Development of a Diversity-Based Approach for the Discovery of Stereoselective Polymerization Catalysts: Identification of a Catalyst for the Synthesis of Syndiotactic Polypropylene
Jun Tian Dr.
Department of Chemistry and Chemical Biology Baker Laboratory Cornell University Ithaca, NY 14853-1301 (USA) Fax: (+1) 607-255-4137
Search for more papers by this authorGeoffrey W. Coates Prof.
Department of Chemistry and Chemical Biology Baker Laboratory Cornell University Ithaca, NY 14853-1301 (USA) Fax: (+1) 607-255-4137
Search for more papers by this authorJun Tian Dr.
Department of Chemistry and Chemical Biology Baker Laboratory Cornell University Ithaca, NY 14853-1301 (USA) Fax: (+1) 607-255-4137
Search for more papers by this authorGeoffrey W. Coates Prof.
Department of Chemistry and Chemical Biology Baker Laboratory Cornell University Ithaca, NY 14853-1301 (USA) Fax: (+1) 607-255-4137
Search for more papers by this authorThis work was supported by the Cornell Center for Materials Research (CCMR), a Materials Research Science and Engineering Center of the National Science Foundation (DMR-9632275), and the Exxon Chemical Corporation. G.W.C. gratefully acknowledges an NSF Career Award (CHE-9875261), a Camille and Henry Dreyfus New Faculty Award, a Research Corporation Research Innovation Award, an Alfred P. Sloan Research Fellowship, an Arnold and Mabel Beckman Foundation Young Investigator Award, a Camille Dreyfus Teacher-Scholar Award, a 3M Untenured Faculty Grant, an IBM Partnership Award, and a Union Carbide Innovation Recognition Award.
Abstract
Combinatorial methods have recently been used to aid in the discovery of new pharmaceuticals and catalysts for organic synthesis. A simple and inexpensive strategy for using combinatorial methods to discover polymerization catalysts is reported, and using it enabled a new catalyst (1, see scheme) for the synthesis of syndiotactic polypropylene to be identified.
References
- 1 Comprehensive Asymmetric Catalysis, Vol. 1–3 ( ), Springer, Berlin, 1999.
- 2 G. W. Coates, Chem. Rev. 2000, 100, 1223–1252.
- 3
B. Jandeleit, D. J. Schaefer, T. S. Powers, H. W. Turner, W. H. Weinberg, Angew. Chem. 1999, 111, 2648–2689; Angew. Chem. Int. Ed. 1999, 38, 2495–2532.
10.1002/(SICI)1521-3757(19990903)111:17<2648::AID-ANGE2648>3.0.CO;2-N Google Scholar
- 4
K. D. Shimizu, M. L. Snapper, A. H. Hoveyda, Chem. Eur. J. 1998, 4, 1885–1889.
10.1002/(SICI)1521-3765(19981002)4:10<1885::AID-CHEM1885>3.0.CO;2-D CAS Web of Science® Google Scholar
- 5 J. M. Newsam, F. Schuth, Biotechnol. Bioeng. 1999, 61, 203–216.
- 6 S. L. Schreiber, Science 2000, 287, 1964–1969.
- 7
F. Balkenhohl, C. von dem Bussche-Hünnefeld, A. Lansky, C. Zechel, Angew. Chem. 1996, 108, 2436–2487; Angew. Chem. Int. Ed. Engl. 1996, 35, 2289–2337.
10.1002/ange.19961082004 Google Scholar
- 8 L. A. Thompson, J. A. Ellman, Chem. Rev. 1996, 96, 555–600.
- 9 J. M. J. Frechét, ACS Poly. Mater. Sci. Eng. 1999, 80, 494.
- 10 For representative examples that demonstrate potential methods for the parallel synthesis of catalyst libraries and their pooled screening for polymerization activity, see:
- 10a
T. R. Boussie, C. Coutard, H. Turner, V. Murphy, T. S. Powers, Angew. Chem. 1998, 110, 3472–3475; Angew. Chem. Int. Ed. 1998, 37, 3272–3275;
10.1002/(SICI)1521-3757(19981204)110:23<3472::AID-ANGE3472>3.0.CO;2-D Google Scholar
- 10b T. R. Boussie, V. Murphy, K. A. Hall, C. Coutard, C. Dales, M. Petro, E. Carlson, H. W. Turner, T. S. Powers, Tetrahedron 1999, 55, 11 699–11 710.
- 11 An elegant mass-spectrometry technique has recently been reported in which a pooled library of living polymerization catalysts was screened for the ability to form high molecular weight polymer:
- 11a
C. Hinderling, P. Chen, Angew. Chem. 1999, 111, 2393–2396; Angew. Chem. Int. Ed. 1999, 38, 2253–2256;
10.1002/(SICI)1521-3757(19990802)111:15<2393::AID-ANGE2393>3.0.CO;2-W Google Scholar
- 11b C. Hinderling, C. Adlhart, P. Chen, Chimia 2000, 54, 232–235.
- 12 Some inventive, alternate high-throughput methods for screening asymmetric catalysts have recently been reported. For example, see:
- 12a G. T. Copeland, S. J. Miller, J. Am. Chem. Soc. 1999, 121, 4306–4307 (a fluorescent sensor approach to identify highly active, and hence selective catalysts);
- 12b
M. T. Reetz, M. H. Becker, H. W. Klein, D. Stockigt, Angew. Chem. 1999, 111, 1872–1875; Angew. Chem. Int. Ed. 1999, 38, 1758–1761 (the use of mass spectrometry to determine the enantioselectivity of reactions involving pseudo-enantiomeric, -prochiral, and -meso substrates).
10.1002/(SICI)1521-3757(19990614)111:12<1872::AID-ANGE1872>3.0.CO;2-G Google Scholar
- 13 For some recent examples of catalytic, asymmetric methods developed by using combinatorial methods, see the following. Diethylzinc additions to aldehydes:
- 13a G. Liu, J. A. Ellman, J. Org. Chem. 1995, 60, 7712–7713;
- 13b C. Gennari, S. Ceccarelli, U. Piarulli, C. Montalbetti, R. F. W. Jackson, J. Org. Chem. 1998, 63, 5312–5313;
- 13c K. L. Ding, A. Ishii, K. Mikami, Angew. Chem. 1999, 111, 519–523; Angew. Chem. Int. Ed. 1999, 38, 497–501;
- 13d A. J. Brouwer, H. J. van der Linden, R. M. J. Liskamp, J. Org. Chem. 2000, 65, 1750–1757; enamide hydrogenation:
- 13e S. R. Gilbertson, X. Wang, Tetrahedron Lett. 1996, 37, 6475–6478; aza-Diels–Alder:
- 13f S. Bromidge, P. C. Wilson, A. Whiting, Tetrahedron Lett. 1998, 39, 8905–8908; alkene epoxidation:
- 13g
M. B. Francis, E. N. Jacobsen, Angew. Chem. 1999, 111, 987–991; Angew. Chem. Int. Ed. 1999, 38, 937–941; C−H insertion:
10.1002/(SICI)1521-3757(19990401)111:7<987::AID-ANGE987>3.0.CO;2-9 Google Scholar
- 13h
K. Burgess, H. J. Lim, A. M. Porte, G. A. Sulikowski, Angew. Chem. 1996, 108, 192–194; Angew. Chem. Int. Ed. Engl. 1996, 35, 220–222; cyanide addition to epoxides:
10.1002/ange.19961080212 Google Scholar
- 13i
B. M. Cole, K. D. Shimizu, C. A. Krueger, J. P. A. Harrity, M. L. Snapper, A. H. Hoveyda, Angew. Chem. 1996, 108, 1776–1779; Angew. Chem. Int. Ed. Engl. 1996, 35, 1668–1671;
10.1002/ange.19961081509 Google Scholar
- 13j K. D. Shimizu, B. M. Cole, C. A. Krueger, K. W. Kuntz, M. L. Snapper, A. H. Hoveyda, Angew. Chem. 1997, 109, 1781–1785; Angew. Chem. Int. Ed. Engl. 1997, 36, 1704–1707; Strecker reaction:
- 13k M. S. Sigman, P. Vachal, E. N. Jacobsen, Angew. Chem. 2000, 112, 1336–1338; Angew. Chem. Int. Ed. 2000, 39, 1279–1281.
- 14 H. B. Kagan, J. Organomet. Chem. 1998, 567, 3–6.
- 15 Interestingly, two milestone discoveries concerning isospecific propylene polymerization foretell the feasibility of this strategy. In Natta's original report of the synthesis of isotactic polypropylene, a multisited heterogeneous titanium-based catalyst produced a mixture of polymer chains. Solvent extraction was used to separate isotactic from atactic chains; later generations of these catalysts were empirically modified to produce only isotactic polypropylene (G. Natta, P. Pino, P. Corradini, F. Danusso, E. Mantica, G. Mazzanti, G. Moraglio, J. Am. Chem. Soc. 1955, 77, 1708–1710). Three decades later, Ewen reported that a mixture of Brintzinger's meso and racemic titanocenes (a “library” of two species) also produced a mixture of polymer chains. Solvent extraction revealed isotactic and atactic polymer, produced by the racemic and meso isomers, respectively ( J. A. Ewen, J. Am. Chem. Soc. 1984, 106, 6355–6364).
- 16 G. Natta, I. Pasquon, A. Zambelli, J. Am. Chem Soc. 1962, 84, 1488–1490.
- 17 J. A. Ewen, R. L. Jones, A. Razavi, J. D. Ferrara, J. Am. Chem. Soc. 1988, 110, 6255–6256.
- 18 T. A. Herzog, D. L. Zubris, J. E. Bercaw, J. Am. Chem. Soc. 1996, 118, 11 988–11 989.
- 19 D. Veghini, L. M. Henling, T. J. Burkhardt, J. E. Bercaw, J. Am. Chem. Soc. 1999, 121, 564–573.
- 20 P. G. Cozzi, E. Gallo, C. Floriani, A. Chiesi-Villa, C. Rizzoli, Organometallics 1995, 14, 4994–4996.
- 21 S. Matsui, Y. Tohi, M. Mitani, J. Saito, H. Makio, H. Tanaka, M. Nitabaru, T. Nakano, T. Fujita, Chem. Lett. 1999, 1065–1066.
- 22 S. Matsui, M. Mitani, J. Saito, Y. Tohi, H. Makio, H. Tanaka, T. Fujita, Chem. Lett. 1999, 1263–1263.
- 23 S. Matsui, M. Mitani, J. Saito, N. Matsukawa, H. Tanaka, T. Nakano, T. Fujita, Chem. Lett. 2000, 554–555.
- 24 J. Strauch, T. H. Warren, G. Erker, R. Fröhlich, P. Saarenketo, Inorg. Chem. Acta 2000, 300–302, 810–821.
- 25 L. Resconi, L. Cavallo, A. Fait, F. Piemontesi, Chem. Rev. 2000, 100, 1253–1345.
- 26 Due to our sub-library searching routine, a stereoselective, mixed-ligand catalyst might have gone undetected. Given the high selectivity of [(L3A)2TiCl2], we have abandoned the search for such a hybrid species.
- 27 For representative examples of chain-end stereocontrol in alkene polymerization, see:
- 27a Y. Doi, Macromolecules 1979, 12, 1012–1013 (polymerization of propene using VCl4/AlEt2Cl at −78 °C produces syndiotactic polymer with [r]=0.87);
- 27b J. A. Ewen, J. Am. Chem. Soc. 1984, 106, 6355–6364 (polymerization of propene using Cp2TiCl2 at −45 °C produces isotactic polymer with [m]=0.85);
- 27c
L. Resconi, L. Abis, G. Franciscono, Macromolecules 1992, 25, 6814–6817 (polymerization of butene using [Cp
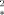 MCl2] (M=Zr, Hf) at −20 °C produces syndiotactic polymer with [r]=0.88);
MCl2] (M=Zr, Hf) at −20 °C produces syndiotactic polymer with [r]=0.88);
- 27d C. Pellecchia, A. Zambelli, Macromol. Rapid Commun. 1996, 17, 333–338 (polymerization of propene using a nickel catalyst at −78 °C produces syndiotactic polymer with [r]=0.89);
- 27e B. L. Small, M. Brookhart, Macromolecules 1999, 32, 2120–2130 (polymerization of propene using an iron catalyst at −20 °C produces isotactic polymer with [m]=0.91).
- 28 M. Brookhart, M. I. Wagner, J. Am. Chem. Soc. 1996, 118, 7219–7220.



39:20<>1.0.co;2-v.cover.gif)
