The Total Synthesis of the Annonaceous Acetogenin, Muricatetrocin C
Darren J. Dixon Dr.
Department of Chemistry University of Cambridge Lensfield Road Cambridge, CB2 1EW (UK) Fax: (+44) 1223-336-442
Search for more papers by this authorSteven V. Ley Prof. Dr.
Department of Chemistry University of Cambridge Lensfield Road Cambridge, CB2 1EW (UK) Fax: (+44) 1223-336-442
Search for more papers by this authorDominic J. Reynolds
Department of Chemistry University of Cambridge Lensfield Road Cambridge, CB2 1EW (UK) Fax: (+44) 1223-336-442
Search for more papers by this authorDarren J. Dixon Dr.
Department of Chemistry University of Cambridge Lensfield Road Cambridge, CB2 1EW (UK) Fax: (+44) 1223-336-442
Search for more papers by this authorSteven V. Ley Prof. Dr.
Department of Chemistry University of Cambridge Lensfield Road Cambridge, CB2 1EW (UK) Fax: (+44) 1223-336-442
Search for more papers by this authorDominic J. Reynolds
Department of Chemistry University of Cambridge Lensfield Road Cambridge, CB2 1EW (UK) Fax: (+44) 1223-336-442
Search for more papers by this authorWe thank the EPSRC (D.J.D. and D.J.R.), the Novartis Research Fellowship (S.V.L.), and the BP Research Endowment (S.V.L.) for generous financial support for this work.
Abstract
References
- 1a F. Q. Alali, X.-X. Liu, J. L. McLaughlin, J. Nat. Prod. 1999, 62, 504;
- 1b L. Zeng, Q. Ye, N. H. Oberlies, G. Shi, Z. Gu, K. He, J. L. McLaughlin, Nat. Prod. Rep. 1996, 13, 275, and references therein.
- 2 M. C. Gonzalez, J. R. Tormo, A. Bermejo, M. C. Zafra-polo, Estornell. D. Cortes, Bioorg. Med. Chem. Lett. 1997, 7, 1113, and references therein.
- 3 D. J. Morré, R. deCabo, C. Farley, N. H. Oberlies, J. L. McLaughlin, Life Sci. 1995, 56, 343.
- 4 D. Decaudin, I. Marzo, C. Brenner, G. Kroemer, Int. J. Oncol. 1998, 12, 141.
- 5 N. H. Oberlies, V. L. Croy, M. L. Harrison, J. L. McLaughlin, Cancer Lett. 1997, 15, 73.
- 6 For recent reviews see:
- 6a G. Cassiraghi, F. Zanardi, L. Battistini, G. Rassu, G. Aappendino, Chemtracts: Org. Chem. 1998, 11, 803;
- 6b R. Hoppe, H. D. Scharf, Synthesis 1995, 1447;
- 6c B. Figadère, Acc. Chem. Res. 1995, 28, 359.
- 7 G. Shi, Z. Gu, K. He, K. Wood, L. Zeng, Q. Ye, J. MacDougal, J. L. McLaughlin, Biorg. Med. Chem. 1996, 4, 1281.
- 8 Independently, Koert and co-workers recently adopted a similar disconnection strategy in their synthesis of the related compounds Muricatetrocin A and B and Mucocin.
- 8a S. Bäurle, U. Peters, T. Friedrich, U. Koert, Eur. J. Org. Chem. 2000, 2207;
- 8b
S. Bäurle, S. Hoppen, U. Koert, Angew. Chem. 1999, 111, 1341; Angew. Chem. Int. Ed. 1999, 38, 1263.
10.1002/(SICI)1521-3757(19990503)111:9<1341::AID-ANGE1341>3.0.CO;2-J Google Scholar
- 9a D. J. Dixon, A. C. Foster, S. V. Ley, D. J. Reynolds, J. Chem. Soc. Perkin Trans. 1 1999, 1631.
- 9b D. J. Dixon, A. C. Foster, S. V. Ley, D. J. Reynolds, J. Chem. Soc. Perkin Trans. 1 1999, 1635.
- 10a M. F. Buffet, D. J. Dixon, S. V. Ley, E. W. Tate, Synlett 1998, 1091;
- 10b M. Chorghade, D. J. Dixon, S. V. Ley, D. J. Reynolds, Synth. Commun. 2000, 30, 1955.
- 11 E. J. Corey, P. L. Fuchs, Tetrahedron Lett. 1972, 3769.
- 12 The various applications of this useful reagent are reviewed by A. S. Thompson in Encyclopedia of Reagents for Organic Synthesis, Vol. 3 ( ), Wiley, New York, 1995, pp. 1957–1960. For a discussion of the reaction mechanism see J.-E. Backväll, M. Sellén, J. Chem. Soc. Chem. Commun. 1987, 827.
- 13 Geometries were obtained in Macromodel using MM2 forcefield. Calculations of eigenvalues carried out in CADPAC using 3-21++G basis set. Calculations kindly carried out by A. G. Leach.
- 14 M. W. Rathke, D. Sullivan, Tetrahedron Lett. 1972, 4249.
- 15 Y. Ito, Y. Kobayashi, T. Kawabata, M. Takosa, S. Terashima, Tetrahedron 1980, 36, 557.
- 16 P. E. Sonnet, Tetrahedron 1980, 36, 557, and references therein.
- 17 E. R. Møller, K. A. Jørgensen, J. Org. Chem. 1996, 61 5770, and references therein.
- 18 T. R. Hoye, P. R. Hanson, L. E. Hasenwinkel, A Ramiraez, Z. Zhaung, Tetrahedron Lett. 1994, 35, 8525.
- 19a M. Nitta, T. Kobayashi, J. Chem. Soc. Perkin Trans. 1 1985, 1401.
- 19b S. Cicchi, A. Goti, A. Brondi, A. Guarna, De Sarlo, Tetrahedron Lett. 1990, 31, 3351.
- 20 K. Takai, K. Nitta, K. Utimoto, J. Am. Chem. Soc. 1986, 108, 7408.
- 21 S. V. Ley, J. Norman, W. P. Griffith, S. P. Marsden, Synthesis 1994, 639.
- 22a D. B. Dess, J. C. Martin, J. Org. Chem. 1983, 48, 4155;
- 22b D. B. Dess, J. C. Martin, J. Am. Chem. Soc. 1991, 113, 7277.
- 23 For preparation of the reagent see: R. T. Lewis, W. B. Motherwell, Tetrahedron 1992, 48, 1465; for a review see: F. Eymery, B. Iorga, P. Savignac, Synthesis 2000, 185.
- 24a T. R. Hoye, P. R. Hanson, A. C. Kovelesky, T. D. Ocain, Z. P. Zhuang, J. Am. Chem. Soc. 1991, 113, 9369;
- 24b K. Sonogashira, Y. Thoda, N. Magihara, Tetrahedron Lett. 1975, 12, 4467.
- 25
Spectroscopic data for synthetic 1, a white amorphous solid: M.p. 65–67 °C (lit. [7] 65–66 °C); [α]
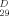 =+5.8 (c=0.38 in CH2Cl2, lit. [7] +6.3 in CH2Cl2); 1H NMR (600 MHz; CDCl3): δ=7.18 (d, 1 H, J=1.1 Hz; H-33), 5.06 (qd, 1 H, J=6.8, 1.1 Hz; H-34), 3.89–3.87 (m, 1 H, H-12), 3.87–3.83 (m, 1 H, H-4), 3.82 (q, 1 H, J=7.2 Hz, H-15), 3.63–3.60 (m, 2 H; H-19, H-20), 3.47–3.43 (m, 1 H; H-16), 2.90 (s (br), 2 H; OH), 2.53 (dt, 1 H, J=15.1, 1.6 Hz; H-3), 2.40 (dd, 1 H, J=15.1, 8.3 Hz; H-3), 2.04–2.01 (m, 1 H; H-13), 2.00–1.97 (m, 1 H; H-14), 1.70–1.40 (m, 6 H; H2-21, H2-18, H2-17), 1.60–1.40 (m, 3 H; H-13, H2-11), 1.62–1.55 (m, 1 H; H-14) 1.50–1.40 (m, 2 H; H2-5), 1.43 (d, 3 H, J=6.8 Hz; H3-35), 1.40–1.20 (m, 32 H; H2-6→H2-10, H2-22→H2-31, 2×OH), 0.88 (t, 3 H; J=6.9 Hz, H3-32); 13C NMR (150 MHz; CDCl3): δ=174.5 (C-1), 151.7 (C-33), 131.2 (C-2), 81.7 (C-15), 79.3 (C-12), 77.9 (C-34), 74.7, 74.4 (C-20, C-19), 74.3 (C-16), 70.0 (C-4), 37.4 (C-5), 33.5–22.7 (C-6→C-11, C-17, C-18, C-21→C-31), 33.4 (C-3), 32.4 (C-13), 28.4 (C-14), 19.1 (C-35), 14.1 (C-32); IR (CDCl3):
=+5.8 (c=0.38 in CH2Cl2, lit. [7] +6.3 in CH2Cl2); 1H NMR (600 MHz; CDCl3): δ=7.18 (d, 1 H, J=1.1 Hz; H-33), 5.06 (qd, 1 H, J=6.8, 1.1 Hz; H-34), 3.89–3.87 (m, 1 H, H-12), 3.87–3.83 (m, 1 H, H-4), 3.82 (q, 1 H, J=7.2 Hz, H-15), 3.63–3.60 (m, 2 H; H-19, H-20), 3.47–3.43 (m, 1 H; H-16), 2.90 (s (br), 2 H; OH), 2.53 (dt, 1 H, J=15.1, 1.6 Hz; H-3), 2.40 (dd, 1 H, J=15.1, 8.3 Hz; H-3), 2.04–2.01 (m, 1 H; H-13), 2.00–1.97 (m, 1 H; H-14), 1.70–1.40 (m, 6 H; H2-21, H2-18, H2-17), 1.60–1.40 (m, 3 H; H-13, H2-11), 1.62–1.55 (m, 1 H; H-14) 1.50–1.40 (m, 2 H; H2-5), 1.43 (d, 3 H, J=6.8 Hz; H3-35), 1.40–1.20 (m, 32 H; H2-6→H2-10, H2-22→H2-31, 2×OH), 0.88 (t, 3 H; J=6.9 Hz, H3-32); 13C NMR (150 MHz; CDCl3): δ=174.5 (C-1), 151.7 (C-33), 131.2 (C-2), 81.7 (C-15), 79.3 (C-12), 77.9 (C-34), 74.7, 74.4 (C-20, C-19), 74.3 (C-16), 70.0 (C-4), 37.4 (C-5), 33.5–22.7 (C-6→C-11, C-17, C-18, C-21→C-31), 33.4 (C-3), 32.4 (C-13), 28.4 (C-14), 19.1 (C-35), 14.1 (C-32); IR (CDCl3):  max=3422 (br OH), 2928, 2855 (C−H), 1733 (C=O) and 1675 (C=C) cm−1; HRMS [M+Na]+ found: m/z: 619.4522, C35H64O7Na required: m/z: 619.4544.
max=3422 (br OH), 2928, 2855 (C−H), 1733 (C=O) and 1675 (C=C) cm−1; HRMS [M+Na]+ found: m/z: 619.4522, C35H64O7Na required: m/z: 619.4544.



39:20<>1.0.co;2-v.cover.gif)
