Parallels in Cation and Anion Coordination: A New Class of Cascade Complexes†
Md. Alamgir Hossain Dr.
Department of Chemistry University of Kansas Lawrence, KS 66045 (USA) Fax: (+1) 785-864-5396
Search for more papers by this authorJosé M. Llinares Dr.
Department of Chemistry University of Kansas Lawrence, KS 66045 (USA) Fax: (+1) 785-864-5396
Search for more papers by this authorSusan Mason Dr.
Department of Chemistry University of Kansas Lawrence, KS 66045 (USA) Fax: (+1) 785-864-5396
Search for more papers by this authorPaula Morehouse
Department of Chemistry University of Kansas Lawrence, KS 66045 (USA) Fax: (+1) 785-864-5396
Search for more papers by this authorDouglas Powell Dr.
Department of Chemistry University of Kansas Lawrence, KS 66045 (USA) Fax: (+1) 785-864-5396
Search for more papers by this authorKristin Bowman-James Prof.
Department of Chemistry University of Kansas Lawrence, KS 66045 (USA) Fax: (+1) 785-864-5396
Search for more papers by this authorMd. Alamgir Hossain Dr.
Department of Chemistry University of Kansas Lawrence, KS 66045 (USA) Fax: (+1) 785-864-5396
Search for more papers by this authorJosé M. Llinares Dr.
Department of Chemistry University of Kansas Lawrence, KS 66045 (USA) Fax: (+1) 785-864-5396
Search for more papers by this authorSusan Mason Dr.
Department of Chemistry University of Kansas Lawrence, KS 66045 (USA) Fax: (+1) 785-864-5396
Search for more papers by this authorPaula Morehouse
Department of Chemistry University of Kansas Lawrence, KS 66045 (USA) Fax: (+1) 785-864-5396
Search for more papers by this authorDouglas Powell Dr.
Department of Chemistry University of Kansas Lawrence, KS 66045 (USA) Fax: (+1) 785-864-5396
Search for more papers by this authorKristin Bowman-James Prof.
Department of Chemistry University of Kansas Lawrence, KS 66045 (USA) Fax: (+1) 785-864-5396
Search for more papers by this authorThis research was sponsored by the Environmental Management Science Program, Offices of Science and Environmental Management, U.S. Department of Energy under Grant DE-FG-96ER62307.
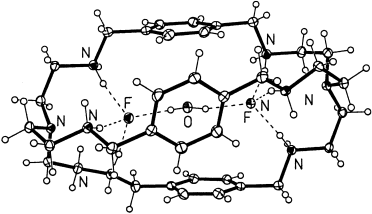
Graphical Abstract
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2002/z18101_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 B. Dietrich, J. Guilhem, J.-M. Lehn, C. Pascard, E. Sonveaux, Helv. Chim. Acta 1984, 67, 91–104.
- 2 Nomenclature: M=m-xylyl, P=p-xylyl, and Fu=furan spacers; E=ethyl links within the tren unit between amine groups; A=amine; cryp=cryptand.
- 3
- 3a S. Mason, T. Clifford, L. Seib, K. Kuczera, K. Bowman-James, J. Am. Chem. Soc. 1998, 120, 8899–8900;
- 3b T. Clifford, A. Danby, J. M. Llinares, S. Mason, N. W. Alcock, D. Powell, J. A. Aguilar, E. García-España, K. Bowman-James, Inorg. Chem. 2001, 40, 4710–4720.
- 4 T. Clifford, S. Mason, J. M. Llinares, K. Bowman-James, J. Am. Chem. Soc. 2000, 122, 1814–1815.
- 5 J. A. Aguilar, T. Clifford, A. Danby, J. M. Llinares, S. Mason, E. García-España, K. Bowman-James, Supramol. Chem. 2001, 13, 405–417.
- 6
- 6a J.-M. Lehn, S. H. Pine, E. I. Watanabe, A. K. Willard, J. Am. Chem. Soc. 1977, 99, 6766–6768;
- 6b J.-M. Lehn, Pure Appl. Chem. 1980, 52, 2441–2459.
- 7
- 7a J. Jazwinski, J.-M. Lehn, D. Lilienbaum, R. Ziessel, J. Guilheim, C. Pascard, J. Chem. Soc. Chem. Commun. 1987, 1691–1692;
- 7b D. McDowell, J. Nelson, Tetrahedron Lett. 1988, 29, 385–386;
- 7c D. Chen, A. E. Martell, Tetrahedron 1991, 47, 6895–6902.
- 8
V. Amendola, E. Bastianello, L. Fabbrizzi, C. Mangano, P. Pallavicini, A. Perotti, A. M. Lanfredi, F. Ugozzoli, Angew. Chem. 2000, 112, 3039–3042;
10.1002/1521-3757(20000818)112:16<3039::AID-ANGE3039>3.0.CO;2-Q Google ScholarAngew. Chem. Int. Ed. 2000, 39, 2917–2920.10.1002/1521-3773(20000818)39:16<2917::AID-ANIE2917>3.0.CO;2-0 CAS PubMed Web of Science® Google Scholar
- 9
X-ray data for 1: A crystal of dimensions 0.64×0.42×0.21 mm was selected for structural analysis. Crystal data: Mr=1161.31; triclinic space group, P
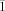 , a=9.8302(11), b=15.9973(19), c=17.417(2) Å, α=84.404(3), β=89.959(3), γ=77.098(2)°, V=2656.5(5) Å3, Z=2, ρcalcd=1.452 g cm−1, F(000)=1232. A total of 12 226 observed reflections (I>2σ(I)) were collected. The refinement converged at R1(F, observed data)=0.0443, and wR2 (F2, all data)=0.1189, and GOF=0.974. Diffraction data were collected using a Bruker SMART APEX CCD area detector mounted on a Bruker D8 goniometer using graphite-monochromated MoKα radiation (λ=0.71073 Å) at 100(2) K.[8] Intensity data were measured as a series of ω and θ oscillation frames each of 0.3° for times of 5 s per frame. The detector was operated in a 512×512 mode and was positioned 5.04 cm from the sample. Coverage of unique data was close to 99.4 % complete to 26.00° in θ. Cell parameters were determined from a least-squares fit of 9504 peaks in the range 2.40<θ<30.58°. Virtually no decay was observed, based on data obtained for a number of peaks monitored at both the beginning and end of data collection. The data were corrected for absorption by the semi-empirical method.[9] Lorentz and polarization corrections were applied. The data were merged to form a set of 15 608 independent data with Rint=0.0248. The space group was determined by statistical tests, and the structure was solved by direct methods and refined by full-matrix least-squares methods on F 2.[10] Hydrogen atom positions were initially determined by geometry and refined by a riding model. Non-hydrogen atoms were refined with anisotropic displacement parameters. CCDC-172118 (1) and CCDC-172119 (2) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12, Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223-336-033; or [email protected]).
, a=9.8302(11), b=15.9973(19), c=17.417(2) Å, α=84.404(3), β=89.959(3), γ=77.098(2)°, V=2656.5(5) Å3, Z=2, ρcalcd=1.452 g cm−1, F(000)=1232. A total of 12 226 observed reflections (I>2σ(I)) were collected. The refinement converged at R1(F, observed data)=0.0443, and wR2 (F2, all data)=0.1189, and GOF=0.974. Diffraction data were collected using a Bruker SMART APEX CCD area detector mounted on a Bruker D8 goniometer using graphite-monochromated MoKα radiation (λ=0.71073 Å) at 100(2) K.[8] Intensity data were measured as a series of ω and θ oscillation frames each of 0.3° for times of 5 s per frame. The detector was operated in a 512×512 mode and was positioned 5.04 cm from the sample. Coverage of unique data was close to 99.4 % complete to 26.00° in θ. Cell parameters were determined from a least-squares fit of 9504 peaks in the range 2.40<θ<30.58°. Virtually no decay was observed, based on data obtained for a number of peaks monitored at both the beginning and end of data collection. The data were corrected for absorption by the semi-empirical method.[9] Lorentz and polarization corrections were applied. The data were merged to form a set of 15 608 independent data with Rint=0.0248. The space group was determined by statistical tests, and the structure was solved by direct methods and refined by full-matrix least-squares methods on F 2.[10] Hydrogen atom positions were initially determined by geometry and refined by a riding model. Non-hydrogen atoms were refined with anisotropic displacement parameters. CCDC-172118 (1) and CCDC-172119 (2) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12, Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223-336-033; or [email protected]).
- 10 Data collection: SMART Software Reference Manual, Bruker-AXS, 6300 Enterprise Dr., Madison, WI 53719-1173, USA, 1994. Data reduction: SAINT Software Reference Manual, Bruker-AXS, 6300 Enterprise Dr., Madison, WI 53719-1173, USA, 1995.
- 11 G. M. Sheldrick, SADABS. Program for Empirical Absorption Correction of Area Detector Data, University of Göttingen, Göttingen (Germany), 1996.
- 12 G. M. Sheldrick, SHELXTL Version 5 Reference Manual, Bruker AXS 5465 E. Cheryl Parkway, Madison, WI 53711-5373, USA, 1994, using International Tables for Crystallography, Vol. C, Kluwer, Boston, 1995, Tab. 6.1.1.4, 4.2.6.8, and 4.2.4.2.
- 13 N. Alcock in Bonding and Structure, Ellis Horwood, West Sussex, England, 1990, p. 316.
- 14 Synthesis, characterization, and structural results for 2 are given in the Supporting Information.
- 15 Protonation constants for PEAcryp have been reported as log K1=9.6(1), log K2=9.00(4), log K3=8.62(8), log K4=7.3(1), log K5=6.7(1), and log K6=6.52(9) using Et4NClO4 as the electrolyte: F. Arnaud-New, S. Fuangswasdi, B. Maubert, J. Nelson, V. McKee, Inorg. Chem. 2000, 39, 573–579.
- 16 R. J. Motekaitis, A. E. Martell, J.-M. Lehn, E.-I. Watanabe, Inorg. Chem. 1982, 21, 4253–4257.
- 17 P. Gans, A. Sabatini, A. Vacca, J. Chem. Soc. Dalton Trans. 1985, 1195–1200.



41:13<>1.0.co;2-h.cover.gif)
