Structural Characterization of a Cyclohexameric meta-Phenyleneethynylene Made by Alkyne Metathesis with In Situ Catalysts
Ping-Hua Ge Dr.
Department of Chemistry and Biochemistry The University of South Carolina Columbia, SC 29208 (USA) Fax: (+1) 803-777-9521
Search for more papers by this authorWei Fu
Department of Chemistry and Biochemistry The University of South Carolina Columbia, SC 29208 (USA) Fax: (+1) 803-777-9521
Search for more papers by this authorWolfgang A. Herrmann Prof.
Institut für Anorganische Chemie Technische Universität München Lichtenbergstrasse 4, 85747 Garching (Germany)
Search for more papers by this authorEberhardt Herdtweck Dr.
Institut für Anorganische Chemie Technische Universität München Lichtenbergstrasse 4, 85747 Garching (Germany)
Search for more papers by this authorCharles Campana Dr.
Bruker AXS, Inc. 5465 East Cheryl Parkway, Madison, WI 53711-5373 (USA)
Search for more papers by this authorRichard D. Adams Prof.
Department of Chemistry and Biochemistry The University of South Carolina Columbia, SC 29208 (USA) Fax: (+1) 803-777-9521
Search for more papers by this authorUwe H. F. Bunz Prof.
Department of Chemistry and Biochemistry The University of South Carolina Columbia, SC 29208 (USA) Fax: (+1) 803-777-9521
Search for more papers by this authorPing-Hua Ge Dr.
Department of Chemistry and Biochemistry The University of South Carolina Columbia, SC 29208 (USA) Fax: (+1) 803-777-9521
Search for more papers by this authorWei Fu
Department of Chemistry and Biochemistry The University of South Carolina Columbia, SC 29208 (USA) Fax: (+1) 803-777-9521
Search for more papers by this authorWolfgang A. Herrmann Prof.
Institut für Anorganische Chemie Technische Universität München Lichtenbergstrasse 4, 85747 Garching (Germany)
Search for more papers by this authorEberhardt Herdtweck Dr.
Institut für Anorganische Chemie Technische Universität München Lichtenbergstrasse 4, 85747 Garching (Germany)
Search for more papers by this authorCharles Campana Dr.
Bruker AXS, Inc. 5465 East Cheryl Parkway, Madison, WI 53711-5373 (USA)
Search for more papers by this authorRichard D. Adams Prof.
Department of Chemistry and Biochemistry The University of South Carolina Columbia, SC 29208 (USA) Fax: (+1) 803-777-9521
Search for more papers by this authorUwe H. F. Bunz Prof.
Department of Chemistry and Biochemistry The University of South Carolina Columbia, SC 29208 (USA) Fax: (+1) 803-777-9521
Search for more papers by this authorU.H.F.B. and P.-H.G. thank the National Science Foundation (CHE 9814118), the Research Corporation, and the EPSCoR Office of the State of South Carolina for generous support. R.D.A. thanks the Alexander von Humboldt Foundation for an award which supported this work.
Abstract
New hexameric cyclophenyleneethynylenes (CPEs, 1) were synthesized by alkyne metathesis of meta-dipropynylated benzenes. Treatment of one of these CPE derivatives (R1=tert-butyl, hexyl, dodecyl; R2=H, methyl) with [Os3(CO)10(MeCN)2] led to a decacarbonyltriosmium–CPE complex in which the planarity of the ring was significantly disrupted, as evidenced by the results of the single-crystal X-ray diffraction analysis.
References
- 1 For excellent reviews see
- 1a J. S. Moore, Acc. Chem. Res. 1997, 30, 402;
- 1b M. M. Haley, J. J. Pak, S. C. Brand, Top. Curr. Chem. 1999, 201, 81.
- 2a
Y. Tobe, N. Utsumi, A. Nagano, K. Naemura, Angew. Chem. 1998, 110, 1347; Angew. Chem. Int. Ed. 1998, 37, 1285.
10.1002/(SICI)1521-3757(19980504)110:9<1347::AID-ANGE1347>3.0.CO;2-L Google Scholar
- 2b
For a strained m-CPE with four phenyleneethynylenes units and its X-ray crystal structure see T. Kawase, N. Ueda, H. R. Darabi, M. Oda, Angew. Chem. 1996, 108, 1658; Angew. Chem. Int. Ed. Engl. 1996, 35, 1556.
10.1002/ange.19961081336 Google Scholar
- 3a O. Y. Mindyuk, M. R. Stetzer, D. Gidalevitz, P. A. Heiney, J. C. Nelson, J. S. Moore, Langmuir 1999, 15, 6897, and references therein;
- 3b J. K. Young, J. C. Nelson, J. S. Moore, J. Am. Chem. Soc. 1994, 116, 10 841;
- 3c J. S. Zhang, D. J. Pesak, J. L. Ludewick, J. S. Moore, J. Am. Chem. Soc. 1994, 116, 4227;
- 3d
O. Y. Mindyuk, M. R. Stetzer, P. A. Heiney, J. C. Nelson, J. S. Moore, Adv. Mater. 1998, 10, 1363.
10.1002/(SICI)1521-4095(199811)10:16<1363::AID-ADMA1363>3.0.CO;2-V CAS Web of Science® Google Scholar
- 4 D. Venkataraman, S. Lee, J. S. Zhang, J. S. Moore, Nature 1994, 371, 591.
- 5
Höger has recently published the structure determination of a related type of ring: S. Höger, V. Enkelmann, Angew. Chem. 1995, 107, 2917; Angew. Chem. Int. Ed. Engl. 1995, 34, 2713.
10.1002/ange.19951072331 Google Scholar
- 6 H. Staab, K. Neunhöffer, Synthesis 1974, 424.
- 7a K. Weiss (Bayreuth) first reported the formation of the macrocycle 2 b in alkyne metathesis mixtures of 1 b by using [(tBuO)3W≡CCMe3][9b];
- 7b K. Weiss, A. Michel, unpublished results, Bayreuth, 1997;
- 7c
K. Weiss, A. Michel, E. M. Auth, U. H. F. Bunz, T. Mangel, K. Müllen, Angew. Chem. 1997, 109, 522; Angew. Chem. Int. Ed. Engl. 1997, 36, 506.
10.1002/ange.19971090517 Google Scholar
- 8
A. Fürstner, O. Guth, A. Rumbo, G. Seidel, J. Am. Chem. Soc. 1999, 121, 11 108;
A. Fürstner, C. Mathes, C. W. Lehmann, J. Am. Chem. Soc. 1999, 121, 9453;
A. Fürstner, G. Seidel, Angew. Chem. 1998, 110, 1758; Angew. Chem. Int. Ed. 1998, 37, 1734.
10.1002/(SICI)1521-3757(19980619)110:12<1758::AID-ANGE1758>3.0.CO;2-I Google Scholar
- 9a L. Kloppenburg, D. Song, U. H. F. Bunz, J. Am. Chem. Soc. 1998, 120, 7973;
- 9b U. H. F. Bunz, L. Kloppenburg, Angew. Chem. 1999, 111, 506; Angew. Chem. Int. Ed. 1999, 38, 478.
- 10 L. Kloppenburg, D. Jones, U. H. F. Bunz, Macromolecules 1999, 32, 4194.
- 11 N. G. Pschirer, W. Fu, R. D. Adams, U. H. F. Bunz, Chem. Commun. 2000, 87.
- 12 T. Mangel, A. Eberhardt, U. Scherf, U. H. F. Bunz, K. Müllen, Macromol. Rapid Commun. 1995, 16, 571. The authors reported the synthesis of 3 b by Pd-catalyzed coupling of the Heck-type.
- 13a
X-ray structure analysis for 2 a: Suitable single crystals for the X-ray diffraction study were grown from n-hexane. C72H72: (937.30): monoclinic P21/a; a=11.3607(3), b=23.5383(6), c=14.1410(3) Å, β=106.661(1)°. V=3622.7(2) Å3, Z=2, ρcalcd=0.859 g cm−3; μ=0.048 mm−1. A clear colorless plate of 2 a (0.5 mm×0.4 mm×0.08 mm) was stored under perfluorinated ether, transferred in a Lindemann capillary, fixed, and sealed. Preliminary examination and data collection were carried out on a Kappa CCD area detector (Nonius; MACH3) equipped with a rotating anode and graphite-monochromated MoKα radiation (Nonius; FR951, λ=0.71073 Å, 2Θmax=50°). The unit cell parameters were obtained by full-matrix least-squares refinements of 11 971 reflections. Data collection was performed at 123 K (exposure time: 100 s per frame; 9 sets, θ and ω scans, Δθ/Δω: 1°; dx: 40.0 mm). A total of 12 551 reflections were collected. Raw data were corrected for Lorentz and polarization effects, not corrected for absorption and decay effects, and scaled with the program DENZO-SMN. After merging (Rint=0.0341), 6366 independent reflections remained and were used for all calculations. All “heavy atoms” of the asymmetric unit were refined anisotropically. All hydrogen atoms were located in the difference Fourier map and refined with individual isotropic temperature parameters. Full-matrix least-squares refinements were carried out by minimizing Σw(F
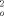 −F
−F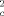 )2 by using the SHELXL-97 weighting scheme and stopped at shift/err<0.001 with R1(all data)=0.0829, Rw2(all data)=0.1587, and GOF 1.050. Neutral atom scattering factors for all atoms and anomalous dispersion corrections for the non-hydrogen atoms were taken from International Tables for Crystallograpy. All calculations were performed on a DEC 3000 AXP workstation and an Intel Pentium II PC, with the STRUX-V system, including the programs PLATON, SHELXS-86, and SHELXL-97. One solvent molecule (C6H14) is highly disordered in the crystal and could not be located and refined correctly. The problem was solved by using the SQUEZZE strategy (A. L. SPEK, PLATON, 1999);
)2 by using the SHELXL-97 weighting scheme and stopped at shift/err<0.001 with R1(all data)=0.0829, Rw2(all data)=0.1587, and GOF 1.050. Neutral atom scattering factors for all atoms and anomalous dispersion corrections for the non-hydrogen atoms were taken from International Tables for Crystallograpy. All calculations were performed on a DEC 3000 AXP workstation and an Intel Pentium II PC, with the STRUX-V system, including the programs PLATON, SHELXS-86, and SHELXL-97. One solvent molecule (C6H14) is highly disordered in the crystal and could not be located and refined correctly. The problem was solved by using the SQUEZZE strategy (A. L. SPEK, PLATON, 1999);
- 13b X-ray structure analysis for 4 a: A cube of 4 a (0.05 mm×0.04 mm×0.05 mm) was grown from dichloromethane. Data were obtained on a Bruker Smart 1000 CCD diffractometer at 298 K. The structure was solved and refined with the programs SHELXS86 and SHELX97 and by using heavy-atom (Patterson) methods. Hydrogen atoms were localized and refined in the riding mode. The crystal was mounted on a glass fiber. Os3O30C82H72 (Mr=1788.00); MoKα radiation; λ=0.71073 Å, graphite monochromator; 2Θmax=22.50°, monoclinic C2/c; a=54.641(5), b=11.7486(10), c=33.250(3) Å, β=120.447(3)°, V=18 401(3) Å3, Z=8, ρcalcd=1.291 g cm−3, μ=41.79 mm−1, absorption correction: SADABS; 12 021 reflections were measured and all reflections with (I=2σ(I)) observed, R=0.0747, Rw=0.1710. Crystallographic data (excluding structure factors) for the structures reported in this paper have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication nos. CCDC-143392 (2 a) and CCDC-143393 (4 a). Copies of the data can be obtained free of charge on application to CCDC, 12 Union Road, Cambridge CB2 1EZ, UK (fax: (+44) 1223-336-033; e-mail: [email protected]).
- 14 The C−C triple bond length in diphenylacetylene is 1.198 Å. A. Mavridis, I. Moustakali-Mavridis, Acta Crystallogr. Sect. B 1977, 33, 3612.
- 15 U. H. F. Bunz, V. Enkelmann, Chem. Eur. J. 1999, 5, 263.
- 16
A. J. Berresheim, M. Müller, K. Müllen, Chem. Rev. 1999, 99, 1747;
M. Müller, C. Kübel, K. Müllen, Chem. Eur. J. 1998, 4, 2099;
10.1002/(SICI)1521-3765(19981102)4:11<2099::AID-CHEM2099>3.0.CO;2-T CAS Web of Science® Google ScholarR. Goddard, M. W. Haenel, W. C. Herndon, C. Krüger, M. Zander, J. Am. Chem. Soc. 1995, 117, 30.
- 17 X. Qiao, M. A. Padula, D. M. Ho, N. J. Vogelaar, C. E. Schutt, R. A. Pascal, Jr., J. Am. Chem. Soc. 1996, 118, 741.



39:20<>1.0.co;2-v.cover.gif)
