Variation in genetic and environmental influences in serum lipid and apolipoprotein levels across the lifespan in Swedish male and female twins
Abstract
The contribution of genetic and environmental factors to variation in lipids and apolipoproteins has been estimated in previous twin and family studies. However, it is unclear whether there are sex and/or age differences in parameter estimates. We investigated a sample selected from the population-based Swedish Twin Registry of 725 like- and unlike-sex twin pairs, ages 17–85. Quantitative genetic methods were used to evaluate sex and age differences in genetic and environmental variation in lipid and apolipoprotein levels in three age groups, 17–49, 50–69, and 70–85. Heritabilities for lipids and apolipoproteins ranged from 35%–74%. Consistent sex differences were found in triglycerides. Females had higher heritabilities (56%) than males (35%) across the age groups. Total phenotypic variation increased across the age groups for cholesterol and apolipoprotein B due to an increase in unique environmental variance components. In contrast, in apolipoprotein A1 variance was highest in the middle age group and no differences were found in the phenotypic variance between age groups for triglycerides. We concluded that differences in phenotypic variation for cholesterol and apolipoprotein B were almost entirely due to the accumulation of environmental experiences throughout life, whereas there were no consistent patterns of differences in phenotypic variance for apolipoprotein A1 and triglycerides. © 2001 Wiley-Liss, Inc.
INTRODUCTION
Twin and family studies have been of major importance in establishing the importance of genetic and environmental effects for cardiovascular disease (CVD) risk factors and mortality [Iselius, 1979; Whitfield and Martin, 1983; Hamsten et al., 1986; Nora et al., 1991; Heller et al., 1993; Goldbourt et al., 1994; Boomsma et al., 1996; Snieder et al., 1997]. However, there are inconsistencies between studies regarding the estimates of genetic and environmental variation across sex and various age groups.
Sex differences in the sources of variation in lipid and apolipoprotein levels have been reported. Heller et al. [1993] found significantly higher heritabilities in women than in men for apolipoprotein B. Sex differences in heritabilities were also reported for triglycerides in a family study [Towne et al., 1993]. In contrast, other family studies have reported no sex differences for lipids and apolipoproteins [Bodurtha et al., 1991; Boomsma et al., 1996]. Such inconsistencies in results could be due to design or type of the study, selection of the study material, or differences in exposures to environmental factors that may have an indirect impact on lipid and apolipoprotein levels (i.e., dietary habits, medication, etc.).
It is well established that the phenotypic variance of most lipids and apolipoproteins increases cross-sectionally across age groups or generations [Reilly et al., 1990; Ericsson et al., 1991; Heller et al., 1993; Boomsma et al., 1996; Snieder et al., 1997]. Significant differences in variances between age groups were reported previously for total cholesterol, HDL, LDL, triglycerides, apolipoprotein A1, and apolipoprotein B. It is important to understand whether the increase in variances across age groups is due to genetic influences, environmental influences, or both.
Genetic and environmental components of variance may also differ across different age groups. Heller et al. [1993] found that heritabilities for apolipoprotein B and triglycerides were significantly lower in an older group of twins than in a middle-aged group of twins. Boomsma et al. [1996] found significantly lower heritabilities in parents compared to twin offspring and suggested that a decrease in heritabilities as people grow old is due to an increase in environmental variance with increasing age. However, in a reanalysis of the twin sample (including even adult twins), heritabilities were found to be similar across generations [Snieder et al., 1997].
The above-mentioned studies have limitations with regard to age and sex effects. They are either lacking opposite-sex twin pairs, which are needed in order to understand the etiology of potential sex differences, or they evaluate restricted age spans instead of the entire lifespan. In this study we will estimate the relative importance of genetic and environmental influences on variation in male and female phenotypes and determine whether or not the same set of genes or environmental experiences influence a trait in males and females. We will use like-sex twin pairs and also a large group of opposite-sex twin pairs, between the ages of 17 and 85, all selected from the Swedish Twin Registry [Cederlöf and Lorich, 1978; Pedersen et al., 1996]. We will also explore age differences in the variation of lipids and apolipoproteins during the lifespan.
METHODS
Subjects
This study uses data gathered from three substudies, based on the Swedish Twin Registry [Pedersen et al., 1996]: Swedish Adoption Twin Study of Aging (SATSA) [Pedersen et al., 1984]; GENDER [Malmberg et al., 1995]; and the Screening Across Lifespan Twin (SALT) pilot study [Svedberg et al., 2000]. Twins in the SATSA were identified as having been reared apart, along with matched pairs of twins who had been reared together [Pedersen et al., 1984]. SATSA is a longitudinal study with a 3-year interval between measurement occasions, each wave containing both questionnaire and in-person-testing components. The biological measurements used in this study were taken from the third in-person testing and the sample is comprised of 216 twin pairs. A more detailed description of blood collection procedures in the SATSA study was given elsewhere [Pedersen et al., 1991]. The GENDER study is comprised of unlike-sex twins that were between the ages of 70 and 79 during data collection [Malmberg et al., 1995]. A questionnaire and a health assessment were collected in order to explore sex differences in health and aging. Biological specimens were available from 226 twin pairs from this study. Finally, 283 twin pairs come from the SALT pilot study, which was conducted in fall 1996 and aimed at screening all major complex diseases. A total of 850 pairs of twins, ages 17 and above, were randomly chosen from the Swedish Twin Registry. They were contacted for a computer-assisted telephone interview concerning health status. They were then asked to go to their local nurses' office for blood sampling.
Informed consent was obtained from all subjects in all three substudies. Zygosity of the like-sex pairs was determined by the use of a set of DNA markers.
Serum Lipids and Apolipoproteins
The subjects were requested to fast 12 hrs before testing. They were also asked about all medication used in the past month. Eighteen of 1,450 individuals reported taking drugs prescribed specifically to lower lipid levels.
All blood samples were processed by the same laboratory. They were frozen at −70°C, prior to processing. Serum triglycerides were measured by an enzymatic determination of glycerol with glycerol-phosphate-oxidase (GPO) after hydrolysis with lipoprotein lipase [Fossati and Prencipe, 1982]. Cholesterol was determined by an enzymatic assay based on cholesterol esterase and cholesterol oxidase conversion followed by a Trinder-type sequence of reaction [Allain et al., 1976]. Apolipoproteins A1 and B were determined by an immunochemical reaction [Riepponen et al., 1987].
Statistical Analysis of Lipids
The distribution of values for triglyceride levels were skewed and therefore transformed logarithmically before statistical analysis. Analyses of lipid values included descriptive statistics and quantitative genetic analysis. They were performed on three age groups in order to test for sex differences within the age groups and to explore differences in genetic and environmental effects between the age groups. The first age group included twin pairs between 17 and 49 years, the second between 50 and 69, and the third between 70 and 85. The youngest age for the SATSA sample was 50, so that age was chosen as a cutoff point for the first age group. The GENDER study comprises opposite-sex twin pairs above the age of 70. With that in mind, 70 years of age was chosen as an arbitrary cutoff for the older age group.
Multiple regression, adjusting for medication, fasting (yes/no), diabetes (yes/no), and age (in years), was applied in order to test for mean differences in lipids and apolipoproteins between the three substudies by sex and age groups. The medication variable was scored for use of diuretics (yes/no), beta-blockers (yes/no), lipid lowering (yes/no), and hormone treatment (yes/no) medicines. There were no apparent systematic differences in the mean values of lipids and apolipoproteins between the substudies. A homogeneity of variance test was used in order to test for variance differences of the lipids and apolipoproteins between substudies by age groups and sex (PROC GLM in SAS). There were no systematic differences between the study samples for any of the lipids or apolipoproteins. Therefore, further statistical analysis proceeded as a joint analysis of the three substudies.
Since lipid and apolipoprotein measurements can be affected by the previously mentioned factors (medication, fasting, diabetes, and age), a multiple regression by sex and age groups was used to assess their linear effects. The residuals were then used in the subsequent quantitative genetic analysis.
Quantitative Genetic Analysis
Twin studies are ideal for estimating genetic and environmental effects of traits and diseases. Identical (monozygotic (MZ)) twins share the same genes, whereas fraternal (dizygotic (DZ)) twins share, on average, half of their segregating genes. A measure of the similarity between twins is the intraclass correlation [Neale and Cardon, 1992]. Comparisons between the intraclass correlations for MZ and DZ twins provide information about the effects that are present.
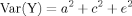
The correlation for MZ twin pairs is assumed to be due to additive genetic and shared environmental factors (a2+c2). The within pair correlation in DZ twins is assumed to be due to the sum of half the genetic and shared environmental factors (½a2+c2). A genetic effect is indicated if twin similarity is greater among MZ than DZ pairs. Heritability is defined as the proportion of total phenotypic variation directly attributable to genetic effects [Falconer, 1989]. Consequently, significant heritability reflects the importance of genetic variation for a trait or disease. The partitioning of phenotypic variance into genetic and environmental effects is usually illustrated in a path diagram. Genetic, shared, and nonshared environmental components are presented as latent variables [Neale and Cardon, 1992]. The genetic correlation (ra) is set to 1 in MZ twins and to 0.5 in like-sex DZ twins. The shared environment correlation (rc) is set to 1 for both groups. By definition there is no correlation for the nonshared environment. Figure 1 illustrates a path diagram for an opposite-sex twin pair. The genetic, shared, and nonshared environmental variance components are noted as am, cm, em, af, cf, and ef, for males and females, respectively. The genetic correlation (ra) is left free for estimation rather than fixed to 0.5. Alternatively, the shared environmental correlation (rc) is set free rather than being fixed to 1.
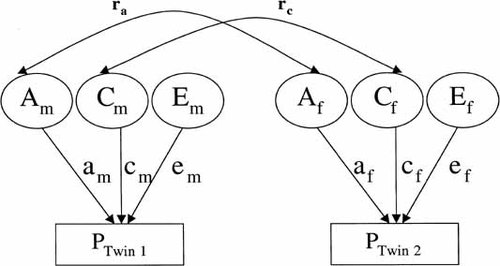
Basic path diagram for an opposite sexed twin pair. Am, Cm, Em, Af, Cf, and Ef are the genetic, shared and unique environmental variance components for males and females, respectively. The genetic correlation, ra, and the shared environmental correlation, rc, are set free to be estimated in the model.
We fitted a series of models in order to test for sex differences [Neale and Martin, 1989]. In the constrained model we assumed equal genetic and environmental variance components for males and females. That is, there were no sex differences in the genetic and environmental influences. The next step was to test whether there were sex differences in the relative importance of these effects by assuming one set of parameters for males in both like- and unlike-sex twin pairs and similarly another set of parameters for females. This model is called the general sex-limitation model. If the latter model fitted better, a scalar sex-limitation model was tested that evaluated whether sex differences in the total phenotypic variance differed only by a scalar component. That is, the genetic and environmental variance in females is a scalar multiple of that in males, af + cf + ef = k(am + cm + em), whereas, for example, heritabilities are the same for both females and males. Further, two additional sex-limitation models were fitted in order to test whether there are different genes or environmental factors influencing phenotypic variation in the sexes. In the first of these two models we allowed not only different variance components for males and females but also the genetic correlation between the members of the opposite-sex twin pairs to vary. For instance, if the genetic correlation is estimated at 0, it indicates that completely different genes influence the trait in males and females. The next model is similar to the previous model, except that now we allowed the shared environmental correlation to vary. It should be noted that there is not enough information in the twin design to estimate both the genetic and environmental correlation at the same time.
We also tested for age differences in the genetic and environmental components by fitting four models. Initially we tested whether a constrained or a free age model fitted the data best in a manner analogous to that for sex differences. In the constrained age model the genetic and environmental components were set equal across the age groups. In the free model, genetic and environmental components between age groups were free to be estimated. That is, we tested whether there are differences in phenotypic variance in the magnitude of genetic and environmental influences, or both, between the age groups. If the free model gave a better fit to data, two age models were further tested. One model assumed different genetic components across the age groups, keeping the unique environmental components stable across the age groups. In the other age model, only the unique environmental components were allowed to be free for estimation, keeping the genetic components stable across the age groups. That is, we test whether differences in phenotypic variance across the age groups are due to differences in variation in genetic or environmental influences, respectively, across the age groups.
For the models fitted, the degrees of freedom and chi-square probability are reported, as well as Akaike's information criterion (AIC), computed as χ2−2df. A model is judged to have a good fit if it has a nonsignificant chi-square and a large negative AIC. The difference in chi-squares between nested models is calculated in order to test which of the two models fits better. A significant chi-square difference indicates that the model with fewer parameters to be estimated fits data worse.
Model-fitting approaches are based on structural equation models, which are implemented in the computer package Mx [Neale, 1994]. Models are applied to the variance-covariance data in order to estimate the genetic and environmental components.
RESULTS
Descriptive Statistics
Means and standard deviations of lipid and apolipoprotein levels by age group and sex are shown in Table I. Analyses utilizing multiple regression models showed significant sex differences in the second and third age groups for cholesterol, in the first age group for apolipoprotein B, in all age groups for apolipoprotein A1, and in the first and second age groups for triglycerides (Table I). Men had lower mean levels of apolipoprotein A1 than women in all three age groups. On the other hand, men had higher mean triglyceride levels than women in all three age groups. There seemed to be no consistent pattern for the differences in total cholesterol and apolipoprotein B across the three age groups.
| Age (years) | ||||||
|---|---|---|---|---|---|---|
| 17–49 | 50–69 | 70–85 | ||||
| N | Mean | N | Mean | N | Mean | |
| Cholesterol (mmol/l) | ||||||
| Men | 133 | 5.07 (±1.10) | 224 | 5.99 (±0.91)a | 299 | 6.09 (±1.13)a |
| Women | 179 | 5.02 (±0.91) | 242 | 6.32 (±1.08) | 373 | 6.73 (±1.22) |
| Apolipoprotein B (g/l) | ||||||
| Men | 133 | 0.97 (±0.27)a | 223 | 1.21 (±0.30) | 298 | 1.16 (±0.31) |
| Women | 179 | 0.86 (±0.21) | 242 | 1.17 (±0.30) | 373 | 1.22 (±0.32) |
| Apolipoprotein A1 (g/l) | ||||||
| Men | 133 | 1.23 (±0.18)a | 223 | 1.33 (±0.22)a | 298 | 1.33 (±0.21)a |
| Women | 179 | 1.33 (±0.21) | 242 | 1.49 (±0.26) | 373 | 1.49 (±0.23) |
| Triglycerides (mmol/l) | ||||||
| Men | 133 | 1.56 (±0.83)a | 224 | 1.74 (±1.10)a | 299 | 1.83 (±0.99) |
| Women | 179 | 1.10 (±0.57) | 242 | 1.35 (±0.80) | 373 | 1.77 (±0.88) |
- * Multiple regression, adjusting for medication, fasting, diabetes, and age, was used in order to test for sex differences in the mean levels of lipids and apolipoproteins within the age groups. Only one twin from a pair was randomly chosen for the analyses to avoid violation of sampling assumptions relating to independence.
- a Indicates significant sex differences in the respective age group, P ≤ 0.05; N = number of twins.
Intraclass Correlations
Intraclass correlations, after adjustment for age, medication, fasting, and diabetes, are presented by age group and sex in Table II. In general, intraclass correlations were higher in MZ than in DZ twins, indicating genetic influences. In the second age group, correlations for triglycerides were higher in females (both MZ and DZ twins) than in males, indicating possible sex differences. Intraclass correlations of the unlike-sex twins gave no consistent indication of sex differences across the various phenotypes. It also appeared that intraclass correlations were lower in the third age group than in the first and second for almost all lipids and apolipoproteins, indicating a possible age effect.
| Men | Women | Unlike-sexed | |||
|---|---|---|---|---|---|
| MZ | DZ | MZ | DZ | ||
| 17 ≤ Age <50 | |||||
| N | 25 | 22 | 45 | 25 | 39 |
| Cholesterol (mmol/l) | 0.55 | 0.27 | 0.60 | 0.09 | 0.41 |
| Apolipoprotein B (g/l) | 0.61 | 0.35 | 0.71 | 0.004 | 0.44 |
| Apolipoprotein A1 (g/l) | 0.41 | 0.03 | 0.47 | 0.11 | 0.22 |
| Triglyceride (mmol/l)a | 0.56 | 0.27 | 0.52 | 0.22 | 0.20 |
| 50 ≤ Age <70 | |||||
| N | 36 | 55 | 34 | 67 | 36 |
| Cholesterol (mmol/l) | 0.77 | 0.28b | 0.58 | 0.17 | 0.08 |
| Apolipoprotein B (g/l) | 0.82 | 0.36 | 0.62 | 0.11 | 0.23 |
| Apolipoprotein A1 (g/l) | 0.80 | 0.22 | 0.71 | 0.33 | 0.04 |
| Triglyceride (mmol/l)a | 0.27 | 0.02b | 0.64 | 0.23 | 0.10 |
| 70 ≤ Age ≤ 85 | |||||
| N | 9 | 21 | 25 | 29 | 223 |
| Cholesterol (mmol/l) | 0.19 | 0.13 | 0.52 | 0.56 | 0.21c |
| Apolipoprotein B (g/l) | 0.32 | 0.28 | 0.58 | 0.50 | 0.28 |
| Apolipoprotein A1 (g/l) | 0.25 | −0.10 | 0.46 | 0.03 | 0.25 |
| Triglyceride (mmol/l)a | 0.13 | 0.31 | 0.53 | −0.04 | 0.29c |
- * All values are corrected for age, medication, fasting and diabetes by multiple regression. MZ, monozygotic or identical twins; DZ, dizygotic or fraternal twins; N, number of twin pairs.
- a Logarithmical values are used.
- b 56 number of twin pairs.
- c 224 number of twin pairs.
Model Fitting
Model fitting results for cholesterol are presented in Table III. For age group 17–49, the constrained model, which sets the variance components to be equal in the sexes, heritability (a2) was estimated at 64%, the shared environment (c2) at 0%, and the nonshared environment (e2) at 36%. There was no significant difference between the general sex-limitation model (where separate variance components for the sexes are estimated) and the constrained model (δχ2 = 1.6; P = 0.66). The AIC for the constrained model also had a larger negative value (AIC = −12.82) than that of the general sex-limitation model (AIC = −8.35), suggesting a better fit of the data in the constrained model, which fitted the data best even for the other two age groups. However, the results for the middle age group were borderline significant and the AIC was lower for the sex-limitation model. In spite of that, estimates of genetic and environmental influences did not deviate much for men and women (heritabilities were 64% and 57%, respectively) in the sex-limitation model, so further analysis was done by using the constrained model for the middle age group.
| Sex models | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age groups | Model | Men | Women | Fit of model | ||||||||||
| a2 | c2 | e2 | a2 | c2 | e2 | χ2 | df | P | AIC | Δχ2 | df | P | ||
| 95% c.i. | 95% c.i. | 95% c.i. | 95% c.i. | 95% c.i. | 95% c.i. | |||||||||
| 17–49 | Constrained | 0.64 | 0 | 0.36 | 11.2 | 12 | 0.51 | −12.82 | ||||||
| 0.30–0.75 | 0–0.26 | 0.25–0.52 | 1.6 | 3 | 0.66 | |||||||||
| Sex-limitation | 0.63 | 0 | 0.37 | 0.64 | 0 | 0.36 | 9.6 | 9 | 0.38 | −8.35 | ||||
| 0.35–0.79 | 0–0.43 | 0.20–0.64 | 0.42–0.78 | 0–0.29 | 0.22–0.58 | |||||||||
| 50–69 | Constrained | 0.65 | 0 | 0.35 | 29.9 | 24 | 0.19 | −18.04 | ||||||
| 0.45–0.76 | 0–0.13 | 0.24–0.49 | 7.4 | 3 | 0.06 | |||||||||
| Sex-limitation | 0.64 | 0.09 | 0.27 | 0.57 | 0 | 0.43 | 22.5 | 21 | 0.37 | −19.55 | ||||
| 0.17–0.83 | 0–0.46 | 0.16–0.46 | 0–0.74 | 0–0.39 | 0.25–0.73 | |||||||||
| 70–85 | Constrained | 0.49 | 0.02 | 0.49 | 11.9 | 12 | 0.46 | −12.13 | ||||||
| 0–0.66 | 0–0.35 | 0.34–0.78 | 5.3 | 3 | 0.15 | |||||||||
| Sex-limitation | 0.23 | 0.04 | 0.73 | 0.18 | 0.39 | 0.43 | 6.6 | 9 | 0.67 | −11.39 | ||||
| 0–0.68 | 0–0.44 | 0.32–0.98 | 0–0.73 | 0–0.65 | 0.25–0.67 | |||||||||
- * a2, c2 and e2, genetic, shared and non-shared environmental components, respectively; constrained, model with same parameters for males and females; sex-limitation, model with separate parameters for males and females; AIC, Aikakes information criteria; Δχ2, the difference in chi-square between the constrained and sex-limitation models.
Next, we tested whether there were significant differences in genetic and environmental components across the age groups for cholesterol. Since the shared environmental component was consistently nonsignificant through all the age groups for all lipids and apolipoproteins examined, it was omitted from the age models. The free age model for cholesterol had a chi-square of 53.0 (the sum of chi-squares for the constrained sex models with the shared environmental component fixed to zero for all age groups: 11.2 + 29.9 + 11.9) with 51° (13 + 25 + 13) of freedom. The free age model fitted significantly better than the constrained age model, in which the parameters were set to be equal across age groups (δχ2 = 38.7; df = 2; P < 0.01), indicating that there were differences in phenotypic variance and/or in the proportion of genetic and environmental variance components between the age groups. However, a model where the unique environmental variance components were allowed to differ across the age groups, keeping the genetic variance components stable, did not fit data worse than the free age model (δχ2 = 1.6; df = 2; P = 0.45). Thus, a model that allowed for variance differences between the age groups due to differences in unique environmental variance components appeared to be the most parsimonious model for cholesterol. Figure 2a depicts the best-fitting age model. According to this model the total phenotypic variance for cholesterol increased across the age groups due to an increase in the unique environmental component, while the genetic component was the same across the age groups.
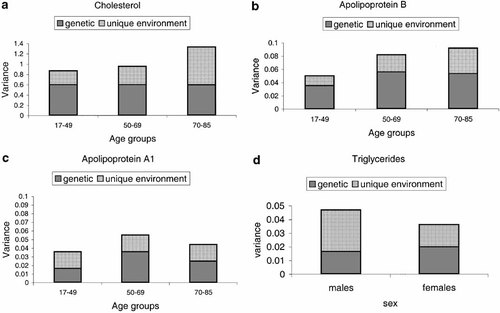
Genetic and unique environmental variance components for cholesterol, apolipoprotein B and A1, and triglycerides, respectively, across the age groups.
There were no sex differences in the variance components of apolipoprotein B in any of the three age groups (Table IV). The chi-square tests for the difference between the constrained and general sex-limitation models were not significant. The constrained model fitted the data best, suggesting the same significant genetic and unique environmental effects influencing the variation in apolipoprotein B in men and women. Table IV shows that the genetic influence was approximately the same in the young and middle age groups and lower in the older age group for apolipoprotein B. The free age model that allowed the genetic and unique environmental components to be free across the age groups fitted the data most parsimoniously for apolipoprotein B (χ2 = 89.9; df = 51; AIC = −12.0). Figure 2b illustrates the increasing phenotypic variance across the age groups according to the best-fitting model for apolipoprotein B.
| Sex models | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age groups | Model | Men | Women | Fit of model | ||||||||||
| a2 | c2 | e2 | a2 | c2 | e2 | χ2 | df | P | AIC | Δχ2 | df | P | ||
| 95% c.i. | 95% c.i. | 95% c.i. | 95% c.i. | 95% c.i. | 95% c.i. | |||||||||
| 17–49 | Constrained | 0.70 | 0 | 0.30 | 26.9 | 12 | 0.01 | 2.99 | ||||||
| 0.42–0.79 | 0–0.22 | 0.20–0.44 | 6.3 | 3 | 0.10 | |||||||||
| Sex-limitation | 0.65 | 0.05 | 0.35 | 0.74 | 0 | 0.26 | 20.6 | 9 | 0.01 | 2.64 | ||||
| 0.11–0.79 | 0–0.45 | 0.20–0.60 | 0.47–0.84 | 0–0 | 0.05–0.43 | |||||||||
| 50–69 | Constrained | 0.68 | 0 | 0.32 | 37.0 | 24 | 0.04 | −10.97 | ||||||
| 0.48–0.77 | 0–0.15 | 0.23–0.44 | 2.4 | 3 | 0.49 | |||||||||
| Sex-limitation | 0.60 | 0.12 | 0.28 | 0.59 | 0 | 0.41 | 34.6 | 21 | 0.03 | −7.43 | ||||
| 0.16–0.82 | 0–0.51 | 0.17–0.42 | 0.26–0.76 | 0–0.18 | 0.23–0.66 | |||||||||
| 70–85 | Constrained | 0.38 | 0.13 | 0.49 | 25.1 | 12 | 0.01 | 1.13 | ||||||
| 0–0.69 | 0–0.41 | 0.31–0.73 | 1.9 | 3 | 0.59 | |||||||||
| Sex-limitation | 0.14 | 0.18 | 0.68 | 0.37 | 0.22 | 0.41 | 23.2 | 9 | 0.01 | 5.19 | ||||
| 0–0.67 | 0–0.49 | 0.33–0.89 | 0–0.75 | 0–0.59 | 0.24–0.68 | |||||||||
- * a2, c2 and e2, genetic, shared and non-shared environmental components, respectively; constrained, model with same parameters for males and females; sex-limitation, model with separate parameters for males and females; AIC, Aikakes information criteria; Δχ2, the difference in chi-square between the constrained and sex-limitation models.
There were also no sex differences for apolipoprotein A1 (Table V). The genetic influence was higher in the middle age group and decreased in the older age group (Table V). Model fitting for age differences showed that the phenotypic variance in apolipoprotein A1 differed across the age groups due to differences in genetic variance, while the unique environmental variance component was held constant across the age groups (δχ2 = 3.4; df = 4; P = 0.49). Phenotypic variance increases between the first and second age groups but decreases between the second and third age groups for apolipoprotein A1. The best-fitting age model is depicted in Figure 2c.
| Sex models | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age groups | Model | Men | Women | Fit of model | ||||||||||
| a2 | c2 | e2 | a2 | c2 | e2 | χ2 | df | P | AIC | Δχ2 | df | P | ||
| 95% c.i. | 95% c.i. | 95% c.i. | 95% c.i. | 95% c.i. | 95% c.i. | |||||||||
| 17–49 | Constrained | 0.45 | 0 | 0.55 | 25.5 | 12 | 0.01 | 1.49 | ||||||
| 0.06–0.60 | 0–0.29 | 0.39–0.74 | 5.4 | 3 | 0.14 | |||||||||
| Sex-limitation | 0.41 | 0 | 0.59 | 0.48 | 0 | 0.51 | 22.1 | 9 | 0.02 | 2.07 | ||||
| 0–0.66 | 0–0 | 0.34–0.93 | 0–0.66 | 0–0 | 0.34–0.76 | |||||||||
| 50–69 | Constrained | 0.74 | 0 | 0.26 | 33.1 | 24 | 0.10 | −14.89 | ||||||
| 0.58–0.82 | 0–0.11 | 0.18–0.39 | 6.1 | 3 | 0.11 | |||||||||
| Sex-limitation | 0.79 | 0 | 0.21 | 0.56 | 0.12 | 0.32 | 27.0 | 21 | 0.17 | −14.99 | ||||
| 0.56–0.88 | 0–0.17 | 0.11–0.37 | 0.06–0.81 | 0–0.47 | 0.19–0.55 | |||||||||
| 70–85 | Constrained | 0.47 | 0 | 0.53 | 13.5 | 12 | 0.33 | −10.48 | ||||||
| 0–0.64 | 0–0.27 | 0.36–0.81 | 2.2 | 3 | 0.53 | |||||||||
| Sex-limitation | 0.39 | 0 | 0.61 | 0.52 | 0 | 0.48 | 11.3 | 9 | 0.25 | −6.69 | ||||
| 0–0.76 | 0–0.39 | 0.23–0.92 | 0–0.73 | 0–0.39 | 0.27–0.80 | |||||||||
- * a2, c2 and e2, genetic, shared and non-shared environmental components, respectively; constrained, model with same parameters for males and females; sex-limitation, model with separate parameters for males and females; AIC, Aikakes information criteria; Δχ2, the difference in chi-square between the constrained and sex-limitation models.
For triglyceride levels, the general sex-limitation model fitted data better in the second age group (Table VI). There was a significant difference between the constrained and the general sex-limitation model (P <0 0.01), showing that there were significant sex differences in the variation of triglycerides. Based on this model, the genetic influence was higher in females than in males (Table VI). The scalar model for testing sex differences in the genetic and environmental components did not fit data better than the general sex-limitation model, nor did the sex-limitation models where the genetic and environmental correlations were set to vary. In the third age group, sex differences were not more apparent with the constrained model fitting than with the sex-limitation model fitting. However, the constrained age model, assuming the same set of genetic and environmental variance components for males in all three age groups and another set of parameters for females in all age groups, fitted data better than the free age model, assuming different genetic and environmental variance components for males and females in all three age groups (δχ2 = 7.9; df = 8; P = 0.44). The latter indicated no differences in the phenotypic variance between age groups for triglyceride levels, but consistent sex differences in the genetic and environmental influences across the age groups. Heritabilities were estimated to 35% and 56% for males and females, respectively. The variance components for triglycerides for the best-fitting age model are shown in Figure 2d.
| Sex models | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age groups | Model | Men | Women | Fit of model | ||||||||||
| a2 | c2 | e2 | a2 | c2 | e2 | χ2 | df | P | AIC | Δχ2 | df | P | ||
| 95% c.i. | 95% c.i. | 95% c.i. | 95% c.i. | 95% c.i. | 95% c.i. | |||||||||
| 17–49 | Constrained | 0.54 | 0 | 0.46 | 1.49 | 12 | 0.24 | −9.06 | ||||||
| 0.10–0.67 | 0–0.34 | 0.33–0.64 | 2.9 | 3 | 0.41 | |||||||||
| Sex-limitation | 0.57 | 0 | 0.43 | 0.41 | 0.10 | 0.49 | 12.0 | 9 | 0.21 | −5.96 | ||||
| 0–0.75 | 0–0.64 | 0.25–0.76 | 0–0.68 | 0–0.58 | 0.32–0.72 | |||||||||
| 50–69 | Constrained | 0.37 | 0 | 0.63 | 31.9 | 24 | 0.13 | −16.03 | ||||||
| 0.05–0.52 | 0–0.21 | 0.48–0.81 | 13.0 | 3 | < 0.01 | |||||||||
| Sex-limitation | 0.19 | 0 | 0.81 | 0.62 | 0 | 0.38 | 18.9 | 21 | 0.59 | −23.14 | ||||
| 0–0.43 | 0–0.27 | 0.57–1 | 0.19–0.76 | 0–0.31 | 0.24–0.60 | |||||||||
| 70–85 | Constrained | 0.44 | 0.03 | 0.53 | 13.5 | 12 | 0.33 | −10.49 | ||||||
| 0–0.64 | 0–0.35 | 0.35–0.81 | 5.8 | 3 | 0.12 | |||||||||
| Sex-limitation | 0.28 | 0.12 | 0.60 | 0.47 | 0.04 | 0.49 | 7.7 | 9 | 0.56 | −10.26 | ||||
| 0–0.67 | 0–0.51 | 0.33–0.86 | 0–0.73 | 0–0.39 | 0.27–0.86 | |||||||||
- * a2, c2 and e2, genetic, shared and non-shared environmental components, respectively; constrained, model with same parameters for males and females; sex-limitation, model with separate parameters for males and females; AIC, Aikakes information criteria; Δχ2, the difference in chi-square between the constrained and sex-limitation models.
DISCUSSION
We have tested for sex and age differences in the relative importance of genetic and environmental factors for variation in lipids and apolipoproteins in a sample of twins from the Swedish Twin Registry. In the current study we found no sex differences in the variation of lipids, except in triglycerides. Further, there were age differences in phenotypic variance for cholesterol and apolipoproteins B and A1 that were due to differences in genetic variance components, unique environmental variance components, or both.
Heller et al. [1993] performed a similar analysis in a sample of like-sex Swedish twins that were reared apart or together (SATSA study) and were between the ages of 50 and 86. The current study sample includes and expands on that sample, comprising both like- and unlike-sex twin pairs at ages 17–85, covering almost the entire lifespan. It also addresses issues of sex and age differences simultaneously with the use of structural equation modeling.
Heritability estimates in our study ranged between 0.35 and 0.74, within the ranges of the previously reported Swedish study. Heritabilities for apolipoprotein B and triglyceride levels were slightly lower than the estimates of Heller et al. [1993], although the age groups were not defined in the same way. In general, heritabilities did not deviate much from those reported in other twin and family studies of total cholesterol, apolipoproteins A1 and B, and triglyceride levels [Iselius, 1979; Rao et al., 1982; Whitfield and Martin, 1983; Hamsten et al., 1986; Nora et al., 1991; Heller et al., 1993; Goldbourt et al., 1994; Snieder et al., 1997]. However, the estimates of the current study are more robust, considering its large sample size.
Twin and family studies suggest an increase in total phenotypic variance with increasing age [Reilly et al., 1990; Ericsson et al., 1991; Heller et al., 1993; Boomsma et al., 1996; Snieder et al., 1997, 1999]. In general, this could be due to an increase in genetic or unique environmental variance, or both. In the present study, model fitting showed that phenotypic variance increases across the age groups for cholesterol due to an increase in unique environmental variance. For apolipoprotein B the increase in phenotypic variance from the first age group to the second is mostly due to changes in genetic variance. However, above the age of 50 the increase in phenotypic variance is almost entirely due to an increase in unique environmental components (Fig. 2b), which is in accordance to the findings of Heller et al. [1993] on the SATSA sample. They reported significantly lower heritabilities in a group of old twins (ages 66–86), compared to a group of young twins (ages 52–65), for apolipoprotein B and triglycerides, due to an increase in unique environmental influences. This could be the result of accumulated environmental experiences over the life course [Nelson and Dannefer, 1992]. An exposure to large environmental variations, such as smoking, diet, stress, and alcohol, might affect lipid metabolism. Thus, it is important to gain information on specific nonshared environmental influences and also genotype by environment interactions [Jarvik et al., 1994]. The age effect could also reflect a weakening of the homeostatic control mechanisms of the lipid system with aging [Reilly et al., 1990; Snieder et al., 1997].
For apolipoprotein A1, differences in phenotypic variance were due to differences in genetic variance components between age groups. That is, different combinations of multiple genes influence lipids and apolipoproteins in different periods of life. However, total phenotypic variance presented a pattern of change that was difficult to interpret. Variance was higher in the middle age group and lower in the third age group. This decrease in variance could reflect cohort differences. Individuals with a genetic predisposition to lipid disorders may die at earlier ages and therefore not be present in the oldest age group (less variability). Although the size of the present twin sample is large, the effects of random variation cannot be fully excluded.
A recent review of twin and family studies concluded that the increase in variance of lipids and apolipoproteins from adolescence to adulthood could be due to interindividual variation in the rise of lipid levels over time and could be explained by an increase in either genetic and/or environmental variance [Snieder et al., 1999]. In accordance with these findings, the increase in total phenotypic variance for cholesterol and apolipoprotein B in the present study was mainly due to an increase in unique environmental variance. Snieder et al. [1999] also pointed out the existence of age-specific genetic influences in some of the lipids and apolipoproteins. The cross-sectional nature of the present study allowed us to view the age dependency of genetic effects by investigating differences in the magnitude of the genetic influence across age groups. The increase in genetic variance for apolipoprotein A1 across the age groups suggested that there may be age-specific genetic influences; however, longitudinal data are necessary to derive further conclusions.
We found no apparent sex differences in the variation of total cholesterol, apolipoprotein A1, and apolipoprotein B. However, there were significant sex differences for triglyceride levels of twins between the ages of 50 and 69. In contrast, Heller et al. [1993] reported significant sex differences in apolipoprotein B. In the current study we have a larger sample size and the added advantage of opposite-sex twins. Therefore, it seems unlikely that sex differences have a major effect on variation in apolipoprotein B. Nevertheless, sex differences in triglycerides are consistent with other studies [Towne et al., 1993]. Heritability was higher in women than in men. Above the age of 50, major changes take place in production of sex hormones in women, which may cause lipid changes. Schaefer et al. [1994] found that postmenopausal women have significantly higher apolipoprotein B and LDL cholesterol levels than premenopausal women, indicating hormonal effects on lipid metabolism.
The shared environmental estimate was almost consistently zero for all lipids and apolipoproteins, indicating that shared environmental influences have no or little impact on variation in adulthood. Snieder et al. [1997] reported a similar lack of shared environmental influence in a group of middle-aged twins, ages 34–63. Interestingly, they found significant shared environmental effects in ages 14–21, which pointed to the possibility of one or more environmental factors that have an influence on family members, only for as long as they live together. Thus, our study strengthens the hypothesis that shared environmental influences are of importance predominantly during the first portion of the lifespan.
One limitation of this study is that our conclusions are drawn from joint cross-sectional studies. Blood samples were collected and analyzed for the three substudies (SATSA, GENDER, and SALT pilot) by the same laboratory but in different time periods. We found no systematic mean and variance differences between substudies when testing by age groups and sex. The cross-sectional nature of this study precluded conclusions to be drawn about changes due to genetic and environmental influences over time.
Nevertheless the results can be used as a platform for future quantitative studies. Taking account of the age-dependent gene expression in lipids and apolipoproteins will improve the power of studies aimed at identifying the location and function of QTLs for components of the lipid system [Snieder et al., 1999]. Sex differences in triglyceride levels suggest that men and women preferably should be considered separately in linkage or association studies of the genes that influence triglyceride levels. Different biological mechanisms might influence triglycerides in men and women.
It has been shown in long-term follow-up studies on the Swedish Twin Registry that the influence of genetic effects on the risk of dying from coronary heart disease decreases at older ages [Marenberg et al., 1994]. The same pattern of decreasing genetic effects was shown in this study for cholesterol. However, a different pattern of change in variation was shown for triglycerides and apolipoprotein A1. To overcome the possible influence of genetic heterogeneity due to selective genetic mechanisms, a longitudinal approach is highly warranted on individuals followed throughout the lifespan.
Acknowledgements
The Swedish Adoption/Twin Study of Aging is supported in part by grants from the National Institute of Aging (R01 AG-04563 and AG-10175) and the MacArthur Research Network on Successful Aging. The Screening Across the Lifespan Twin pilot study was supported by Astra Hässle AB and Pharmacia. The GENDER study of men and women's health was supported by the MacArthur Foundation Research Network on Successful Aging, the Axel and Margret Ax:son Johnson Foundation, the Swedish Council for Social Research, and the Swedish Foundation for Health Care Sciences and Allergy Research.




