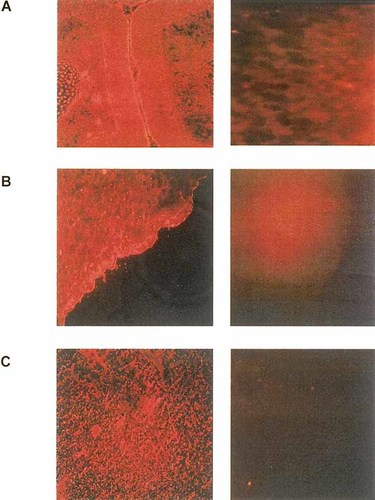
Immunohistochemical localization of the 5-HT4 receptor in brain (A), skin (B) and heart (C) of fetal mice. Blanks were performed by incubating the antibodies in the presence of 25 μg peptide used as immunogen before revealing the tissues. Left panels showing the specific binding, right panels, the nonspecific binding.
The hearts of the pups who died at birth were also compared to those of their healthy littermates. Macroscopically, the smaller size of the hearts from the diseased mice suggested a less advanced development. Microscopic examination confirmed the underdevelopment of the ventricles which correlates with a disorganized hyperplasia of the myocardium.
3 Discussion
To determine the pathogenicity of an autoimmune response, the following criteria must be fulfilled: (1) autoantibodies must be shown to interact with a specific auto-antigen; (2) autoantibodies must interfere with the function of the autoantigen and (3) autoantibodies must be able to induce the pathological symptoms of the disease. The first two criteria were met for the anti-5-HT4 autoantibodies found in lupus patients 4. With the observations presented here, we satisfy the third criterion. We have thus defined neonatal lupus as an autoimmune anti-5-HT4 receptor disease.
Since the major isotype of the anti-peptide antibodies was IgG1, transplacental transport of pathogenic antibodies to the fetus is plausible. As in humans, anti-receptor autoantibodies do not induce apparent symptoms in the mothers but have a pathogenic effect in the offspring. Since the target receptor, at least in the adult mouse heart, is known not to be functionally uncoupled from its effector mechanisms 8, we may presume that the 5-HT4 receptor is only of importance during fetal development and at birth. This was confirmed by the immunohistochemical detection of the 5-HT4 receptor in the fetal target tissues. Interestingly, the 5-HT4 receptor was no longer detectable in the heart of 5-day-old pups. The disease is thus a fetal disease which is reflected in the infertility of mice with high antibody titers, pointing towards death in utero, in the stillbirths of pups from immunized animals and in the transient symptoms of the surviving pups which are apparently normal 12 days after birth.
The main symptom of neonatal lupus in humans is the presence of an atrioventricular block associated with bradycardia. Only 13 % of the pups from mothers immunized with the peptides derived from the 5-HT4 receptor showed atrioventricular block, while the bradycardia was a more general symptom. It could be that atrioventricular block resulted mainly in death in utero or at birth, while bradycardia was better tolerated or might be only a sequel of fetal antibody-induced pathology. Interestingly, a significant number of pups showed a longer QTc interval. Recently, it has been shown that QT inverval prolongation is prevalent in infants without congenital heart block from asymptomatic anti-SSA / Ro-positive mothers. This is a predictor of malignant ventricular arrhythmias 9. While no direct causal relation can be found between 5-HT4 receptor-blocking antibodies and ventricular arrhythmias, the occurrence of the symptom both in children from SSA-Ro-positive mothers and the pups from 5-HT4 receptor peptide-immunized mice substantiate the importance of receptor recognition by autoantibodies in the disease. Myocardial hyperplasia, observed in one of the stillborn pups, is compatible with increased electrical instability.
Although skin rash and neurological disorders are relatively rare in neonatal lupus, these symptoms were predominant in the pups from mice immunized with the 5-HT4 receptor peptides. As with the heart symptoms, they were transient. Since the 5-HT4 receptor is mainly a brain receptor 10, it is not surprising that functional anti-5HT4 receptor antibodies could interfere with brain development. To our surprise, the receptor is also present in fetal skin which could explain the skin symptoms observed.
Finally, it must be emphasized that the pups from mice immunized with Ro52-derived peptides showed strikingly less symptoms and that only bradycardia occurred in some of them. A possible explanation is that only a subset of anti-Ro52 antibodies induced by the peptides cross-react with the 5-HT4 receptor, resulting only in a minor pathological effect. This hypothesis is supported by the fact that anti-R18L antibodies did not recognize the 5-HT4 receptor-derived peptide C15Q. Alternatively, antibodies directed against the human Ro52 should only cross-react slightly with the mouse Ro52 protein, considered as the target of pathogenic antibodies 11.
The presence of autoantibodies is a necessary but not a sufficient requirement of the induction of the disease. Indeed, healthy and diseased pups could be found in the same litter, suggesting that fetal factors are also responsible for the development of the disease. This is compatible with the low incidence of the human disease, in which only 1 to 3 % of the anti-Ro52 antibody-positive mothers give birth to children with neonatal lupus.
In conclusion, the experimental model of neonatal lupus presented here will enable us to search for immunotherapeutic and pharmacotherapeutic approaches for the prevention of the disease.
4 Materials and methods
4.1 Peptides
Four peptides, corresponding to sequences derived from the 5-HT4 receptor (G21V, C15Q) and the Ro52 protein (R18L, C15T), were synthesized in a automatic peptide synthesizer as previously described 12, their purity assessed by HPLC and their identity by mass spectrometry.
4.2 Immunization and induction of the antibodies in mice
Female BALB / c mice, 5 weeks old at the start of the experiment, were immunized with 25 μg peptide and 200 μg methylated BSA. Priming injections were performed i. p. in CFA. Two booster injections of 25 μg peptide were given within 2 weeks interval in IFA. After 5 weeks interval, a final booster injection was given. One week after the last booster injection, mice were mated. The mice were bled 1 week before and 1 week after each booster injection. Serum samples were tested by ELISA for the presence of anti-peptide antibodies.
4.3 Immunochemical analysis
Enzyme immunoassays and competition assays were performed as previously described 4. Immunoblots were performed on membrane proteins of CHO cells transfected with the 5-HT4 receptor 7 as previously described 4 using rabbit anti-mouse antibodies (Jackson Laboratories, San Diego, CA) instead of goat anti-rabbit antibodies.
4.4 in vivo cardiac physiology measurements
Three-lead electrocardiograms (ECG) (DI, DII, DIII derivations) were recorded from conscious neonates using four silver micro-electrodes attached to a body clip. ECG were digitized at a sampling rate of 1000 Hz for each. Digital files were recorded at least for 5 min for each mouse and analyzed with commercially available software (Notocord). ECG were evaluated according to standard criteria. QT interval was corrected for the beating frequency using Bazett's formula. Parameters were considered abnormal when they were under the lower (heart rate) or upper (RR, PQ, QRS, QT intervals) limit of the lowest and highest value in the control mice, respectively.
4.5 Immunohistochemistry
Heart, brain and whole trunk tissues from mouse fetus were collected at the 12th and 16th embryonic day and at day 5 after birth. They were prepared according to the standard procedures and cut at 5 μm with a Leica cryostat at – 20 °C, then fixed for 5 min in paraformaldehyde. After washing with PBS-Tween-20 (1 %) for 5 min the tissues were permeabilized with 2 % Triton X-100 for 3 min, then saturated with 1 % BSA in PBS-Tween-20 (1 %) buffer for 1 h at room temperature. The saturated tissues were incubated with or without peptide for 2 h at room temperature with a dilution of 1 / 200 of polyclonal antibodies raised against the C-terminal sequence of the 5-HT4 receptor 13. After several washes with PBS-Tween (1 %), they were revealed with Texas red-conjugated goat anti-rabbit antibodies (Molecular Probes, Eugen, OR) at a dilution of 1 / 200 for 1 h at room temperature.
Acknowledgements
The authors thank Adolphe Alonso and Michel Toussaint for technical assistance in developing an ECG device adapted to mouse neonates. Dr. Bernard Lorber has helped us to videoscan the animals, Ms. F. Goltzène and Dr. A. Jelali for help in the histological studies, Dr. J. P. Briand in peptide synthesia and Dr. G. Pruijn for providing us with the recombinant Ro52 protein. We acknowledge Drs. S. Muller and H. Partidos for helpful comments on the manuscript. This work was partially supported by a grant from Fondation de France. P.E. was supported by a grant from the Fondation pour la Recherche Médicale.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




