Effects of noise and low-concentration carbon monoxide exposure on rat immunity
Xiaojun She, Xiujie Gao, and Kun Wang are contributed equally to this work.
Abstract
Objective
To evaluate the immunotoxicity and effects of noise and/or low-concentration carbon monoxide (CO) exposure on immune organs and immune functions in rats.
Methods
Male Wistar rats exposed to 98 dB(A) white noise and/or 100 ppm CO 4 h/d for 30 d were used to determine the pathological changes in the thymus and spleen, and variations in leukocyte counts, inflammatory factors, and immunoglobulin (Ig) concentrations.
Results
The boundaries of the cortex and medulla of the thymus were unclear following noise and combined exposure. The pathological changes in spleen after CO and combined exposure included blurred boundaries of red-pulp and white-pulp, disappearance of normal splenic nodules and neutrophil infiltration. After exposure to noise and in combination, leukocyte and lymphocyte counts decreased significantly. After exposure to low-concentration CO and in combination, serum IgM and IgG levels decreased significantly, but the levels of tumor necrosis factor-α and interferon-γ levels increased significantly. Eosinophils and IgA levels decreased significantly following exposure to noise and/or low concentration of CO, while the level of interleukin-1 increased significantly. Monocytes increased significantly only under noise or CO exposure, but not under combined exposure.
Conclusions
Noise and/or low-concentration CO exposure may suppress innate and adaptive immune functions and induce inflammatory responses. Noise exposure mainly affected the innate immune function of rats, whereas low-concentration CO exposure mainly affected adaptive immune functions. Combined exposure presented higher immunotoxicity than noise or CO alone, suggesting that exposure to noise and low-concentration CO in the living and working environments can affect the immune system.
1 INTRODUCTION
Noise and a low concentration of carbon monoxide (CO) are common hazards in daily and occupational environments; they often exist simultaneously in barbecue shops, metallurgy workshops, some working environments requiring diesel engines, etc. It has been reported that traffic police on duty on the road are exposed to noise up to 71.63-88.51 dB(A) and an average CO concentration of 1.5-7.7 ppm for 8 h.1 Studies have shown that approximately half of all enterprises have both noise and low-concentration CO pollution in their production environments, with noise and CO ranking first and second, respectively, among hazard factors.2 In addition, firemen, fishermen, and forestry workers are also groups that are constantly exposed to noise and low-concentration CO for a long time. As noise and low-concentration CO are common environmental pollutants, studies should be performed to examine their influence on the body.
The immune system is not only the most effective defense against the invasion of pathogens but also participates in regulating the functions of various cells, organs, and systems in the body. A study comparing the effects of noise exposure for 3 days and 4 weeks on the immune function of mice showed that 3 days noise exposure enhanced the proliferation ability of lymphocytes and increased the concentration of serum immunoglobulin (Ig) M (IgM); 4 weeks noise exposure inhibited adaptive immunity.3 Acute exposure to low CO concentration can stimulate the generation of granulocytes in the bone marrow and increase immunity,4 and subchronic exposure inhibits innate immune function in the body.5 Not only low CO concentration but also high CO concentration has immunotoxicity to human and experimental animals.6 The impact of noise on the immune system of the body is not only related to the exposure dose but also to species7 and individual sensitivity.8 The immune function of C57BL6 mice was inhibited upon noise exposure, while BALB/c mice showed no significant changes.7 Therefore, the effects of noise and CO on immune function share some similarities but may differ given that sound is a physical factor and CO is a chemical factor.
Several studies on animals and humans have shown that noise or CO have adverse effects on immune function. However, those conclusions are based on research on just one of the two factors; therefore, the combined effects and severity of noise and CO on the immune system or the target organ have never been directly inferred. Thymus and spleen are important organs of the immune system as they are the sites of the immune response.9, 10 Leukocytes, including granulocytes, monocytes, and lymphocytes, are the first line of defense against pathogen invasion.11 Igs and cytokines are immunoactive substances that regulate the immune response.12 Therefore, we focused on the effects of subchronic noise and/or low-concentration CO exposure on the thymus, spleen, leukocytes, Igs, and pro-inflammatory factors.
2 MATERIALS AND METHODS
2.1 Animals
A total of 40 healthy male Wistar rats with a body mass of 200-220 g were provided by Beijing Vital River Laboratory Animal Technology Co., Ltd. [license number – SCXK (Beijing)-2016-0006]. The rats were maintained in the animal room of the Tianjin Institute of Environmental and Operational Medicine. They had free access to food and water and were subjected to light (8:00-20:00)/dark (20:00-8:00) cycles for 12 h. The temperature was 25 ± 2℃ and humidity was 50% ± 5%. After 3 d of adaptive feeding, the rats were randomly divided into the following groups: control group, noise group, CO group, and co-exposure group, with 10 rats per group. Operations involving the use and care of animals were approved by the Laboratory Animal Ethics Committee of the Institute of Environmental and Occupational Medicine.
2.2 Conditions of noise and CO exposure
Noise exposure: White noise was generated by a noise generator (BK 3560C, B&K Instruments) and transmitted to the ears of the rats by connecting the power amplifier and speaker (500-6000 Hz). The conical loudspeaker (YH 50-10B, Tianjin Zhenmei Electroacoustic Equipment Co., Ltd.) was suspended 30 cm above the rats' heads. Rats in groups noise and co-exposure were placed in rectangular wire cages and arranged in a circle. The left and right ears of rats were exposed to noise with an intensity of 98 ± 1.0 dB(A), which were monitored every hour with a sound level meter (BSWA 308, BSWA Technology Co., Ltd.). The rats were exposed to the noise for 4 h/d (14:00-18:00) for 30 consecutive days. Meanwhile, rats in the control and CO groups were placed in an environment with background noise lower than 40 dB(A) (alone in a rectangular wire cage).
CO exposure: CO gas used was generated by mixing 20% CO gas with air in an air mixing tank. When the CO concentration reached 100 ppm, the mixed gas was imported into the self-made dynamic gas poisoning chamber at a flow rate of 50 mL/min. Exposure began when the CO concentration reached 100 ppm in the chamber after approximately 10 min. The 20% CO gas used was directly obtained from CO gas cylinders (Tianjin Saimeite Special Gas Co., Ltd.). Air in the air mixing tank was from an air compressor (4 × 550W –120 L, Outstanding Air Compressor Co., Ltd.). Rats in the CO and co-exposure groups were placed separately in rectangular wire cages, and then placed into the dynamic gas poisoning chamber. The environment in the dynamic gas poisoning chamber was controlled as follows: CO concentration 100 ± 5 ppm, oxygen concentration 20% ± 1%, temperature 25 ± 2℃, and humidity 50% ± 10%. During exposure, a multi-functional gas detector (BH-4S, Baoshi Precision Instrument Co., Ltd.) was used to monitor the CO concentration, oxygen concentration, temperature, and humidity in real-time. The CO exposure time was 4 h/d (8:00-12:00) for 30 d. Meanwhile, rats in the control and noise groups were placed in a clean air environment in individual rectangular wire cages.
2.3 Blood sample preparation
After 30 d of treatment with noise/CO, the rats were anesthetized by intraperitoneal injection of sodium pentobarbital (50 mg/kg body weight) and blood samples were collected from their abdominal aorta. The blood samples were placed in a vacuum vessel for testing, along with EDTA as an anticoagulant. A portion of the blood samples was placed in a vacuum vessel without the anticoagulant. The samples were placed at room temperature for 90 min and centrifuged at 4℃ at 1000 g for 10 min, after which the serum was collected and stored at −80℃ until testing.
2.4 Leukocyte counting
Blood samples with EDTA (anticoagulant) were used to count leukocytes using an automatic blood cell analyzer (BC-5100, MINDRAY Biological Medical Electronic Co., Ltd.). Calibration, quality control, and maintenance of the blood cell analyzer were carried out according to the quality control requirements of the blood analyzer (ISO 15189). Quality control testing was performed twice per day to ensure data reliability.
2.5 Determination of concentrations of IgA, IgM, IgG, tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), and interferon-γ (IFN-γ)
The concentrations of IgA, IgM, and IgG in the serum samples were determined using an IgA, IgM, and IgG ELISA kit, respectively (Abcam). Serum concentrations of TNF-α, IL-1, and IFN-γ were also determined using an TNF-α, IL-1, and IFN-γ ELISA kit, respectively (AngleGene). The procedures were performed according to the kit instructions. Absorbance was measured at 450 nm using a microplate reader (SpectraMax M5, Molecular Devices).
2.6 Pathological observation of thymus and spleen
The thymus and spleen were separated; part of the thymus and spleen were immediately placed in 4% neutral formaldehyde solution for 24 h for fixation. Fixed tissues were paraffin embedded and cut into 5-µm continuous sections. The tissue sections were stained according to the protocol for hematoxylin and eosin (HE) staining. The histomorphological changes were observed under an optical microscope (Olympus BX51).
2.7 Statistical analysis
SPSS 22.0 statistical software (SPSS, Inc) was used for statistical analysis. Levene's test was used for homogeneity of variance. One-way analysis of variance was used to compare groups. The least significant difference (LSD) method was used for statistical testing when the variance was homogeneous, whereas Dunnett's method was used for statistical testing when the variance was heterogeneous. Data were expressed as the mean ± standard deviation. P < .05 was considered to indicate statistical significance.
3 RESULTS
3.1 Effects of noise and/or CO exposure on pathological structure of thymus and spleen
The thymus is an important central immune organ composed of the cortex and medulla. There are many lymphocytes and epithelial reticular cells in the cortex. The medulla contains many reticular cells, some macrophages and lymphocytes. As shown in Figure 1A, the thymic tissues of the control and CO groups showed an overall normal structure, with well-defined cortex and medulla, reticular cells, and lymphocytes visible; the boundary of the cortex and medulla of the thymus in the noise group was not clearly demarcated; and the boundary of the cortex and medulla of the thymus tissue in the co-exposure group was unclear and showed arterial congestion and dilation.
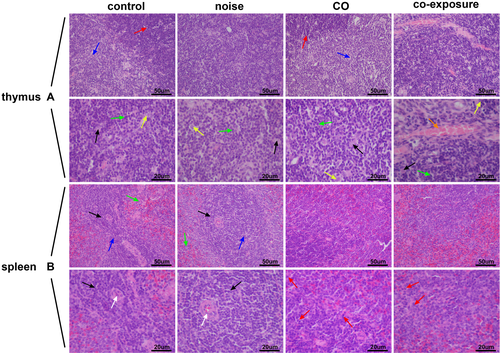
The spleen is the largest peripheral immune organ in the body. It is composed of the white pulp, red pulp, and marginal area. As shown in Figure 1B, in the control group, the splenic tissue morphology and structure were normal, the splenic nodules had clear structures, the boundary of the red- and white-pulp were clear, there was no obvious necrosis, and the splenic nodules did not expand or shrink significantly; there was no obvious damage to the structure of the spleen tissue in the noise group, with clear boundary between the red- and white-pulp and the morphology was almost normal; and the boundary between the red- and white-pulp of the CO and co-exposure groups was fuzzy, the normal splenic nodules disappeared, and neutrophil infiltration was observed in the tissues.
3.2 Effects of noise and/or CO exposure on serum leukocytes
Effective immune protection requires rapid recruitment of leukocytes to infected regions and the sites of surgery; thus, the leukocyte count in circulation is a common indicator of the body's immune and inflammatory status. The number of leukocytes and lymphocytes in the noise and co-exposure groups was reduced significantly (P < .05), whereas those in the CO group did not change compared to the control group (Figure 2A,B). Neutrophils in the noise group were reduced significantly (P < .001) compared to the control group, whereas neutrophils were reduced significantly in the co-exposure group (P < .05) compared to in the CO group (Figure 2C). Further, eosinophils in the noise, CO, and co-exposure groups were decreased significantly (P < .05, P < .01, P < .01, respectively) compared to the control group (Figure 2D). Basophils in the noise, CO, and co-exposure groups showed no significant changes (P > .05) compared to the control group (Figure 2E). Monocytes were increased significantly in the noise and CO groups (P < .05, P < .01), whereas those in the co-exposure group showed no change (P > .05) compared to the control group (Figure 2F).
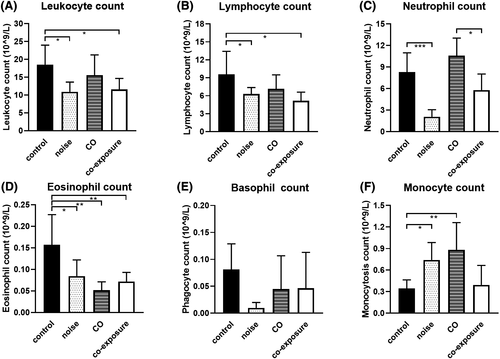
The results showed that the number of leukocytes, neutrophils, and lymphocytes was decreased, whereas the number of monocytes was increased after noise exposure. CO exposure resulted in a decrease in the number of eosinophils and increase in the number of monocytes. Co-exposure reduced the number of leukocytes, eosinophils, and lymphocytes in rats, indicating that both noise and CO exposure individually cause abnormalities in the leukocyte system in rats, with noise exposure showing stronger effects.
3.3 Effects of noise and/or CO exposure on humoral immunity
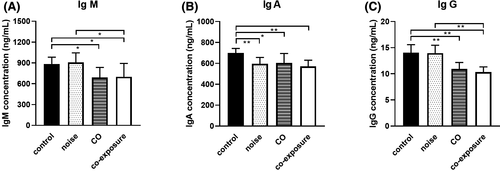
Determination of serum IgM, IgA, and IgG levels is the most common method for examining humoral immune function. IgM levels in the CO and co-exposure groups were significantly lower (P < .05) compared to the control group (Figure 3A). IgA levels in the noise, CO, and co-exposure groups were significantly lower (P < .01, P < .05, P < .01, respectively) than those in the control group (Figure 3B). The concentration of IgG in the CO and co-exposure groups was significantly lower (P < .01) compared to that in the control group. IgM and IgG concentrations in the co-exposure group were significantly reduced (P < .05, P < .01, respectively) compared to those in the noise group (Figure 3A,C). These results indicate that noise and CO exposure inhibited humoral immune function in rats, with co-exposure showing a greater effect.
3.4 Effects of noise and/or CO exposure on inflammatory factors
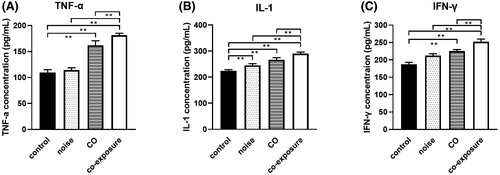
The concentrations of TNF-α and IFN-γ in the CO and co-exposure groups were significantly higher (P < .01) than those in the control group (Figure 4A,C). The concentrations of IL-1 in the noise, CO, and co-exposure groups were increased significantly (P < .01) compared to the control group (Figure 4B). The concentrations of TNF-α, IL-1, and IFN-γ in the co-exposure group were higher than those in the noise and CO groups (P < .01) (Figure 4A–C). These results show that noise and CO can increase the expression of inflammatory factors, indicating that both factors increase the cellular immune function of rats and promote the inflammatory response in the body; however, co-exposure induced a stronger inflammatory response.
4 DISCUSSION
Noise and a low concentration of CO are unavoidable environmental pollutants in modern society, so it is necessary to study their effects on the organisms. Conclusions about the effects of low concentrations of CO on the body are often drawn from studies on air pollution, cigarette smoke, or combustion smoke; therefore, it is difficult to avoid the influence of other harmful substances. At present, there are limited data on the combined toxicity of noise and low-concentration CO, especially on their combined immunotoxicity. This study investigated the effects of subchronic noise and/or low-concentration CO exposure on immune organs, and on the innate and adaptive immune function in rats. Our results suggest that there is immunotoxicity associated with subchronic exposure to noise and/or a low concentration of CO, manifested as changes in the structure and function of the thymus and spleen, the number of leukocytes, and in the level of serum Igs and pro-inflammatory cytokines, which may be related to hypothalamic-pituitary-adrenal axis (HPA) and the sympathetic adrenal medulla system (SNS).13, 14
The thymus and spleen are important immune organs in the body; structural and functional changes in the thymus and spleen directly affect the functions of T and B lymphocytes. In this experiment, noise led to structural abnormalities in the thymus, suggesting that noise exposure is involved in reducing the number or damaging the function of T lymphocytes. However, we observed no pathological damage to the spleen following exposure to noise; although structural abnormalities were observed previously.15 This may be related to the characteristics of the noise, exposure time, and rat strain used in each study. A previous study found that mild CO exposure can reduce the number of leukocyte-common antigen cells in the spleen; this may result in T- and B-lymphocyte dysfunction.16 In this experiment, CO cause damage to the spleen structure, leading to the reduction and disappearance of splenic nodules; it also suggests that CO exposure may cause a reduction in the number of B lymphocytes, leading to a decrease in humoral immunity. Co-exposure to noise and CO result in morphological and structural abnormalities of both the thymus and spleen.
Leukocytes in the blood reflect the body's functional state and resistance to perturbations from the outside world.17 Granulocytes play a role in the phagocytosis of allogenic organisms. Neutrophils account for 50-60% of leukocytes and can resist exogenous allogenic organisms by producing enzymes and reactive oxygen species. Monocytes play a major role in defending the host from invading pathogens. They constitute the non-specific immunity of the body.18 Lymphocytes are involved in cellular and humoral immunity. We found that both noise and CO can cause a decrease in the number of immune cells in the body, indicating that a decrease in the ability to phagocytose or kill bacteria, and a decrease in the immune response to infection. A study showed that after 4 h of acute exposure to 100 dB noise, leukocyte count decreased but the phagocytosis of neutrophils increased.19 Another acute noise exposure experiment showed that the ability of neutrophils to release superoxide anions decreased, and the ability of monocytes to secrete IL-1 decreased upon noise exposure.20 In addition, rats exposed to noise for 2 months showed a significant increase in the number of neutrophils, whereas the number of lymphocytes decreased significantly.21 Therefore, both acute and subchronic noise exposure tends to cause change in the number of leukocytes, but there is no consistent result on the change in different leukocyte types. Epidemiological studies have found that CO concentration in the air is correlated with the distribution of leukocytes in the population, decreased total granulocyte count, decreased naïve CD4+ T cell ratio, and increased the B lymphocyte ratio.5 The CO intoxications showed higher leukocytes and neutrophils count, whereas the number of lymphocytes decreased significantly.22 It could be seen that high-concentration and low-concentration CO could have different effects on the leukocyte system, which may be different from the concentration and duration of CO exposure. In this experiment, monocytes in the blood increased upon exposure to either noise or CO, while there was no change upon co-exposure; this may be due to the increased inflammatory response of the body caused by co-exposure, inducing monocytes in the circulation to mobilize to the inflammatory sites of the body and to play a pro-inflammatory or anti-inflammatory role.23 In most studies, noise or CO exposure has inconsistent changes in the distribution and function of leukocyte system, which may be caused by differences in the parameters of noise and CO, different subjects, as well as different amplitude and duration of increase in catecholamine and glucocorticoids in the body.24, 25
Subchronic noise exposure has a certain impact on humoral immune function, manifested as a significant reduction in IgA levels and no significant changes in IgG and IgM. CO exposure and co-exposure to noise and CO have a serious impact on humoral immune function; they can reduce IgA, IgG, and IgM levels significantly. The decrease in humoral immune function due to noise and/or CO exposure is to some extent consistent with the decrease in the number of lymphocytes circulating. The humoral immune function of the rats exposed to CO alone or co-exposed to noise and CO is seriously damaged; this is confirmed by the shrinkage or disappearance of the spleen nodules, indicating that B lymphocytes are more susceptible to environmental factors and stress.
TNF-α, IL-1, and IFN-γ are among the most common pro-inflammatory cytokines and are positively correlated with the severity of inflammation, which may contribute to a variety of diseases. Noise can increase the expression of inflammatory factors, chemokines, and adhesion molecules in the cochlea,26 brain,27 and serum,28 indicating that noise could cause local and systemic inflammatory response. Research on smoking suggests that CO exposure is associated with inflammatory response.29 In this study, pro-inflammatory cytokines increased upon either noise or CO exposure, but were higher upon co-exposure to noise and CO, indicating that noise and/or CO could induce inflammatory response and co-exposure may induce inflammatory response more intensely. Hence, we speculated that long-term exposure to noise and/or CO may cause inflammation-related chronic diseases,30 such as cardiovascular disease, asthma, type 2 diabetes, and cancer.
Noise exposure and high-concentration or low-concentration CO exposure are external stresses that can activate the HPA and SNS, leading to changes in catecholamine and corticosterone levels.14, 15, 31, 32 It has been shown that adrenergic receptor blockers and corticosterone antagonists can be given to inhibit the changes to the distribution of leukocytes in peripheral blood caused by stress,33 hence suggesting that the changes to the distribution of leukocytes in this study may be related to the two systems activated by noise and CO exposure. The effects of HPA on immune function involve cell proliferation, cytokine secretion, and antibody generation. Catecholamines inhibit the proliferation of splenic T lymphocytes34 and inhibit CD8+ T lymphocytes via the β2-AR pathway.35 Therefore, we suggest that the reduction in innate and adaptive immune functions caused by noise and CO exposure may be related to HPA and SNS activation.
Overall, subchronic noise and/or low-concentration CO stimulation causes disorders of the neuroendocrine system, including changes in the number of immune cells in peripheral blood, cytokines, and Ig level, resulting in the impairment of innate and adaptive immunity functions.
5 CONCLUSION
This study confirmed that both innate and adaptive immunity are affected by noise and/or low-concentration CO exposure. Moreover, noise exposure has a greater damage effect on the leukocyte system, whereas CO exposure causes an obvious damage to humoral immunity. Co-exposure of noise and CO is more immune-toxic than exposure to either factor independently. This immunotoxicity can lead to a reduction in the resistance to infection, cause immune disorders, or result in the development of autoimmune diseases.36 However, the signaling pathways involved in this kind of immunotoxicity need to be studied further. Lastly, we think that the immune function of the noise and co-exposed population needs to be further followed upon; the health standards of the workplace or environment also need to be taken into account.
ACKNOWLEDGMENTS
This study is funded by a grant (AWS16J004) and the Natural Science Foundation of China (No. 81102100). We thank Prof. Danfeng Yang for assistance with CO exposure.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
AUTHOR CONTRIBUTIONS
X.S, X.G, and K.W drafted the manuscript; X.S, X.G, and K.W analyzed the data; X.S, H.Y, and K.M performed the research; and B.C and Z.X designed the research. All authors have approved the manuscript to be published.
DISCLOSURE
Approval of the research protocol: N/A. Informed Consent: N/A. Registry and the Registration No. of the study/Trial: N/A. Animal Studies: Operations involving the use and care of animals were approved by the Laboratory Animal Ethics Committee of the Institute of Environmental and Occupational Medicine, Tianjin, China.




