Expression of SPARC/osteonectin/BM4O in the human gut: Predominance in the stroma of the remodeling distal intestine
Abstract
SPARC is a glycoprotein of the extracellular matrix that exhibits a number of biological functions such as disruption of cell adhesion and modulation of matrix metalloprotease expression. These properties, in concert with the expression of the molecule during development, repair, and neoplastic progression, suggest that SPARC has an important role in remodeling in a variety of tissues. However, the role of SPARC in the intestine is unclear since the development expression and tissular origin of SPARC in this organ appears to be species-dependent. As a first step to investigate the function of SPARC in the tissues of the intestine, we have analyzed its expression at the protein and mRNA levels in the human fetal and adult small intestinal and colonic mucosa as well as in intestinal cell models. Our results show that SPARC expression is differentially regulated during development and along the length of the human intestine. In the colon, SPARC was predominantly found at the epithelial–mesenchymal interface at the fetal stage, below detection levels in the normal adult, but re-expressed in the stroma of colonic tumors. In the small intestine, low levels of SPARC expression were observed at an early stage of morphogenesis (between 9 and 11 weeks) but expression was not detected at subsequent developmental stages nor was it induced in the mucosa of Crohn's disease. While SPARC appeared to be produced mainly by mesenchymal and stromal cells in the intact intestine it was not detected in colon cancer cells. Taken together, these results indicate that SPARC is subject to an onco-fetal pattern of expression in the stroma of the colonic mucosa while its expression is much more restricted in the small intestine, suggesting a differential involvement of this molecule in the extracellular matrix remodeling occurring along the length of the developing and diseased human intestinal mucosa. J. Cell. Biochem. 81:463–476, 2001. © 2001 Wiley-Liss, Inc.
Secreted protein, acidic and rich in cysteine (SPARC), also termed as osteonectin or BM4O, is a well characterized calcium-binding extra-cellular matrix (ECM) glycoprotein exhibiting counteradhesive effects that is secreted by many different cell types [Sodek, 1993; Lane and Sage, 1994; Yan and Sage, 1999]. Indeed, while initially identified as one of the major noncollagenous protein in bone matrix [Termine et al., 1981], SPARC mRNA and protein were detected in a number of non-mineralized developing tissues in the mouse [Holland et al., 1987, Sage et al., 1989a, 1989b] pig [Tung et al., 1985; Domenicucci et al., 1988; Maillard et al., 1992], and human [Metsaranta et al., 1989; Mundlos et al., 1992; Porter et al., 1995]. Encoded by a single gene, SPARC has been highly conserved among species from C. elegans to primates [Lane and Sage, 1994]. The analysis of the modular structure of this 43 kD glycoprotein revealed three distinct domains on which a number of biological activities, such as disruption of cell adhesion, regulation of cell proliferation and induction of matrix metalloprotease production, have been mapped [Sage, 1997]. These diverse activities appear to take place through specific interactions between SPARC and growth factors, other ECM molecules as well as cell-surface proteins [Lane and Sage, 1994; Yan and Sage, 1999]. Surprisingly, SPARC-null mice were generally found indistinguishable from their wild-type counterparts in viability, fertility, body size and weight [Gilmour et al., 1998; Norose et al., 1998]. The lack of a clear phenotype, which has also been found in tenascin-null mice [Saga et al., 1992], could be the result of a functional compensation by one or more of the four other SPARC-related proteins identified to date [Yan and Sage, 1999].
Similar to other anti-adhesive molecules, such as tenascin and thrombospondin [Sage and Bornstein, 1991; Chiquet-Ehrismann, 1995a], SPARC has raised considerable interest as a potential key factor involved in tissue development [Nomura et al., 1988; Huynh et al., 1999] and remodeling [Salonen et al., 1990]. Accordingly, its spatial and temporal patterns of expression have been analyzed in great detail in different species. Consistent with studies in murine [Holland et al., 1987; Nomura et al., 1988; Sage et al., 1989b], porcine and bovine [Domeniccui et al., 1988, Maillard et al., 1992] tissues, SPARC expression has been detected in several mineralized and non-mineralized human fetal tissues [Metsaranta et al., 1989; Mundlos et al., 1992; Porter et al., 1995]. While SPARC expression is more restricted in adults, it is moderately expressed in steroidogenic and endothelial cells, some mesenchymal derivatives such as chondrocytes and muscle cells, and a subset of epithelial cells such as pancreatic and epidermal cells [Porter et al., 1995; Hunzelmann et al., 1998]. Notably, SPARC is over-expressed in a variety of neoplastic tissues by the tumor cells and by stromal cells of surrounding tumor, suggesting that its expression con-tributes to some aspect of tumor progression [Wewer et al., 1988; Porter et al., 1995]. Overall, these observations support the concept that SPARC is associated with morphogenesis and remodeling in several tissues.
An exception to this seems to be the human intestinal mucosa. Indeed, while identified in both the fetal and adult intestinal mucosa of the mouse [Sage et al., 1989b], as well as in the fetal pig intestine [Maillard et al., 1992], SPARC was reported to be rarely expressed or absent in the fetal human gut even at stages of intense villus and crypt morphogenesis [Mundlos et al., 1992; Porter et al., 1995]. Normal human adult small intestinal and colonic mucosa were also found to be mostly devoid of SPARC [Porte et al., 1995; Porter et al., 1995] as in the rat [Puolakkainen et al., 1999]. On the other hand, both protein and transcripts were found to be over-expressed in colorectal cancers [Wewer et al., 1988; Porte et al., 1995; Porter et al., 1995] but the cell type involved in SPARC production (malignant cells and/or surrounding stromal cells) appears controversial. Furthermore, in the rat, SPARC was not observed in association with the epithelium of the experimentally healing small intestine [Puolakkainen et al., 1999]. Thus, SPARC expression is not clearly associated with the mucosal remodeling that takes place in the intestine during development and in disease. As a first step to investigate the role of SPARC in the physiopathology of the intestine we have systematically analyzed the expression of SPARC protein and mRNA at various stages of development, in normal and diseased adult proximal and distal human intestine and in cell models of intestinal epithelium and mesenchyme. Our results show that SPARC expression in the mucosa is differentially regulated along the length of the human intestine. Indeed, SPARC was predominantly found in the distal intestine according to an onco-fetal pattern of expression. Furthermore, it was not detected in colon cancer cells and appears to be mainly produced by mesenchymal cells in the intact intestine.
METHODS
Tissues
Specimens of small intestine and colon from 16 fetuses ranging in age from 9 to 20 weeks (post-fertilization) were obtained after legal abortion. Seven control samples of adult small intestine (jejunum and ileum; 4 samples) and colon (3 samples) were obtained from non-diseased parts of resected segments (resection margins) for pathologies other than Crohn's disease or colon cancer (bowel obstructions, primary lymphoma, diverticulosis). Eight adult specimens of ileum with Crohn's disease were used in the study. For each patient, samples from both inflamed and uninflamed (resection margin) areas were obtained, as described previously [Bouatrouss et al., 2000]. Samples of adult colon were obtained from nine patients who underwent surgical treatment for colon carcinoma. For all patients, samples were obtained from the primary tumor and from the corresponding non-diseased part (resection margin, at least 10 cm distant from the lesion). Diagnosis of the various pathologies and stag-ing of colon tumors were determined by a pathologist. The project was in accordance with protocols approved by the Institutional Human Research Review Committee for the use of human material. Only tissues obtained in less than 1 h were used in the present study.
In some experiments, fetal small intestinal and colonic epithelia were separated from mesenchyme using Matrisperse (Collaborative Biomedical Products, Becton Dickenson Labware, Mississauga, Ontario, Canada) as described previously [Perreault et al., 1998].
Cell Culture
Three epithelial cell lines, which together recapitulate the entire crypt-villus axis of the fetal intestine [Pageot et al., 2000], were used in this study. The HIEC-6 cell line has been generated from normal fetal human intestine [Perreault and Beaulieu, 1996]. These cells express a number of crypt cell markers but no villus cell markers [Desloges et al., 1998; Perreault and Beaulieu, 1998; Basora et al., 1999) and appear to be unable to differentiate. HIEC-6 cells are thus considered as intestinal stem-like cells [Quaroni and Beaulieu, 1997; Pageot et al., 2000]. The HIEC cells were used at passages 5–10 and were grown as described [Perreault and Beaulieu, 1996]. The tsFHI cell line was derived from fetal human intestinal epithelial cells conditionally immortalized with a temperature-sensitive large T antigen [Quaroni and Beaulieu, 1997]. while tsFHI cells are phenotypically similar to HIEC-6 cells when grown under permissive conditions (31°C), they acquire some morphological and functional characteristics similar to those found in the upper part of the crypt such as a stronger expression of dipeptidylpeptidase IV and the loss of proliferative capacity, under non-permissive conditions (39°C) [Quaroni and Beaulieu, 1997; Tian and Quaroni, 1999]. The tsFHI cells were used at passages 20–30 and grown as described [Quaroni and Beaulieu, 1997]. Finally, the Caco-2/15 cell line, a stable clone of the parent Caco-2 cell line [Pinto et al., 1983], has been characterized elsewhere [Beaulieu and Quaroni, 1991; Vachon and Beaulieu, 1992]. Although of cancerous origin (see below), these cells are unique in that upon confluence they spontaneously undergo a gradual villuslike enterocytic differentiation process, similar to that observed in the epithelium of the intact fetal small and large intestine [Ménard and Beaulieu, 1994; Pageot et al., 2000]. Cells between passages 54 and 72 were cultured in plastic dishes as described [Vachon and Beaulieu, 1992] and analyzed at 0–15 days of postconfluence.
Human intestinal mesenchymal (HIM) cells derived from the fetal intestine were characterized elsewhere [Vachon et al., 1993]. Subconfluent HIM cells were used at passage 3–4. A series of colon carcinoma cell lines were also used. HT29, T84, LS123, HCT116, LoVo, and Colo201 cell lines were grown according to the instructions provided by the ATCC (Rockville, MD) and use at 80–90% confluence as previously described [Basora et al., 1998].
Immunofluorescence Staining
The preparation and Optimum Cutting Temperature embedding compound (OCT; Tissue Tek, Miles Laboratories, Elkart, IN) embedding of specimens for cryo-sectioning and immunostaining were performed as previously described [Beaulieu et al., 1991; Beaulieu, 1992]. Cryosections 2–3 μm thick were cut on a Jung Frigocut 2800N cryostat (Leica Canada Inc., Saint-Laurent, Qué.), spread on silane-coated glass slides then air-dried for 1 h at room temperature before storage at −80°C. Tissue sections were fixed in ethanol (10 min, −20°C). Rabbit anti-porcine SPARC antibodies [Tung et al., 1985; Domenicucci et al., 1988] were diluted 1:100 in PBS (pH 7.4) containing 10% Blotto. FITC-conjugated goat anti-rabbit IgG (Boehringer Mannheim Canada, Laval, Quebec) was used as the secondary antibody at a working dilution of 1:25. Sections were then stained with 0.01% Evan's blue in PBS. Preparations were mounted in glycerol-PBS (9:1) containing 0.1% paraphenylene diamine and viewed with a Reichert Polyvar 2 microscope (Leica, Canada) equipped for epifluorescence. In all cases, no immunofluorescent staining was observed when primary antibodies were omitted or replaced by the appropriate non-immune sera.
Western Blot Analysis
Sodium dodecyl sulfate (SDS)/12% PAGE and Western blotting were performed as described previously [Beaulieu et al., 1989; Vachon and Beaulieu, 1995]. Briefly, proteins from cells in culture were directly solubilized in sample buffer containing 5% β-mercaptoethanol. Separated proteins (100 μg/lane) were transferred onto nitrocellulose (ImmunoSelect, Gibco/BRL, Burlington, Ontario, Canada) and stained with Ponceau red to localize molecular weight markers (44–220 kD range, BioRad, Mississauga, Ontario, Canada). Membranes were blocked overnight at room temperature in PBS (pH 7.4) containing 10% Blotto and incubated with the anti-SPARC antibody diluted 1:200 in the blocking solution. After washing in PBS, membranes were incubated with alkaline phosphatase-conjugated goat anti-rabbit IgG (BioRad, Mississauga, Ontario, Canada), further washed and finally incubated with a chromogenic substrate for alkaline phosphatase detection according to the instructions of the manufacturer.
RT-PCR
Total RNAs were prepared from cell lines using TriZOL (Gibco-BRL), and from tissues according to Clontech's Atlas Total RNA Isolation protocol (Clontech, Palo Alto, CA). The integrity of the RNA was verified by ethidium bromide staining and the quantities were determined spectrophotometrically. The reverse transcriptase Superscript (Gibco-BRL) and 0.5 μg of oligo-(dT)12–18 (Pharmacia) were added to 5 μg of total RNA, as described elsewhere [Beaulieu et al., 1994]. For SPARC, we used the sense primer 5′-TCTCTCTTTAACCCTCCCC-3′ and the antisense primer 5′-GAAAAAGTGGAGTTGGTGAA-3 which span the region of 995 to 1843 [Swaroop et al., 1988] to amplify a band of 848 bp. Primers for S14 amplification were described elsewhere [Basora et al., 1997]. Single stranded cDNA was amplified by Touchdown PCR [Don et al., 1991] in PCR buffer (Pharmacia) containing 0.25 μM of both sense and antisense primers in the presence of 250 μM dNTPs and 2.5 U of Taq (Roche; obtained from Pharmacia) for 20 cycles of denaturation (1 min at 94°C), annealing (1 min from 65°C declining by 1°C every second cycles to 56°C) and extension (2 min at 72°C) in a thermal cycler (Perkin-Elmer DNA Thermal Cycler model 480). SPARC was further amplifled by an additional 12 cycles decreasing to an annealing temperature of 50°C. S14 amplification was used as a control for SPARC to normalize the amounts of input RNA.
Data Presentation and Statistical Analysis
The data presented are from a minimum of three separated experiments. Results were analyzed by Student's t-test and were considered significantly different at P < 0.05.
RESULTS
SPARC Expression in the Intact Human Intestine
The expression of SPARC was first investigated by RT-PCR in fetal and adult human intestine. SPARC mRNA transcripts were detected at comparable levels in the small intestine and colon at both studied stages (Fig. 1). To evaluate SPARC protein expression, the rabbit anti-SPARC antibody was first characterized on Western blots. A predominant band at 43 kD was detected in cell lysates of the mesenchymal HIM cells (Fig. 2, lane 4), which, corresponded to the classical form of human SPARC [Porter et al., 1995]. In epithelial cells, a fainter 43 kD band was detected in HIEC and tsFHI cells (Fig. 2, lanes 1 and 2) but not in Caco-2 cells (Fig. 2, lane 3). The 60 kD band observed in all samples (Fig. 2, asterisk) was identified as bovine serum albumin from the serum used in the culture medium. Using the same antibody for indirect immunofluorescent staining of human intestinal sections, SPARC was localized predominantly in the smooth musculature in both developing and adult small intestine (Fig. 3) and colon (Fig. 4). A second site of SPARC distribution appears to be in the mucosa at the epithelial–mesenchymal interface. In the small intestine, a weak staining of the epithelial–mesenchymal interface was seen at early stages of development mainly in the intervillous area (Fig. 3B) but was not detected along the crypt–villus axis at mid-gestation (Fig. 3C) or in the adult tissues (Fig. 3D). In the colon, the staining of the epithelial–mesenchymal inter-face. was much stronger (Fig. 4). SPARC was also detected along the entire epithelial–mesenchymal interface before (Fig. 4A) and at early stages of villus formation (Fig. 4B and C). At mid-gestation, immunolabeling at the base of all epithelial cells was still apparent (Fig. 4D), although consistently weaker than observed at earlier stages, whereas staining was not detected in adult tissues (Fig. 4E). These observations have been summarized in Table I.
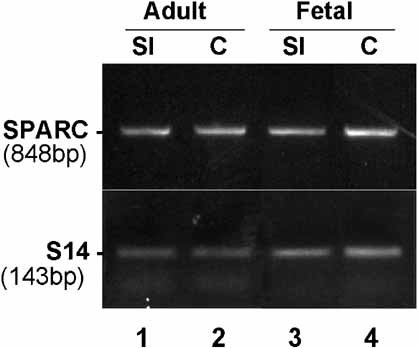
Representative RT-RCR analysis for the detection of the SPARC mRNA in the intact adult and fetal small intestine (SI) and colon (C). cDNAs were transcribed from total RNAs and amplified with primer sets specific for human SPARC. S14 was used to ensure comparable quantities of starting material.
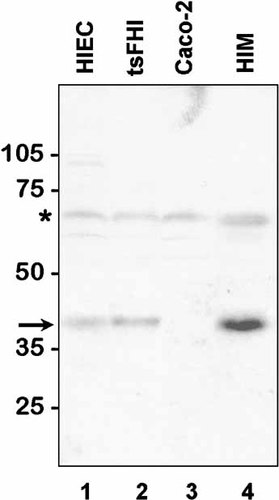
Western blot analysis of human intestinal cell lines for the expression of SPARC. Total proteins from cell lysates (∼100 µg/lane) were separated on SDS-PAGE under reducing conditions and transferred to nitrocellulose membrane for the detection of SPARC with an anti-pig SPARC antiserum. The 43 kD antigen (arrow) was predominant in the mesenchymal HIM cells (lane 4), in epithelial cell lines, SPARC was detectable in the two crypt-like HIEC and tsFHI cells (lane 1 and 2) while absent in Caco-2/15 cells (lane 3). The 60 kD band observed in all samples (asterisk) was identified as bovine serum albumin from the serum used in the culture medium.
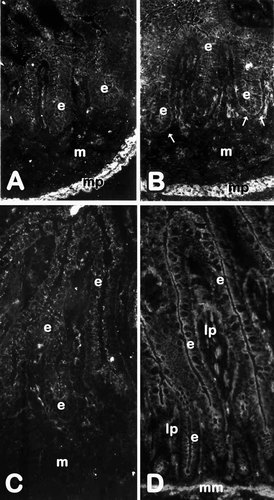
Expression and distribution of SPARC in the developing and adult small intestine. Representative immunofluorescence micrographs of human jejunum at 9 (A), 11(B), and 18(C) weeks of gestation and adult (D) stained with an anti-pig SPARC antiserum. At young stages of fetal development, specific staining is apparent at the epithelial (e) -mesenchymal (m) interface (arrows in B), in cellular elements of the mesenchyme (m) as well as in the muscularis propria (mp). At mid-gestation (C) and in adult (D), the antigen is detected in cellular elements of the mesenchyme (m) and lamina propria (lp), respectively, as well as in the muscularis propria (not shown) and muscularis mucosa (mm), but remains below detection levels at the base of the epithelium (e). A–D: X152.
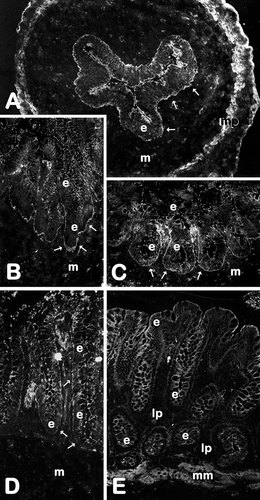
Expression and distribution of SPARC in the developing and adult colon. Representative immunofluorescence micrographs of human colon at 9 (A), 11 (B), 13(C), and 18 (D) weeks of gestation and adult (E) stained with an anti-pig SPARC antiserum. At young stages of fetal development, specific staining is clearly seen at the epithelial (e) -mesenchymal (m) interface (arrows in A–C) as well as in the muscularis propria (mp). At mid-gestation (D), the antigen is still detected at the epithelial–mesenchymal interface (arrows) as well as in cellular elements of the mesenchyme (m). In the adult (E), SPARC immunoreactivity is observed in the muscularis mucosa (mm) and in a few elements of the lamina propria (lp) but remains below detection levels at the base of the epithelium (e). A–D: X152.
| Segment | Tissue | Stage1 | Site | SPARC2 | Tenascin-C3 |
|---|---|---|---|---|---|
| Small intestine | Smooth musculature | All | All | +++4 | +++ |
| Epithelial–stromal; interface | Fetal (9–14 w) | Villus core | ± | ++ | |
| Fetal (16–20 w) | Villus core | ± | +++ | ||
| Crypt region | ± | ++ | |||
| Adult | Villus core | ± | +++ | ||
| Crypt region | ± | + | |||
| Epithelium | All | All | − | − | |
| Colon | Smooth musculature | All | All | +++ | +++ |
| Epithelial–stromal interface | Fetal (9–14 w) | Villus core | +++ | ± | |
| Fetal (16–20 w) | Villus core | ++ | ± | ||
| Crypt region | ++ | ± | |||
| Adult | Upper region of the glands | ± | +++ | ||
| Lower region of the glands | ± | ++ | |||
| Cancer | Stroma | +++ | +++ | ||
| Epithelium | All | All | − | − |
- 1 Analyzed in the 9–20 week fetal intestine and in adult by indirect immunofluorescence.
- 2 This study
- 3 Adapted from Bélanger and Beaulieu [2000].
- 4 Scale from strong staining (+++) to weak (±) or negative (−).
Epithelial Versus Mesenchymal Origin of SPARC in the Intestinal Mucosa
The respective contributions of epithelial and mesenchymal tissues to SPARC expression at the epithelial–mesenchymal interface was investigated by RT-PCR on epithelial and mesenchymal fractions freshly isolated from the fetal small intestine and colon using a non-enzymatic dissociation method [Perreault and Beaulieu, 1998]. The mRNA transcript for SPARC was detected in a much larger and significant (P < 0.05) proportion in the mesenchymal tissue compared to epithelia for both the proximal and distal fetal intestine (Fig. 5).
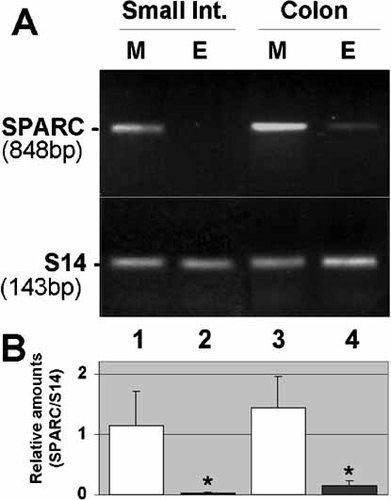
Distribution of SPARC transcripts in fetal intestinal tissues. (A) Representative RT-PCR analysis of SPARC mRNA in mesenchymal (M, lanes 1 and 3) and corresponding epithelial (E, lanes 2 and 4) fractions isolated from fetal small intestine (lane1 and 2) and colon (lanes 3 and 4) at mid gestation. S14 transcripts was determined to ensure complementary DNA integrity and to compare amounts of starting RNA material in the various samples. (B) Amounts of SPARC transcripts in paired mesenchymal and epithelial fractions (n = 3 separated experiments) for both small intestine and colon were determined relative to S14. Statistical difference between M and E were noted (asterisks; P < 0.05).
RT-PCR analysis on intestinal cells in vitro supported the in vivo results indicating that mesenchymal cells are an important source of SPARC (Fig. 6, lane 7). Analysis of SPARC mRNA also revealed significant expression by the two intestinal crypt-like cell lines HIEC and tsFHI (Fig. 6, lanes 1–4), but not the villus-like cell line Caco-2 (Fig. 6, lanes 5–6), consistent with the Western blotting experiments (Fig. 2).
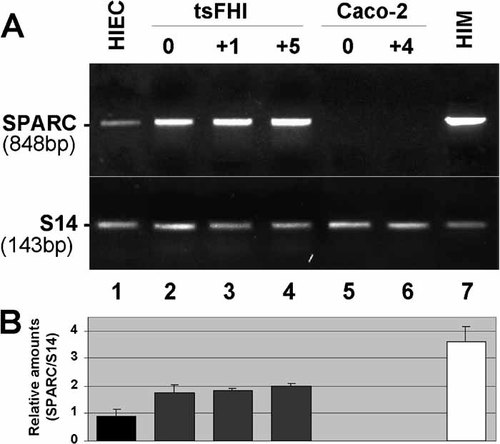
Expression of SPARC transcripts in human intestinal cell lines. (A) Representative RT-PCR analysis of SPARC mRNA in epithelial HIEC cells (lane 1), tsFHI cells grown under permissive (0; lane 2) and non-permissive conditions for cell proliferation after one day (+1; lane 3) and five days (+5; lane 4), and Caco-2/15 cells at confluence (0; lane 5) and four days post-confluence (+4; lane 6), as well as intestinal mesenchymal cells (HIM; lane 7). (B) Amounts of SPARC transcripts in intestinal cells were determined relative to S14 (n ≥ 3).
SPARC in the Diseased Intestinal Mucosa
SPARC expression in relation with mucosal remodeling was further investigated in two intestinal pathologies: small intestinal-related Crohn's disease and colon carcinoma. In Crohn's disease, a substantial disorganization of the crypt-villus axis occurs in the mucosa, which includes villus shortening and/or disappearance, crypt hyperplasia and redistribution of basement membrane molecules [Bouatrouss et al., 2000]. However, under this condition, SPARC immunostaining was absent at the epithelial–stromal interface (not shown) as observed in normal tissues (see Fig. 3D). RT-PCR analysis revealed that the SPARC transcript also remains expressed at comparable levels between inflamed and non-inflamed regions (Fig. 7).
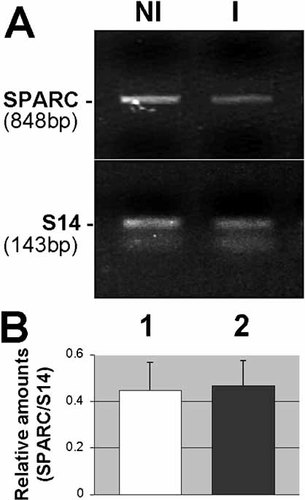
Expression of SPARC transcripts in the small intestine from patients with Crohn’s disease. (A) Representative RT-PCR analysis of SPARC mRNA in non-inflamed (NI; lane 1) and corresponding inflamed (I; lane 2) Crohn’s disease specimens. (B) Amounts of SPARC transcript in paired non-inflamed (lane 1) and inflamed (lane 2) specimens (n = 6) were determined relative to S14.
In contrast to normal adult tissues, expression of SPARC was observed in colon cancers (Fig. 8). Indirect immunofluorescence staining of colon carcinomas revealed a particularly strong expression of the molecule in the stroma surrounding the glandular structures (Fig. 8A). Expression of SPARC in colon tumors was confirmed at the transcript level by RT-PCR analyses of RNA extracted from primary tumors and corresponding resection margins (Fig. 8B, C; lanes 1,2, respectively). Similar to the Caco-2 cell line, which was found to be negative for SPARC expression (Fig. 2, lane 3; Fig. 6, lanes 5 and 6), the five other colon carcinoma cell line tested for SPARC expression were also found to lack the SPARC transcript.
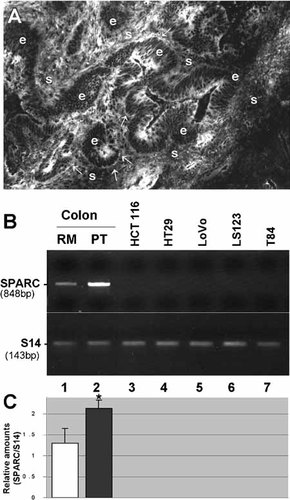
Expression of SPARC from patients with colon cancers and in adenocarcinoma cell lines. (A) Representative immunofluorescence micrographs of the primary tumor of human colon carcinoma stained with an anti-pig SPARC antiserum. Specific staining is detected in the stroma (s) as well as at the epithelial (e) -stromal (s) interface at various sites (arrows). (B) Representative RT-PCR analysis of SPARC mRNA in resection margin (RM, lane 1) and corresponding primary tumor (PT, lane 2) from human colon as well as in the adenocarcinoma cell lines HCT116, HT29, LoVo, LS123, and T84 (lanes 3–7). (C) Amounts of SPARC transcripts in paired resection margin (lane 1) and primary tumor (lane 2) specimens (n = 5) were determined relative to S14. Statistical difference between RM and PT was noted (asterisk; P < 0.05).
DISCUSSION
In this study, we have shown that SPARC is expressed in the human intestine according to a relatively complex spatial and temporal pattern. While constitutively present in the smooth musculature of the entire intestine, it has been found at the epithelial–stromal interface according to a typical onco-fetal pattern of expression that is mainly restricted to the distal intestine.
The constitutive expression of SPARC in the enteric smooth musculature is in good agreement with previous studies [Porter et al., 1995; Hunzelmann et al., 1998; Puolakkainen et al., 1999]. SPARC expression in relation to the intestinal epithelial–stromal interface appears more controversial since its presence at this site seems to be species-dependent. Indeed, for the intestinal epithelium, SPARC expression was observed intracellularly in both embryonic and adult mice [Sage et al., 1989b] while no appreciable immunoreactivity or mRNA were found in the human [Mundlos et al., 1992; Porter et al., 1995] or the rat [Puolakkainen et al., 1999]. As far as the small intestine is concerned, our observations are consistent with these previous reports in the human. Indeed, as shown in Figure 3, SPARC was found only marginally detected by immunostaining on cryosections, in both time and intensity, in the mucosa of the human proximal intestine. Similarly, and in agreement with the phenomenon described in the healing rat small intestine [Puolakkainen et al., 1999], SPARC was not found to be induced in the mucosa of the Crohn's diseased small intestine, although subject to intense remodeling [Bouatrouss et al., 2000]. The situation was clearly different in the colon where, although not significantly observed into epithelial cells, SPARC was consistently found to be deposited at the epithelial–mesenchymal interface at all studied fetal stages.
These observations, suggesting that human intestinal mucosal cells can synthesize SPARC, were further investigated in order to delineate the respective mesenchymal and epithelial contribution to the expression of the molecule. We first used intestinal cell culture models, which present the advantage of examining pure and well-characterized derivatives from either the epithelium or the mesenchyme of the human intestine. The three human intestinal epithelial cell lines HIEC [Perreault and Beaulieu, 1996], tsFHI [Quaroni and Beaulieu, 1997], and Caco-2/15 [Beaulieu and Quaroni, 1991; Vachon and Beaulieu, 1992] that together can recapitulate the intact crypt–villus axis [Pageot et al., 2000], as well as the mesenchymal cell cultures HIM derived from the fetal human intestine [Vachon et al., 1993], were used. The Caco-2/15 and HIM cell models have been used successfully in the past to study the tissular origin of type IV collagen chains, laminin subunits, tenascin-C, and fibronectin in the human intestine [Vachon et al., 1993; Beaulieu et al., 1994; Perreault et al., 1998; Simoneau et al., 1998]. Analysis of these two cell types showed that SPARC was produced by mesenchymal HIM cells while being absent from the epithelial Caco-2/15 cell line at both protein and transcript levels. However, the two other epithelial cell lines HIEC and tsFHI were found to express some SPARC under the same conditions. This finding remains unclear at this time but it is noteworthy that both cell lines have been derived from human fetal small intestinal crypt cells [Perreault and Beaulieu, 1996; Quaroni and Beaulieu, 1997]. Whether SPARC expression in these particular cells is the manifestation of a normal phenomenon specifically occurring in crypt cells of the intact fetal intestine, or the result of a cell culture-related adaptive mechanism [Lane and Sage, 1994], as shown also for other ECM molecules [Chiquet-Ehrismann et al., 1995b], are among the possibilities. To further investigate the tissular origin of SPARC deposition at the epithelial–mesenchymal interface, we used a second approach based on the recently described non-enzymatic dissociation method for the epithelium and mesenchyme of the human fetal intestine [Perreault et al., 1998]. RNA analysis from freshly isolated epithelial and mesenchymal fractions revealed very low relative amounts of the transcript in the intestinal epithelium suggesting that the SPARC detected at the epithelial–mesenchymal interface in the intact intestine is predominantly produced by the adjacent mesenchymal cells. A mesenchymal origin for SPARC at the human intestinal epithelial–mesenchymal interface is readily conceivable in the light of previous studies that have shown a main, if not exclusive, mesenchymal origin for the α1(IV) and α2(IV) chains of collagen and tenascin-C [Vachon et al., 1993; Beaulieu et al., 1994; Simoneau et al., 1998], two components of the intestinal epithelial basement membrane [Probstmeier et al., 1990; Beaulieu, 1997]. However, a minor contribution of SPARC by a specific subset of epithelial cells, such as crypt cells, cannot be excluded.
The clear prevalence of SPARC expression for the mucosa of the distal intestine, as observed herein, appears of potential functional importance since it is among the first ECM molecules identified in the developing human intestinal mucosa to be mainly confined to the colon. Indeed, most ECM molecules studied to date, including the basement membrane components such as type IV collagens, laminins, and fibronectins were found ubiquitously expressed in both fetal small intestinal and colonic mucosa [Beaulieu, 1997, 1999]. This observation was not unexpected in regard to the great similarity of the crypt–villus architecture and epithelial functionality for both segments during the first two trimesters of gestation [Ménard and Beaulieu, 1994; Babyatsky and Podolsky, 1999]. It is only after 30 weeks that the colonic mucosa undergoes the remodeling process that will provide its definitive glandular structure [Ménard and Beaulieu, 1994; Babyatsky and Podolsky, 1999]. While it would be premature to propose a specific role for SPARC in this process, it is noteworthy that tenascin-C, another anti-adhesive ECM molecule, has also been found to be differentially expressed along the length of the human intestine. However, in contrast to SPARC, tenascin-C was found to be expressed at the epithelial–mesenchymal interface of the developing human small intestinal mucosa but absent in fetal colonic villi, suggesting that tenascin-C may be dispensable for the process of villus formation but required for their maintenance [Desloges et al., 1994; Bélanger and Beaulieu, 2000]. In this regard, the strictly complementary patterns of SPARC and tenascin-C expression at the epithelial–mesenchymal interface of the developing and adult small intestine and colon, as summarized in Table I, are striking and may suggest a functional relation between the two anti-adhesive molecules. It is also of interest to note that colon cancer is the only circumstance in which co-expression of SPARC and tenascin-C was observed in the region of the epithelial–mesenchymal interface (Table I).
Overexpression of SPARC and tenascin-C in relation to human colon cancer progression is a relatively well-documented phenomenon [Wewer et al., 1988; Riedl et al., 1992; Porte et al., 1995; Porter et al., 1995; Iskaros et al., 1997; Kressner et al., 1997]. Interestingly, both molecules were found to be abundant in the stromal fibroblasts adjacent to colon carcinomas emphasizing the existence of particular inter-actions between neoplastic and stromal cells. However, their individual role in cancer progression may differ since tenascin-C has been suggested to play a protective function in preventing tumor invasion [Iskaros et al., 1997; Kressner et al., 1997] while SPARC, which activates the expression of matrix-degrading metalloproteinases [Tremble et al., 1993], is likely to promote it [Porte et al., 1995; Ledda et al., 1997]. These putative contradictory functions would be consistent with the fact that under normal circumstances, tenascin-C is present in close vicinity to the epithelium in the adult colon [Lohi et al., 1996; Beaulieu, 1997] while SPARC expression appears to be repressed at this site [Porte et al., 1995; this work].
Taken together, these observations indicate that SPARC is present at the epithelial–mesenchymal interface of the fetal distal intestinal mucosa, absent in its normal adult counter-part, and then re-expressed in colon cancers. Resurgence of a fetal pattern of epithelial gene expression in colon adenocarcinoma cells has been reported for a number of markers including CEA [Gold and Freeman, 1965], brush border hydrolases such as sucrase–isomaltase [Ménard and Beaulieu, 1994] as well as the α9β1 integrin [Basora et al., 1998]. However, SPARC appears to be principally expressed by stromal cells. This is, to our knowledge, the first ECM molecule to be identified as being expressed according to a strict onco-fetal pattern in the stroma of the human intestinal mucosa. In the light of its expression in relation to the morphogenetic process in the developing intestine, in particular in the colon, and with colon cancer progression, in concert with its well-recognized anti-adhesive properties and its potential for the activation of the expression of matrix-degrading metalloproteinases [Yan and Sage, 1999], SPARC is likely to play a key onco-fetal role in ECM remodeling of the human colonic mucosa.
Acknowledgements
The authors thank Dr J. Gosselin, Dr M. Lessard and Dr J. Poisson for their cooperation in providing tissue specimens and for the characterization of the pathological tissues, and F.E. Herring-Gillam for reviewing the manuscript.




