Isolation and nucleotide sequence of a gene encoding tRNA nucleotidyltransferase from Kluyveromyces lactis
Abstract
A gene (KlCCA1) encoding ATP(CTP):tRNA specific tRNA nucleotidyltransferase (EC 2.7.7.25) was isolated from Kluyveromyces lactis by complementation of the Saccharomyces cerevisiae cca1-1 mutation. Sequencing of a 2665 bp EcoRI–SpeI restriction fragment revealed an open reading frame potentially encoding a protein of 489 amino acids with 57% sequence similarity to its S. cerevisiae homologue. Southern hybridization revealed a single copy of KlCCA1 in the K. lactis genome. KlCCA1 was able to complement both the mitochondrial and cytosolic defects in the cca1-1 mutant, suggesting that, as in S. cerevisiae, the K. lactis gene encodes a sorting isozyme that is targeted to mitochondria and the nucleus and/or cytosol. An altered KlCCA1 gene encoding a tRNA nucleotidyltransferase that lacked its first 35 amino acids was able to complement the nuclear/cytosolic but not the mitochondrial defect in the S. cerevisiae cca1-1 mutant, suggesting that the 35 amino-terminal amino acids are necessary for targeting to mitochondria but are not required for enzyme activity. Our results suggest that the mechanisms for production and distribution of mitochondrial and nuclear/cytosolic tRNA nucleotidyltransferase in K. lactis differ from those seen in S. cerevisiae. This DNA sequence has been assigned GenBank Accession No. AF207744. Copyright © 2000 John Wiley & Sons, Ltd.
Introduction
While the yeasts Kluyveromyces lactis and Saccharomyces cerevisiae are closely related evolutionarily, based on their nuclear DNA (Van de Peer et al., 1992), they show differences in mitochondrial genome composition and in requirements for mitochondrial DNA. The mitochondrial genome of K. lactis (39 kbp) is approximately half the size of the S. cerevisiae mitochondrial genome (Skelly et al., 1991). In addition, the mitochondrially encoded RNase P RNA of K. lactis appears to be less than half the size of its S. cerevisiae counterpart (Wise and Martin, 1991). These contrasts in mitochondrial characteristics suggest that the two yeasts may differ in aspects of mitochondrial biogenesis. We are interested specifically in the contribution of nuclear gene products to mitochondrial biogenesis in K. lactis and the targeting of these proteins to mitochondria.
In S. cerevisiae a single nuclear gene (CCA1) encodes multiple forms of ATP (CTP):tRNA-specific tRNA nucleotidyltransferase that are targeted to the nucleus, mitochondrion and cytosol (Chen et al., 1992). We wanted to determine whether the K. lactis CCA1 homologue also encodes multiple forms of tRNA nucleotidyltransferase and to see if mechanisms for targeting this enzyme in K. lactis are similar to those in S. cerevisiae. To begin our study, we isolated the gene encoding tRNA nucleotidyltransferase in K. lactis by complementation of the S. cerevisiae cca1-1 mutation (Aebi et al., 1990). We show here that the K. lactis gene is capable of complementing not only the nuclear/cytosolic defect in the S. cerevisiae cca1-1 mutant but also the mitochondrial defect, indicating that this protein is targeted to both the nucleus/cytosol and mitochondrion in S. cerevisiae and suggesting that the protein is also targeted to multiple locations in K. lactis.
In S. cerevisiae three in-frame ATGs (at amino acids 1, 10 and 18) at the 5′ end of the CCA1 open reading frame, in combination with multiple transcription start sites, lead to the production of multiple forms of tRNA nucleotidyltransferase (Wolfe et al., 1994). Proteins arising from ATG2 or ATG3 provide nuclear and cytosolic activity (Wolfe et al., 1994), while protein produced from ATG1 is required for mitochondrial protein synthesis (Chen et al., 1992), indicating that the sequences between ATG1 and ATG2 are necessary for efficient mitochondrial targeting. In K. lactis, two in-frame ATGs are found near the 5′ end of the open reading frame encoding tRNA nucleotidyltransferase. However, the position of these ATGs (at amino acids 1 and 4) and the results of site-directed mutagenesis (described here) make it unlikely that alternative use of these codons generates proteins that are sorted differently in K. lactis. Therefore, targeting of tRNA nucleotidyltransferase to multiple locations in K. lactis most likely is achieved by mechanisms different from those used in S. cerevisiae.
Materials and methods
E. coli strains XL-2 Blue (Stratagene) and JM109 (Yanish-Perron et al., 1985) and Saccharomyces cerevisiae strain NT33-5 (Shanmugam et al., 1996; relevant genotype cca1-1 ura3) were used as the recipients for bacterial (Capage and Hill, 1979) and yeast (Schiestl and Gietz, 1989) transformations, respectively. The K. lactis genomic library was kindly supplied by Dr M. Stark, University of Dundee. Plasmids utilized included pRS316 (Sikorski and Hieter, 1989), p426 (Mumberg et al., 1995) and pBluescript II KS+ (Stratagene). Growth media for E. coli and yeast were described by Sambrook et al. (1989) and Sherman (1991), respectively.
Plasmid DNA was isolated from E. coli by the alkaline lysis method (Sambrook et al., 1989) and from yeast by the method of Ward (1990). K. lactis genomic DNA was isolated by the method of Philippsen et al. (1991). Plasmid KL16-24-1 was identified by complementation of the S. cerevisiae cca1-1 mutation and a 2665 bp EcoRI–SpeI fragment was subcloned into pRS316 to generate pKLES. For the polymerase chain reaction (PCR) the following oligonucleotides were purchased from BioCorp Inc., Montreal, PQ, Canada: KLCCA3 TCTAGAATTCGTGTTTCAAAAG (complementary to the 3′ end of the gene), KLCCA2 TTATACTCGACTAGTTTCAAAATGGTAG(eliminating ATG1), and KLCCAE TATAATAAAACTAGTGCAATGACTGAACCTTTGG (converting amino acid 35 to an ATG). A hot start was used to begin the PCR and 35 cycles of 94°C/1 min, 26°C/1.5 min and 72°C/2 min were performed with plasmid KL16-24-1 as template. The resulting products were digested with EcoRI and SpeI, gel-purified (Bewsey et al., 1991) and ligated into EcoRI- and SpeI-digested p426, generating pK2 and pKE, respectively. At the same time, a DNA fragment extending from the XbaI site (position 970) to the SpeI site (position 2665) of pKES was subcloned into p426, generating pKXS. Sequencing was either done in house with Sequenase version 2 (Amersham) and [α-35S]dATP, or commercially by Bio S&T Inc., Lachine, Canada.
Alignments, computer modelling and predictions were carried out using the software provided with PCGENE and through GenBank.
DNA was transferred from agarose gels to Biotrans+ membranes and hybridization was carried out as recommended by the supplier (ICN Biomedicals Canada, Mississauga, ON, Canada). Plasmid KXS was digested with XbaI and SpeI, the insert isolated from an agarose gel (Bewsey et al., 1991), labelled using [α-32P]dCTP with the multiprimeTM DNA labelling system (Amersham), and used as probe.
Results and discussion
We isolated the gene encoding tRNA nucleotidyltransferase from K. lactis by complementation of a temperature-sensitive mutation (cca1-1) in S. cerevisiae. Introduction of a YCp50-based K. lactis library into S. cerevisiae strain NT33-5 produced 61 400 Ura+ transformants, of which 66 were temperature-resistant. Plasmid KL16-24-1 was isolated from one temperature-resistant transformant that showed concomitant loss of uracil prototrophy and temperature resistance under non-selective conditions. The 7.9 kbp insert in KL16-24-1 supported growth of the cca1-1 mutation at the non-permissive temperature on either a fermentable or non-fermentable carbon source. This indicated that the cloned K. lactis sequence provided active enzyme for both mitochondrial and nuclear/cytosolic CCA addition.
The gene coding for the S. cerevisiae tRNA nucleotidyltransferase is interesting because it codes for the mitochondrial, cytosolic and nuclear forms of tRNA nucleotidyltransferase. We carried out Southern analysis to see if the K. lactis genome contains multiple genes coding for tRNA nucleotidyltransferase or a single gene that could encode a protein targeted to multiple intracellular destinations. A single band was apparent in EcoRI or SpeI restriction digests (data not shown) and fragments of expected size were apparent in each double digest (Figure 1). These data suggest that a single gene coding for tRNA nucleotidyltransferase is present in the K. lactis genome. However, we cannot exclude the possibility that other genes coding for tRNA nucleotidyltransferase are present but differ enough in DNA sequence from KlCCA1 to elude detection. Gene disruption and gene replacement experiments will be required to address this possibility, but will be complicated by the fact that K. lactis is petite-negative.
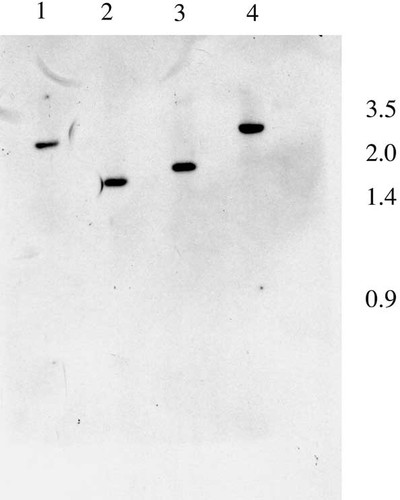
Southern analysis. Genomic DNA isolated from K. lactis was digested with EcoRI and XbaI (lane 1), SpeI and XbaI (lane 2), SpeI and XcmI (lane 3), or SpeI and EcoRI (lane 4), resolved on a 1% agarose gel, transferred to a membrane and probed with the 32P-labelled 1.7 kb XbaI–SpeI fragment of pKL16-24-1. Numbers on the right represent size markers of lambda DNA digested with EcoRI and HindIII. The genomic XbaI–SpeI fragment is smaller than the XbaI–SpeI probe because the XbaI site at position 1183 is methylated in E. coli and is not cleaved.
Plasmid KLES, generated by cloning a 2665 bp EcoRI–SpeI fragment of plasmid KL24-16-1 into pRS316, was found to contain sufficient sequence to complement the S. cerevisiae cca1-1 mutation. Sequencing of this fragment revealed an open reading frame of 1467 bp that extended from an ATG at position 1007 to a TGA at position 2474 and potentially could encode a protein of 489 amino acids (Figure 2). This is about 10% smaller than the 546 amino acid open reading frame in the S. cerevisiae CCA1 gene (Aebi et al., 1990) and represents the smallest eukaryotic tRNA nucleotidyltransferase identified to date. Convincing evidence that this open reading frame codes for the K. lactis tRNA nucleotidyltransferase is provided by the high level of identity (49%) with the predicted amino acid sequence of S. cerevisiae tRNA nucleotidyltransferase (Figure 3) and the presence of conserved amino acid motifs characteristic of nucleotidyltransferases (Holm and Sander, 1995; Martin and Keller, 1996; Yue et al., 1996). Previous sequence comparisons showed that amino acid identity among tRNA nucleotidyltransferases is confined primarily to the amino-terminal 25 kDa portion of the protein with significant divergence after this point (Aebi et al., 1990; Shanmugam et al., 1996; Yue et al., 1996; Martin and Keller, 1996). This is clearly the case for the K. lactis enzyme, where amino acid identity is confined primarily to the amino-terminal half of the protein, such that the majority of the gaps in sequence alignment are located at the carboxy-terminal portion of the protein.

Nucleotide sequence and predicted amino acid sequence of a Kluyveromyces lactis genomic EcoRI–SpeI fragment encoding tRNA nucleotidyltransferase. Standard one-letter abbreviations for amino acids are used. Numbers indicate position in nucleic acid sequence.

Similarity of the K. lactis (KL), S. cerevisiae (SC) and E. coli (EC) tRNA nucleotidyltransferases. Standard one-letter abbreviations for amino acids are used. (*) indicates a position that is perfectly conserved. (.) indicates a position that is well conserved. (-) indicates a gap introduced to optimize alignments. Numbers indicate position in each protein sequence. The conserved DXD motif shown to be required for Sulfolobus shibatae tRNA nucleotidyltransferase activity (Yue et al., 1998) is shown in bold.
As in S. cerevisiae, the predicted sequence of the K. lactis protein includes an amino-terminal extension that is not found in the E. coli enzyme. The amino terminus of the E. coli protein aligns approximately with amino acid 35 of the K. lactis sequence and with amino acid 61 of the S. cerevisiae sequence (Figure 3). Aebi et al. (1990) first proposed that these amino-terminal amino acids could function in S. cerevisiae as a mitochondrial targeting signal, and Chen et al. (1992) confirmed this by showing that removal of the first 9 or 17 amino acids did not effect cytosolic and nuclear enzyme activity, but mitochondrial activity was reduced or lost. Although the N-terminus of the K. lactis sequence does contain some hydrophobic, basic and hydroxylated amino acids suggestive of a mitochondrial targeting signal (von Heijne et al., 1989), a computer search (Gavel and von Heijne, 1990) failed to identify this sequence as a potential mitochondrial targeting signal. Interestingly, a similar analysis of the S. cerevisiae N-terminus (Wolfe et al.1996) also failed to find the relatively high hydrophobic moment and α-helical amphipathic structure characteristic of mitochondrial targeting signals.
Because the gene encoding K. lactis tRNA nucleotidyltransferase complements a mitochondrial defect in the S. cerevisiae cca1-1 mutant (Figure 4) the protein must be imported into mitochondria. As in S. cerevisiae, the protein predicted from the K. lactis sequence has more than one in-frame start codon (Figure 2). However, unlike S. cerevisiae, where the first, second and third in-frame start codons are separated by eight and seven amino acids, respectively, the two K. lactis start codons are separated by only two amino acids. We found that K. lactis tRNA nucleotidyltransferase generated from a PCR product lacking ATG1, and presumably missing its first three amino acids, allowed growth of the cca1-1 mutant at the non-permissive temperature on both fermentable and non-fermentable carbon sources, indicating that the first three amino acids are not required for activity of the enzyme or for import into mitochondria (Figure 4). In contrast, expression of a PCR product designed to generate tRNA nucleotidyltransferase lacking its first 35 amino acids allowed growth only on the fermentable carbon source (Figure 4), suggesting that although these amino-terminal amino acids are not required for enzyme activity, they are necessary for mitochondrial targeting. The ability of the K. lactis gene to complement both the mitochondrial and nuclear/cytosolic defects in the S. cerevisiae cca1-1 mutant suggests that, as in S. cerevisiae, the KlCCA1 gene encodes a sorting isozyme.
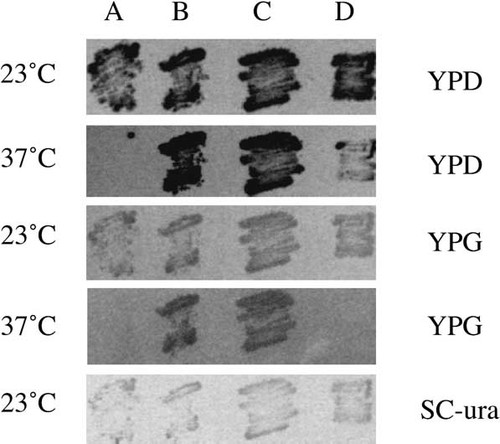
Complementation of S. cerevisiae cca1-1 mutation with K. lactis clones and constructs. Growth characteristics of strain NT33-5 transformed with: (A) plasmid p426 alone (control); (B) plasmid pKXS (containing the entire KlCCA1 open reading frame); (C) plasmid pK2 (containing the PCR product coding for tRNA nucleotidyltransferase lacking ATG1); or (D) plasmid pKE (containing the PCR product coding for tRNA nucleotidyltransferase lacking its first 35 amino acids), at 23°C or 37°C on YPD (containing glucose, a fermentable carbon source) or YPG (containing glycerol, a non-fermentable carbon source) or at 23°C on SC-ura (synthetic complete medium lacking uracil). Transformants were patched on SC-ura medium, replica-plated to designated media and incubated for 2 days. The darkness of the patches on rich media can be attributed to pink pigmentation that results from an ade2 mutation present in strain NT33-5.
A common method for producing ‘sorting isozymes’ (Gillman et al., 1991), i.e. products of one gene that carry out analogous functions at more than one intracellular destination, is the use of heterogeneous transcripts that produce protein products with or without mitochondrial targeting signals (see Small et al., 1998). The S. cerevisiae CCA1 gene contains three in-frame ATG codons (Aebi et al., 1990). Transcription initiation sites have been mapped upstream and downstream of the first in-frame ATG (Wolfe et al., 1994) and when transcripts containing the first ATG are translated they produce a longer form of the protein that is targeted to mitochondria. The shorter transcripts lacking this first ATG produce the cytosolic and nuclear forms of the enzyme (Wolfe et al., 1994). Because the KlCCA1 gene contains two potential start codons separated by only two amino acids, it seems unlikely that a similar process could occur in this gene. It is difficult to imagine that two amino acids are sufficient to encode a complete mitochondrial targeting signal, and our data (Figure 4) suggest that this is not the case. Perhaps the KlCCA1 situation more closely resembles that of the S. cerevisiae TRM1 gene, where two forms of N2,N2-dimethylguanosine-specific tRNA methyltransferase (a long form containing 17 additional amino-terminal amino acids and a short form lacking these amino acids) are generated from a single gene (Ellis et al., 1987). Both forms of this enzyme contain mitochondrial targeting information; however, the long form is more efficiently targeted to the mitochondrion, while the short form is shared between the mitochondrion, cytosol and nucleus (Ellis et al., 1989). For KlCCA1 it is possible that when the first in-frame ATG is used, the additional three N-terminal amino acids present create a more efficient targeting signal. However, it seems unlikely that these three amino acids could make a significant difference in targeting efficiency. It is clear from our experiments that the presence or absence of these amino acids has no dramatic effect on complementation of the S. cerevisiae cca1-1 mutation since both forms allowed efficient growth of NT33-5 on a non-fermentable carbon source (Figure 4). Perhaps the situation for KlCCA1 is more similar to that seen with the S. cerevisiae MOD5 gene. In this case, one form of the MOD5 gene product is generated containing a less than optimal mitochondrial targeting signal, such that the protein is distributed between the mitochondrion and the cytosol (Boguta et al., 1994). In fact, even for the S. cerevisiae tRNA nucleotidyltransferase, some of the enzyme containing the mitochondrial targeting signal can be found outside of the mitochondrion (Wolfe et al., 1996). Perhaps a single form of the protein containing an inefficient mitochondrial targeting signal is generated from KlCCA1, such that it is not imported efficiently into the mitochondria, so that a significant amount remains accessible in the cytosol or can be imported into the nucleus.
Further analysis will be required to determine which start codon(s) is(are) used for synthesis of the K. lactis tRNA nucleotidyltransferase. It is unlikely that the third in-frame start codon is used because it is downstream of the conserved DXD motif (Figure 3) that has been shown to be required for tRNA nucleotidyltransferase activity (Yue et al., 1998).
In S. cerevisiae, tRNA nucleotidyltransferase is known to function in the nucleus because tRNAs restricted to the nucleus have been shown to contain CCA ends (Knapp et al., 1979). However, no nuclear localization signal has been identified on the S. cerevisiae tRNA nucleotidyltransferase. Since the K. lactis gene complements the cca1-1 mutation, it is likely targeted to the nucleus in S. cerevisiae. However, analysis of the KlCCA1 sequence did not reveal a typical nuclear localization signal and further experiments will be required to determine whether this enzyme is targeted to the nucleus in K. lactis.
Acknowledgements
This work was supported by the Natural Sciences and Engineering Research Council (NSERC) of Canada. We are grateful to Dr M. Stark for providing the Kluyveromyces lactis genomic library and to Dr L. Sahlman for critically reading the manuscript.




