Biomarker analysis demonstrates a hypoxic environment in the castrated rat ventral prostate gland
Abstract
Within the first 24 h after castration of an adult male rat, the vascular system of the ventral prostate gland undergoes a degenerative process that drastically reduces blood flow to the tissue. Since the vascular degeneration precedes the loss of the prostatic epithelium (by apoptosis), we have proposed that the onset of epithelial cell apoptosis in this tissue is caused by an ischemic/hypoxic environment resulting from the loss of blood flow. In order to further evaluate the extent to which ischemia/hypoxia might be a factor in apoptosis of the prostate epithelium after castration, we analyzed for biomarkers of cellular hypoxia in rat ventral prostates during the first 3 days following castration. Ventral prostate tissues removed from hypoxyprobe-1-treated adult male rats (uncastrated controls; surgically castrated for 24, 48 or 72 h, or sham-castrated for equivalent times) were directly analyzed for evidence of hypoxia by in situ immunohistochemical evaluation of hypoxyprobe-1 adduct formation in the prostate cells. Protein extracts from these tissues were also tested for expression of the 120 kDa hypoxia-inducible factor-1-α (HIF-1-α) protein as well as for expression of mitogen-activated protein kinase (MAPK) and c-Jun N-terminal kinase (JNK) proteins using a Western blot assay. The tyrosine phosphorylation status of the latter signaling molecules was also evaluated by Western blotting using anti-tyrosine phosphate antibodies. Our results showed that epithelial cells of the rat ventral prostate stained positively for hypoxyprobe-1 adducts at all times after castration, whereas cells in control tissues were unstained by this procedure. In addition, the prostatic expression of HIF-1-α protein was increased approximately 20-fold at 48 h after castration compared to control tissues. Finally, although prostatic MAPK and JNK protein expression was unaltered during the early period after castration, phosphorylation of the JUN kinase protein was significantly elevated, indicating that this stress-activated cellular signaling pathway becomes more active subsequent to castration. These results support our proposal that early castration-induced degeneration and constriction of the vascular system of the rat ventral prostate gland leads to reduced oxygenation of prostatic epithelial cells and the activation of hypoxic cellular signaling in these cells through upregulation of HIF-1-α expression and stimulation of the JUN kinase signaling pathway. J. Cell. Biochem. 81:437–444, 2001. © 2001 Wiley-Liss, Inc.
The mammalian prostate gland requires androgenic steroids for its embryonic development and postnatal growth as well as for its maintenance in the adult state. This latter dependence is manifest by the striking effects of castration (androgen-withdrawal) on the adult tissue. In the adult male rat, often utilized as a laboratory animal model to study the effects of androgens on the prostate, the ventral prostate gland undergoes a remarkable regression process after castration, losing greater than 90% of its mass [Colombel and Buttyan, 1995]. Coincident with the drastic loss of tissue mass after castration, the ventral prostate ceases its production of seminal proteins and undergoes a remodeling process characterized by the loss of the vast majority of prostatic cells through the programmed cell death process known as apoptosis. Indeed, it has been estimated that over 85% of the cells of the ventral prostate gland will undergo apoptosis over the 2-week period following castration. The activation of prostate cell apoptosis by castration is especially pronounced in the epithelial cell compartment of the tissue where the entire population of differentiated secretory epithelial cells will be deleted through this process.
Since the effects of androgenic steroid stimulation on any given cell are mediated by an intracellular androgen receptor (AR) protein, it has been commonly assumed that those cells in the prostate that express AR are the cells most likely to suffer the effects of androgen withdrawal. Indeed, immunohistochemical analysis of AR expression in the rat ventral prostate has demonstrated AR expression in the prostate epithelial cells as well as in smooth muscle cells and a subset of prostatic fibroblasts [Prins and Birch, 1993]. But recent studies of the rat prostate model have called into question the concept that castration directly induces apoptosis of the prostate epithelial cells and have led to a proposal that this is, instead, the potential results of a hypoxic environment in the prostate caused by a drastic reduction of prostate blood flow when androgens are withdrawn [Buttyan et al., 1999].
This hypothesis was proposed based on the outcome of a series of experiments in which blood flow to the rat ventral prostate was measured as a function of time after castration [Shabsigh et al., 1998]. As was reported in these studies, ventral prostate blood flow was found to be reduced by 35% within 18 h after castration and by greater than 50% within 24 h after castration. Other (non-androgen sensitive) rat tissues did not experience any significant change in blood flow in these experiments nor did the ventral prostate if the castrated rats were immediately replenished with testosterone (by pharmacological intervention) after their surgery, thus indicating that the rapid blood flow change measured in the castrated rat ventral prostate was not the simple consequence of gonadal surgery. The drastic reduction of blood flow to the ventral prostate gland after castration was subsequently shown to coincide with some key physical changes in the prostatic vascular system, including an early degeneration (via apoptosis) of the capillary network that surrounds the prostatic glands [Shabsigh et al., 1999] as well as a drastic constriction of larger vascular elements in the tissue that survived castration [Hayek et al., 1999]. Temporal comparison of apoptosis rates in the prostatic endothelial cell compartment with apoptosis rates in the epithelial cell compartment of the castrated ventral prostate showed that endothelial cell apoptosis occurred significantly earlier (peaking at 24 h after castration) than the onset of epithelial cell apoptosis (peaking at 72 h after castration) [Shabsigh et al., 1999], thus identifying the possibility that the epithelial cell apoptosis associated with castration might be the secondary results of an ischemic/hypoxic environment in the prostate brought on by the drastic and chronic loss of blood flow to the tissue.
In order to further test this hypotheses, we studied the rat ventral prostate model for biomarkers of hypoxia during the very early period (the first 72 h) after castration. Utilizing a small molecular agent (hydroxyprobe-1) that forms protein adducts in hypoxic cells (cells with [O2] < 10 mg Hg at 37°C), we tested whether such adducts might be formed in the epithelial cells of the prostate or other rat tissues after castration. Other experiments were performed to determine whether castration affects the expression of the hypoxia-inducible transcription factor, HIF-α, in rat prostate tissue. Finally, we evaluated expression and phosphorylation status of two members of the “stress-activated kinase” signaling pathways, mitogen-activated protein kinase (MAPK) and c-Jun N-terminal kinase (JNK), after castration in order to evaluate whether either of these cellular signaling pathways might become more active in the prostate after castration. Previous studies have demonstrated that hypoxia activates the JNK signaling pathway in tissues and cultured cells [Yin et al., 1997; Pombo et al., 1994].
MATERIALS AND METHODS
Laboratory Animals
Mature male Sprague-Dawley rats (325–350 g) were maintained in a controlled environment with food and water available ad libitum under 12 h light/dark cycles. Upon arrival, animals were randomly assigned to one of three different experimental groups including one group that was studied intact (control, unoperated), another a group that received surgical castration under anesthesia, and a third group that was sham-castrated (sham-operated controls) under anesthesia as previously described [Shabsigh, et al., 1998]. The animals in the latter two groups were studied at 24, 48 or 72 h after the surgical procedure. In one experiment, all groups (5 rats per time point) received an intraperitoneal injection of aqueous hypoxyprobe-1 (60 mg/kg pimonidazole hydrochloride, Natural Pharmacia International Inc, Research Triangle Park, NC) solution exactly one hour before sacrifice. In a second experiment, animals (5 per time point) were simply sacrificed to obtain tissue for subsequent protein extraction and analysis. Necropsy was performed immediately upon expiration of the animal and tissue specimens were immediately placed in fixative (10% phosphate-buffered formalin) for subsequent dehydration and embedding or were frozen under liquid nitrogen before being stored in a −80°C freezer.
Immunohistochemistry for Hydroxyprobe-1 Adducts
Formalin-fixed, paraffin-embedded tissues were sectioned (5 μM) and attached to slides. The sections were then rehydrated and immunostained using a monoclonal antibody (Hypoxyprobe-1 Mab1, Natural Pharmacia International, Inc.), that detects adducts of hypoxyprobe-1 in hypoxic cells. Immunostaining was performed according to the manufacture's recommendations. Antibody binding was revealed by peroxidase-labeled secondary antibody against mouse IgG using the Vectastain immunohistochemical staining kit (Vector Laboratories Inc, Burlingame, CA)
Protein Extraction and Western Blotting for HIF1-α, MAPK and JNK
Frozen tissues were pulverized under liquid nitrogen and the frozen powder was then thawed and homogenized (using a Polytron tissue homogenizer, Brinkmann Instruments Inc., Westbury, NY) in five volumes of ice-cold RIPA buffer (50 mM Tris–HCl, pH 8.0, 300 mM NaCl, 0.1% sodium dodecylsulfate, 1% NP-40 and 0.5% sodium deoxycholate). The homogenate was stored on ice for 40 minutes prior to the removal of nuclear and other debris by centrifugation at 14,000 × g for 15 min. Protein concentrations in the extracts were determined using the Bio-Rad Protein Assay system (BioRad Labs, Inc., Richmond, CA). Aliquots of extracts containing 50 μg of protein were solubilized in loading buffer (10% glycerol, 2% SDS, 60 mM Tris, pH 6.8, 0.01% bromophenol blue, and 100 mM DTT) and were boiled for 5 minutes prior to electrophoresed on 12% SDS-polyacrylamide gels. The proteins in the gel were electrophoretically transferred to nitrocellulose mebranes (Hybond, Amersham, Inc., Arlington Heights, IL) to produce Western blots in 25 mM Tris–HCl, pH 8.0, 192 mM glycine, and 20% methanol at 100 Volt for one hour. Membranes were blocked in 10% non-fat milk powder (in TBS-T, 20 mM Tris–HCl, pH 7.6, 136 mM NaCl, 0.3% Tween-20) at 4°C overnight and was then incubated for 1 h with a mouse monoclonal antibody against human HIF1-α (Novus Biologicals, Littleton, CO) diluted 1:1,000 in TBS-T buffer at 37°C (for detection of HIF1-α), or with rabbit polyclonal antibodies against human MAPK (diluted 1:500, New England Biolabs, Beverly, MA) or human JNK (diluted 1:500, New England Biolabs). Subsequently the Western blot tested for HIF1-α expression was cleaned by incubation in a denaturing buffer (0.2% SDS, 0.1 mM DTT) at 70° for 30 min followed by several washings in TBS-T and re-blocking with 10% milk solution and the blot was then reprobed for smooth muscle actin using (mouse monoclonal diluted 1:2000, Sigma Chemical Co., Inc., St Louis, MO). Likewise, the Western blot previously probed for MAPK was cleaned and reprobed for phospho-MAPK (rabbit polyclonal recognizing MAPK thr-183/tyr-185, diluted 1:500, New England Biolabs) and the Western blot previously probed for JNK was cleaned and reprobed for phospho-JNK (rabbit polyclonal recognizing JNK thr-180/tyr-182, diluted 1:500, New England Biolabs) (obtained from New England Biolabs, Beverly, MA). After extensive washing with TBS-T buffer, membranes were incubated with a secondary antibody (sheep anti-mouse IgG-horseradish peroxidase complex for HIF1-α and phospho-JNK or goat anti-rabbit IgG-horseradish peroxidase for MAPK, phospho-MAPK and JNK (Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Chemoluminescent detection of antibody binding was done using the ECL western blotting analysis system (Amersham, Inc.). The autoradiograph was analyzed with the molecular dynamics scanning densitometer (Sunnyvale, CA).
RESULTS
Formation of Hypoxyprobe-1 Adducts in Cells of the Castrated Rat Ventral Prostate Gland
Hypoxyprobe-1 is a substituted 2-nitrominidazole (pimonidazole hydrochloride) that is freely water soluble and widely used to demonstrate hypoxic conditions in tissues (in vivo) and cultured cells. Once injected into a laboratory animal or human, it is rapidly distributed to all tissues in the body, but only forms adducts with proteins in cells having oxygen concentrations less than 14 μM, which is equivalent to a pO2 of 10 mmHg at 37°C [Arteel et al., 1998]. Many types of tumor cells, as well as certain cells in the normal liver, kidney and skin have been found to be reactive to hypoxyprobe-1 and these cells can be easily detected using immunohistochemical techniques employing a commercially available monoclonal antibody that recognizes hypoxyprobe-1-protein adducts [Cline et al., 1990]. Control (intact or sham-operated) and castrated rats (at 24, 48 or 72 h subsequent to surgery) were treated with a hypoxyprobe-1 injected one hour prior to sacrifice (5 rats per time point). Individual tissues obtained from these rats were fixed dehydrated and embedded, then sectioned for immunohistochemistry using the anti-hypoxyprobe-adduct antibody. The results of this immunohistochemical analysis (Fig. 1) showed that all cells in the ventral prostate tissues obtained from the control rats were unstained by the antibody, whereas some epithelial cell regions within the renal cortex from kidneys obtained from these same control rats were consistently found to stain positive for hypoxyprobe-adducts, as should be expected (not shown here). In contrast, the cytoplasmic compartments of epithelial cells in the ventral prostates of castrated rats examined (at all time points) were found to be positively stained by this antibody. These results suggest that the epithelial cells within the intact and sham-operated rat ventral prostates were in a normoxic environment whereas the epithelial cells of the early castrated rat ventral prostates were exposed to a significantly more hypoxic environment.
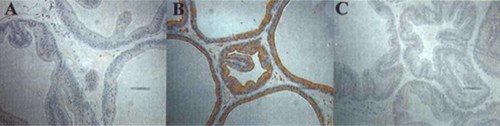
Immunohistochemical detection of Hypoxyprobe-1 adducts in ventral prostate tissues from rats. Ventral prostate tissues were obtained from (A) control (unoperated) rats or (B) from 48-h castrated rats or (C) from 48-h sham-operated rats that had been treated for one hour before sacrifice with hypoxyprobe-1. The tissues were fixed, embedded and sectioned, and were immunostained using an antibody that recognizes hypoxyprobe-1 protein adducts. Most of the epithelial cells in castrated ventral prostates (B) were strongly immunostained by this procedure whereas the cells in the control tissues (A and C) were unstained.
Effects of Castration on the Expression of Hypoxia-Inducible Factor-1-α in the Rat Ventral Prostate Gland
Exposure of cultured mammalian cells to a hypoxic environment rapidly induces expression of a 120-kDa protein referred to as HIF1-α [Semenza, 2000]. HIF1-α forms dimers with a constitutively expressed protein known as HIF1-β (also referred to as ARNT) and, together, this complex acts as a nuclear transcription factor that induces the expression of several different gene products in hypoxic cells that helps to sustain them during transient exposure to hypoxic conditions (exemplified by vascular endothelial growth factor [VEGF], erythropoietin, heme oxygenase-1, inducible nitric oxide synthase and other glycolytic enzymes).
Western blotting methods were used to determine whether the expression of HIF1-α protein was altered in the rat ventral prostate gland during the first three days after castration. As demonstrated by the results of the Western blot (Fig. 2), prostatic HIF-1-α levels were significantly increased during the first 24 h after castration, and this protein continued to accumulate in the tissue, with almost a 20-fold increase by 48 h after castration. This level remains stable through 72 h after castration. Control probing of the Western blot for smooth muscle actin showed that expression of this control gene product was not altered in these specimens (Fig. 2).

Expression of HIF1-α protein in the rat ventral prostate as a function of time after castration. Western blots were prepared using protein extracts made from individual ventral prostates obtained from control (unoperated) rats or rats at 24, 48 or 72 h after castration (as indicated). These Western blots were first probed for expression of the 120 kDa HIF1-α protein (upper panel) using an anti-HIF1-α antibody. The Western blots were cleaned and then reprobed with an antibody that detects smooth muscle actin (42 kDa) (lower panel). Densitometric evaluation of these blots indicate that HIF1-α expression is induced over 20-fold in the 48-h castrated rat ventral prostate.
Effects of Castration on the Expression and Phosphorylation of Prostatic MAPK and JNK
Certain physiological stresses (such as UV light, heat shock or ischemia) can activate signaling pathways regulated by “stress kinases” including the 38-kDa mitogen-activated protein kinase (MAPK) or the 54 and 46 kDa forms of c-jun N-terminal kinase (JNK) [Garay et al., 2000]. As with other cellular signaling pathways that operate via protein kinase action, activation of the MAPK or JNK signaling pathways is associated with increased phorphorylation of the signaling proteins themselves.
In order to determine whether either of these kinase signaling pathways might be activated in the prostate by castration, we performed Western blot analysis to measure expression of the generic MAPK or JNK proteins or the phosphorylated forms of each of these proteins. Equal aliquots of protein in extracts from rat prostates at various times after castration were co-electrophoresed by SDS-PAGE and were transferred to nitrocellulose to produce Western blots. These blots were first probed for total MAPK or JNK protein using rabbit polyclonal antibodies made against these proteins. The results (Fig. 3a, b) demonstrate that prostatic expression of the 38-kDa MAPK protein or the 54 and 46 kDa species of JNK protein were essentially unchanged by early castration. These blots were subsequently cleaned and reprobed for phospho-MAPK and phospho-JNK using specific polyclonal antibodies that only recognize the phosphorylated forms of these proteins. Whereas the expression of phosphorylated form of MAPK was unchanged after castration, the phorphorylation of the JNK protein species were found to increase on castration. Densitometric evaluation of the films made from these blots demonstrated that the levels of p-JNK (46-kDa band) increased almost 10-fold 48 h after castration (Fig. 3c). These results demonstrate that the prostatic JNK signaling pathway is activated by castration and it is this particular stress-activated signaling pathway that was previously shown to be activated in cultured cells by exposure to hypoxic conditions.
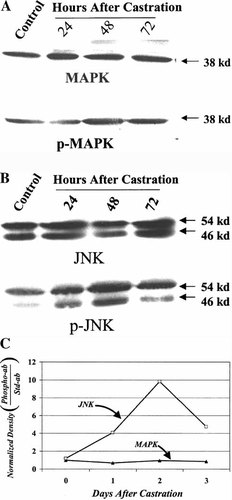
Expression and activity of ventral prostate MAPK and JNK as a function of time after castration. A: Western blots were prepared using protein extracts made from individual ventral prostates obtained from control (unoperated) rats or rats at 24, 48 or 72 h after castration (as indicated). A blot was first probed with an antibody against MAPK (38 kDa) (upper panel) and was subsequently cleaned and probed for p-MAPK (lower panel) using an antibody that specifically recognizes the phosphorylated form of the protein. B: A Western blot probed for expression of JNK (54 and 46 kDa) (upper panel) was subsequently cleaned and reprobed with an antibody against p-JNK. C: Quantification of ratio of normalized density (density at time after castration/density of control band) of 38-kDa MAPK bands/normalized p-MAPK band or normalized density of 46-kDa JNK band/normalized p-JNK band demonstrates induction of JNK phosphorylation and activation of JNK signaling pathway after castration.
DISCUSSION
The epithelial cells of the prostate gland are known to be highly dependent on androgenic steroids for their survival and, in the model of the rat ventral prostate gland, the vast majority of these cells will undergo apoptosis after castration [Colombel and Buttyan, 1995]. A similar sensitivity to androgens is often demonstrated by human prostate cancer cells and, for this reason, therapies that deplete the body of androgens are often used in the treatment of advanced forms of this disease. The precise physiological and cellular mechanisms involved in the activation of prostate cell apoptosis (benign or malignant) upon androgen deprivation remains unresolved. However, based on experimental studies demonstrating that one of the earliest physiological changes in the normal rat prostate is a drastic reduction of blood flow to the tissue, we have proposed that it is the hypoxic environment of the prostate subsequent to the early loss of blood flow that is responsible for initiating the apoptotic demise of the epithelial cells in this tissue [Lekas et al., 1997; Buttyan et al., 1999]. Indeed, this hypothesis is supported by a study of the kinetics of rat prostate cell apoptosis after castration in which it was shown that tissue blood flow loss and capillary regression occurred significantly earlier than the onset of apoptosis of the epithelial cells in this tissue [Shabsigh et al., 1999].
In the experiments reported here, we again used the rat prostate model to evaluate whether the epithelial cells of the tissue might experience hypoxia subsequent to castration. Our results of the study of biomarkers of hypoxia in the rat ventral prostate after castration strongly supports the concept that this tissue does indeed become hypoxic after castration and that the epithelial cell compartment is especially affected by this hypoxia. First, we showed that the hypoxia-indicating reagent, hypoxyprobe-1 (pimonidazole hydrochloride), readily forms adducts with the proteins of rat prostate epithelial cells after castration but not before. This is direct evidence of the hypoxic environment of the post-castrated prostate because this substance (hypoxyprobe-1) is only known to form adducts in cells whose oxygenation is less than 14 μM [Raleigh et al., 1996]. Secondly, we found that the rat prostate cells mount a typical hypoxia response after castration, increasing their expression of the HIF1-α protein by 20-fold within 48 h after castration. This action is a normal response of mammalian cells to hypoxia and, under some conditions, can help the cell survive the stress of hypoxia by inducing expression of gene products that alter the cellular metabolism as well as increasing the expression of certain angiogenic growth factors, such as VEGF, that might ultimately assist in re-establishing a suitable blood supply to the tissue [Iyer et al., 1998]. Indeed, the induction of HIF1-α reported here to be maximal on the second day after castration, is consistent with a rebound in the expression of VEGF-A noted previously in the rat ventral prostate on the third day after castration [Burchardt et al., 2000]. Finally, we have demonstrated that the JNK signaling pathway in the rat prostate is highly activated by castration, in a similar manner to which this signaling pathway has previously been shown to be activated in tissues or cultured cells by hypoxia [Scott et al., 1998; Seko et al., 1997]. While it is not clear what role JNK signaling activation plays in hypoxia-induced apoptosis, a recent experimental study has demonstrated that antisense oligonucleotides against JNK were capable of suppressing apoptosis of human renal cells exposed to hypoxia, suggesting that the activation of this signaling pathway might be critical for the apoptotic response to hypoxia [Garay et al., 2000]. Furthermore, it should be pointed out that one of the end points of the JNK-signaling pathway is phosphorylation of the c-jun protein and increased activity of the AP-1 transcription complex (made up of c-fos and c-jun dimers). Past experimentation establishing the early induction of c-fos and c-jun expression in the rat prostate after castration [Buttyan et al., 1988; Marti et al., 1994] and increased AP-1 transcriptional activity is consistent with the activation of the JNK signaling pathway by castration. Moreover, recently it was demonstrated that increased AP-1 transcriptional activity was likely to be crucial for the onset of mouse prostate cell apoptosis (after castration) based on the observation that male c-fos knockout mice do not have prostate regression nor prostate cell apoptosis after castration [Feng et al., 1998]. The apparent requirement for AP-1 transcriptional activation in apoptosis of prostate cells subsequent to castration suggests that activation of JNK signaling, aside from being a simple biomarker of hypoxia in the castrated rat ventral prostate gland, might also have an important function in the apoptotic signaling process in the epithelial, or other cells of this tissue.
In summary, the results of this study, at least, support the concept that hypoxia contributes to the onset of apoptosis that occurs in the prostate gland following ablation of androgenic steroids. Other, more direct actions on prostate epithelial cells caused by the lack of androgens, still have the potential of contributing to the extensive loss of cells that occurs when the prostate undergoes castration-induced regression. Evidence that growth factor expression is rapidly altered in the prostate after castration allows for the possibility that these changes directly participate in the cellular decision through which the prostate epithelial cells activate their endogenous apoptotic process. These changes include reduction in the local expression of certain cell survival supportive factors (such as epidermal growth factor and basic fibroblast growth factor) as well as acute induction of certain cell inhibitory factors (transforming growth factor-β) [Kyprinaou and Isaacs, 1989; Nishi et al., 1996]. Combined, these drastic alterations in the growth factor environment of the prostate after castration are expected to exert an important influence on the apoptotic signaling pathway in prostate epithelial cells that might be further advanced by the rapid onset of tissue hypoxia.
Acknowledgements
This work was supported by a generous donation from the T.J. Martell Foundation for AIDS and Cancer Research (New York) as well as by a grant from the National Institutes of Health (R01-DK56808 to R.B.). Ahmad Shabsigh is the recipient of The Seymore Milstein Fellowship for Prostate Cancer Research and Mohamed Ghafar is the recipient of The Irving White Fellowship for Prostate Cancer Research.