Structural Characterization of Novel Olefinic Cation Radicals: X-ray Crystallographic Evidence of σ–π Hyperconjugation
Jay K. Kochi
Department of Chemistry University of Houston Houston, TX 77204-5641 (USA) Fax: (+1) 713-743-2709
Search for more papers by this authorRajendra Rathore
Department of Chemistry University of Houston Houston, TX 77204-5641 (USA) Fax: (+1) 713-743-2709
Search for more papers by this authorChengjian Zhu, and
Department of Chemistry University of Houston Houston, TX 77204-5641 (USA) Fax: (+1) 713-743-2709
Search for more papers by this authorSergey V. Lindeman
Department of Chemistry University of Houston Houston, TX 77204-5641 (USA) Fax: (+1) 713-743-2709
Search for more papers by this authorJay K. Kochi
Department of Chemistry University of Houston Houston, TX 77204-5641 (USA) Fax: (+1) 713-743-2709
Search for more papers by this authorRajendra Rathore
Department of Chemistry University of Houston Houston, TX 77204-5641 (USA) Fax: (+1) 713-743-2709
Search for more papers by this authorChengjian Zhu, and
Department of Chemistry University of Houston Houston, TX 77204-5641 (USA) Fax: (+1) 713-743-2709
Search for more papers by this authorSergey V. Lindeman
Department of Chemistry University of Houston Houston, TX 77204-5641 (USA) Fax: (+1) 713-743-2709
Search for more papers by this authorWe thank the National Science Foundation and the Robert A. Welch Foundation for financial support.
Abstract
A 29° twist of the central olefinic bond is observed in the sesquihomoadamantene cation radical (SH+.). A careful comparison of the X-ray structures of neutral SH (right) and its cation radical (left) reveal the twist is not a result of relieving steric strain. Moreover, the changes in the bond lengths in SH+.—the central C−C bond lengthens by 5 pm and the four single C−C bonds directly attached to the olefinic carbonatoms shorten by 3 pm—provide the first experimental evidence for the (classical) hyperconjugative stabilization of cationic charges.
References
- 1a S. F. Nelsen, Acc. Chem. Res. 1987, 20, 269, and references therein;
- 1b T. Kim, G. A. Mirafzal, J. Liu, N. L. Bauld, J. Am. Chem. Soc. 1993, 115, 7653;
- 1c S. F. Nelsen, R. Akaba, J. Am. Chem. Soc. 1981, 103, 2096;
- 1d E. L. Clennan, W. Simmons, C. W. Almgren, J. Am. Chem. Soc. 1981, 103, 2098;
- 1e C. M. Jefford, A. F. Boschung, Tetrahedron Lett. 1976, 4771;
- 1f R. Akaba, H. Sakuragi, K. Tokumaru, Tetrahedron Lett. 1984, 25, 665;
- 1g E. Bosch, J. K. Kochi, J. Am. Chem. Soc. 1996, 118, 1319.
- 2a N. L. Bauld, Tetrahedron 1989, 45, 5307;
- 2b N. L. Bauld, J. Am. Chem. Soc. 1992, 114, 5800;
- 2c J. P. Dinnocenzo, D. A. Conlon, J. Am. Chem. Soc. 1988, 110, 2324;
- 2d M. Schmittel, C. Wohrle, J. Org. Chem. 1995, 60, 8223, and references therein.
- 3a M. Tabata, A. Lund, P. O. Samskog, S. Lunell, M. B. Huang, J. Polym. Sci. A 1988, 26, 2725, and references therein;
- 3b see also: N. Salhi-Benachenhou, J. R. Alvarez-Idaboy, S. Lunell, L. A. Eriksson, Theor. Chem. Acc. 1997, 97, 282;
- 3c H. Zipse, J. Am. Chem. Soc. 1995, 117, 11 798;
- 3d S. S. Shaik, A. Pross, J. Am. Chem. Soc. 1989, 111, 4306;
- 3e M. S. Workentin, N. P. Schepp, L. J. Johnston, D. D. Wayner, J. Am. Chem. Soc. 1994, 116, 1142.
- 4a R. S. Mulliken, C. C. Roothaane, J. Chem. Rev. 1947, 41, 219;
- 4b R. S. Mulliken, C. C. Roothaane, Tetrahedron 1959, 5, 253.
- 5a S. Lunell, M.-B. Huang, Chem. Phys. Lett. 1990, 168, 63;
- 5b N. Salhi-Benachenhou, B. Engels, M.-B. Huang, S. Lunell, Chem. Phys. 1998, 236, 53, and references therein;
- 5c L. A. Eriksson, S. Lunell, R. J. Boyd, J. Am. Chem. Soc. 1993, 115, 6896;
- 5d O. Takahashi, O. Kikuchi, J. Chem. Phys. 1994, 100, 1350.
- 6a A. J. Merer, L. Schoonveld, J. Chem. Phys. 1968, 48, 522;
- 6b A. J. Merer, L. Schoonveld, Can. J. Phys. 1969, 47, 1731.
- 7 H. Koppel, W. Domcke, L. S. Cederbaum, W. V. Niessen, J. Chem. Phys. 1978, 69, 4252.
- 8a K. Toriyama, M. Okazaki, Appl. Magn. Reson. 1996, 11, 47, and references therein;
- 8b M. Shiotani, Y. Nagata, J. Sohma, J. Am. Chem. Soc. 1984, 106, 4640; however, see: F. Gerson, G. Gescheidt, S. F. Nelsen, L. A. Paquette, M. F. Teasley, L. Waykole, J. Am. Chem. Soc. 1989, 111, 5518.
- 9a F. Gerson, J. Lopez, R. Akaba, S. F. Nelsen, J. Am. Chem. Soc. 1981, 103, 6716;
- 9b T. Clark, M. F. Teasley, S. F. Nelsen, H. Wynberg, J. Am. Chem. Soc. 1987, 109, 5719;
- 9c T. Clark, S. F. Nelsen, J. Am. Chem. Soc. 1988, 110, 868;
- 9d S. F. Nelsen, M. F. Teasley, D. L. Kapp, C. R. Kessel, L. A. Grezzo, J. Am. Chem. Soc. 1984, 106, 791;
- 9e S. F. Nelsen, C. R. Kessel, J. Am. Chem Soc. 1979, 101, 2503.
- 10a H. Prinzbach, G. Gescheidt, H.-D. Martin, R. Herges, J. Heinze, G. K. S. Prakash, G. A. Olah, Pure Appl. Chem. 1995, 67, 673, and references therein;
- 10b H. Prinzbach, B. A. R. C. Murty, W.-D. Fessner, J. Mortensen, J, Heinze, G. Gescheidt, F. Gerson, Angew. Chem. 1987, 99, 488; Angew. Chem. Int. Ed. Engl. 1987, 26, 457;
- 10c
G. Gescheidt, R. Herges, H. Neumann, J. Heinze, M. Wollenweber, M. Etzkorn, H. Prinzbach, Angew. Chem. 1995, 107, 1109; Angew. Chem. Int. Ed. Engl. 1995, 34, 1016;
10.1002/ange.19951070922 Google Scholar
- 10d M. Etzkorn, F. Wahl, M. Keller, H. Prinzbach, F. Barbosa, V. Peron, G. Gescheidt, J. Heinze, R. Herges, J. Org. Chem. 1998, 63, 6080, and references therein.
- 11a T. Shida, Y. Egawa, A. Kubodera, T. Kato, J. Chem. Phys. 1980, 73, 5963;
- 11b M. Kira, H. Nakazawa, H. Sakurai, J. Am. Chem. Soc. 1983, 105, 6983;
- 11c Y. Itagaki, M. Shiotani, A. Hasegawa, H. Kawazoe, Bull. Chem. Soc. Jpn. 1998, 71, 2547;
- 11d O. West, J. Am. Chem. Soc. 1997, 119, 5713;
- 11e A. D. Trifunac, D. W. Werst, J. Am. Chem. Soc. 1996, 118, 9444;
- 11f H. D. Roth, Top. Curr. Chem. 1992, 163, 131, and references therein.
- 12a D. J. Bellville, N. L. Bauld, J. Am. Chem. Soc. 1982, 104, 294;
- 12b M. J. Shephard, M. N. Paddon-Row, J. Phys. Chem. 1995, 99, 3101;
- 12c S. D. Chemerisov, D. W. Werst, A. D. Trifunac, Chem. Phys. Lett. 1998, 291, 262;
- 12d F. Gerson, P. Felder, R. Schmidlin, H. N. C. Wong, J. Chem. Soc. Chem. Commun. 1994, 1659;
- 12e T. Nishinaga, K. Komatsu, N. Sugita, H. J. Lindner, J. Richter, J. Am. Chem. Soc. 1993, 115, 11642.
- 13 Tetraanisylethylene cation radical is the only known example which has been characterized crystallographically and it also shows a twist of 30° around the central C−C bond, see: R. Rathore, S. V. Lindeman, A. S. Kumar, J. K. Kochi, J. Am. Chem. Soc. 1998, 120, 6931.
- 14a H. Wynberg, E. Boelema, J. H. Wieringa, J. Strating, Tetrahedron Lett. 1970, 3613;
- 14b E. Boelema, H. Wynberg, J. Strating, Tetrahedron Lett. 1971, 4029
- 15 X-ray structure of sesquihomoadamantene has been determined at room temperature, see: W. H. Watson, A. Nagl, Acta Crystallogr. Sect. C 1987, 43, 2465.
- 16
The one-electron oxidation of SH to its purple cation radical can also be carried out electrochemically at E
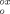 =1.36 V versus SCE in anhydrous dichloromethane (containing tetra-n-butylammonium hexafluorophosphate as the supporting electrolyte) at −78 °C.
=1.36 V versus SCE in anhydrous dichloromethane (containing tetra-n-butylammonium hexafluorophosphate as the supporting electrolyte) at −78 °C.
- 17 For a general procedure for the oxidation of organic donors using NO+SbCl6−, see: R. Rathore, J. K. Kochi, J. Org. Chem. 1994, 60, 4399.
- 18 The identity of liberated NO was confirmed by UV/Vis and IR spectroscopy (W. G. Fateley, H. A. Bent, B. Crawford, Jr., J. Chem. Phys. 1959, 31, 204).
- 19 For a list of donors which afford stable cation radical salts, see: R. Rathore, A. S. Kumar, S. V. Lindeman, J. K. Kochi, J. Org. Chem. 1998, 63, 5847.
- 20 In addition, a very weak absorption at λ>750 nm (see Figure 1) accounts for the purple color of the cation radical salt. The ESR spectrum of SH+. SbCl6− in dichloromethane at −70 °C was the same as that obtained by Nelsen and co-workers in a mixture of trifluoroacetic acid and trifluoroacetic anhydride using the electrochemical method.[9b,c]
- 21 Note that the remote b′ bonds which remain unchanged in the cation radical lie almost orthogonal to the p orbitals and thus do not participate in stabilizing the cationic charge, compare: J. F. King, R. Rathore, J. Am. Chem. Soc. 1990, 112, 2001.
- 22a J. Rosenbaum, M. C. R. Symons, Proc. Chem. Soc. 1959, 92;
- 22b see also: S. F. Nelsen, M. F. Teasley, D. L. Kapp, C. R. Kessel, L. A. Grezzo, J. Am. Chem. Soc. 1984, 106, 791.
- 23 Theoretical calculations of olefinic cation radicals are in progress with Professor P. von R. Schleyer.
- 24 G. M. Sheldrick, SHELSX-86, program for structure solution, University of Göttingen, Germany, 1986.



39:20<>1.0.co;2-v.cover.gif)
