Comments on biological asymmetry
Abstract
Gross forms of asymmetry of biological structure, hence of development, are generally considered abnormalities of conformation with “perfect” symmetry, whether bilateral or radial, being regarded as the “ideal” form. This notion, primarily a cultural deceit of neo-Platonic origin, can easily be shown to be wrong or at best only skin-deep by any student of anatomy or surgery who finds the heart not in the midline but, most of the time on the left, liver on the right, gut coiled and disposed in a certain direction with appendix in the right lower quadrant, and so forth. Indeed, since the beginning of Amphioxus, a major effect of evolutionary developmental modification has been the abolition of the visceral symmetry which characterized this cephalochordate with introduction of a specific pattern of asymmetry called laterality determination.
This embryonic process, which is beginning to yield its universal molecular basis, is probably not responsible for another type of biological phenomenon designated fluctuating asymmetry well known to anthropologists (on the basis of quantitative studies of morphometric traits of teeth, appendicular skeleton, dermatoglyphics) and well-known to the ancients who in their most beautiful Hellenistic sculptures introduced deliberate asymmetries into facial structure and expression. Photographic images constructed of 2 right or 2 left facial halves may differ to a starling degree from the authentic face (Fig. 1). The relatively random nature of fluctuating asymmetry makes it less likely to be under strong natural selection.
1. Middle panel: Frontal view of face of a normal man. Left panel: “Artificial” face constructed out of two right halves of the same face. Right panel: Face constructed out of two left halves. A careful study of the right and left panels makes it easier to appreciate the actual degree of asymmetry present in the unaltered middle image/face.
However, in addition to laterality determination and fluctuating asymmetry, there are additional forms of biological asymmetry which have other biological bases such as Lyonization, somatic/clonal mosaicism, mosaic aneuploidy/polyploidy, chimaerism, and developmental “resistance” seen with especial clarity in virtually every hereditary limb malformation.
In this paper we will attempt to enumerate the causal forms and bases of biological asymmetry. © 2001 Wiley-Liss, Inc.
INTRODUCTION
Surely the almost “perfect” symmetry of the vertebrate body early during blastogenesis with its branchiomeric and mesomeric vascular system equal on right and left, must be a recapitulation of the phylogenetically oldest types of chordates or cephalochordates such as Amphioxus (Branchiostoma lanceolatum, Fig. 2a,b) whose very gonads are metamerically segmented in a bilaterally symmetrical manner. This fortunate animal has solved its problems with health insurance for cardiological care since it has no heart, but rather pumps blood, each branchial arch at a time, through a bilaterally symmetrical arrangement of bulbilli or branchial hearts.

Bilaterally symmetrical early (?ancestral) chordate Branchiostoma lanceolatum, the lancet fish or Amphioxus. a: upper panel: Fins (Fn), segmentation of body musculature (Ms) and of gonads (Go). An: anus; Oc: oral or buccal cirri: Bpo: branchial porus. Lf : lateral folds. Middle panel: Neural tube (Nt), notochord (Nc), oral cavity (Or), branchial pharynx (Bph), liver (Li), midgut (Mg), hindgut (Hg); Lower panel: Circulatory system. Aortic roots (Ar), Carotids (Ca), bulbilli (B), branchial arteries (Ba), hepatic/portal circulation and capillaries (Lc), portal vein (Pv), intestinal capillaries (Ic). b: left panel: cross-section through branchial pharynx; right: through midgut region. Abbreviations as above, plus: Dn: Dorsal (sensory) nerve; Vn: ventral (motor) nerve; M: muscle; Ce: celom; Ne: nephric canal; Br: branchial cleft; Pe: peribranchial space; Ov: Ovary; Ao: aorta. Modified from Kühn [1967].
However, in another form of marine chordate, the tunicates, a highly asymmetrical sessile adult form was identified as chordate only through study of its tadpole-like, more symmetrical larval form which becomes highly altered during attainment of sexual maturity, a striking example of evolutionary changes of symmetry in response to selective forces. But, then, it is not only the “early metazoans”, but also unicellular animals that have evolved highly differentiated morphogenetic asymmetries as in Plasmodium with its complex life cycle, the ciliate Paramecium, and many others.
It is tempting to postulate that the “invention” of the neural crest (the 4th germ layer [Hall, 2000]) as the single most critical evolutionary change in the chordates leading to the true vertebrates was the deciding event that imposed permanent lateral asymmetry on the visceral situs, leaving paraxial structures in a relatively undisturbed metameric, bilaterally symmetrical arrangement. This is true of the locomotory appendages, gonads, trunk organization, facial structures and sensory organs, but less so of lungs and kidneys.
The molecular machinery regulating the development of laterality (or lateral asymmetry) of heart, lungs/bronchi and viscera is being unraveled in all of its complexity and, as known to date, is reviewed in Varlet and Robertson [1997], Rankin et al. [2000], Garcia-Castro et al. [2000], Rodriguez-Esteban et al. [1999], Lohr et al. [1997], Srivastava [1997], and was detailed at this conference by Bisgrove and Yost [2001] and Hamada et al. [2001]. These data in several vertebrates make it clear that laterality determination, like all other inductive processes during gastrulation and neurulation, also is a process of pattern formation as defined by Davidson and his coworkers [Davidson et al., 1995; Peterson et al., 1997, 2000; Cameron et al., 1998] and reviewed in Opitz et al. [2001], Opitz and Clark [2000], Opitz and Rauch [1999], and Opitz [1993] as pertinent to blastogenesis in general, and more specifically during cardiogenesis. Since such defects of laterality determination as dextrocardia, asplenia, atrial isomerism or polysplenia are causally non-specific and highly heterogenous, and furthermore may occur or be produced in many different species of vertebrates, they are, by definition, field defects. The fact that variants of these malformations may at times occur in the same family suggested that in such families poly- and asplenia were genetically identical, hence, the formulation of the concept of polyasplenia to designate this field defect [Opitz, 1985].
Since polyasplenia is a quintessential defect of blastogenesis, it is, by definition, a polytopic anomaly [Martínez-Frías et al., 1998] and must be expected and frequently is found in combination with other defects of blastogenesis either in syndromal form or in what had been designated formerly as “associations”, but presently is designated simply as “polytopic field defects”. Thus, it is not surprising that in such a complex defect as lumbosacral agenesis (LSA) a laterality defect was found in 2/15 cases [Bohring et al., 1999: case 5, born to an insulin-depended woman, with LSA and malformations of other cervical and thoracic vertebrae, abnormal ribs and pelvis, congenital heart defect, pulmonary and renal abnormalities, imperforate anus and situs inversus with stomach and aorta on the right; case 14: LSA with multiple other vertebral anomalies, rib and pelvic defects, congenital heart and pulmonary defects, renal anomaly, imperforate anus, left pulmonary isomerism, diaphragmatic hernia, complete situs inversus and polysplenia].
OTHER ASYMMETRIC DEFECTS OF BLASTOGENESIS
…not related to the process of laterality determination are seen at times in fetal pathology including limb-body wall defects, the hemifacial microsomias, the Goldenhar anomaly [Opitz and Faith, 1969], with associated predisposition to monozygotic twinning, the multiple, bizarre anomalies in the acardia/acephalus form of twinning representing primarily resorbtive phenomena [Opitz et al., 2001], the Hanhart anomaly (Fig. 3) [Herrmann et al., 1976; Bersu et al., 1976], the Poland anomaly [Freire-Maia et al., 1973], etc. However, the most severe form of such blastogenetic disturbance was reported by Carranza et al. [1998] (Fig. 4) in a fetus who seemed to manifest complete absence of the left half of the body with fusion of the right into a circular shape with normal right arm and leg, but only half a face and single cardiac tube. A second infant in that report had gross deficiency and abnormalities also predominantly of left organs with rudimentary blind left ventricle, persistent left superior vena cava, absence of ductus arteriosus, transposition of great vessels with such midline anomalies as large umbilical hernia/exomphalocele, imperforate anus, absence of gallbladder and pancreas with large area of left cerebral pachygyria. Whatever the mechanism, the above entities would appear to be pure defects of blastogenesis, i.e. of induction of gross structure with apparently normal subsequent histogenesis of the malformed organs and parts of the body, something that is true of virtually all fetuses/infants with multiple defects of blastogenesis.

Hanhart anomaly or complex (“aglossia-adactylia syndrome”). Patient 1 of Herrmann et al. [1976] with microstomia, microglossia, micrognathia, imperforate anus and grossly asymmetrical limb involvement, postulated as a defect of Blechschmidt's “ectoderm ring”. Reproduced with the kind permission of the authors and the European Journal of Pediatrics and its publisher, Springer-Verlag.
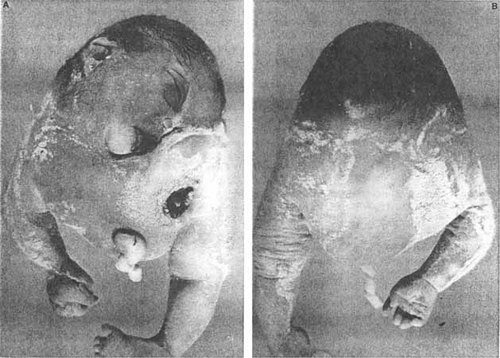
Hemibaby, lacking the left half of the body, published by Carranza et al. [1998]. Reproduced with the kind permission of the authors and the American Journal of Medical Genetics, and its publisher, Wiley-Liss. Inc.
DYSPLASTIC BLASTOGENESIS
On rare occasions morphogenesis may be disturbed by early defects of histogenesis, i.e. dysplasias, as in sacrococcygeal teratomas and epignathus anomalies. One of the most spectacular forms of such blastogenetic dysplasia was studied by Durkin-Stamm et al. [1978] at the University of Wisconsin involving two infants with gross malformations of external genitalia, limbs and spina bifida, parts which were infiltrated with teratomatous tissue which underwent sudden malignant transformation rapidly leading to death at about age 1 year (Fig. 5a,b). Drs Byrne and Carey [1995] at the University of Utah have studied a similar infant, and in all 3 it can be presumed that the teratomatous cell population, migrating and dividing in an uncontrolled manner, infiltrated the progenitor fields or budding primordia interfering with their ontogeny during blastogenesis and organogenesis.
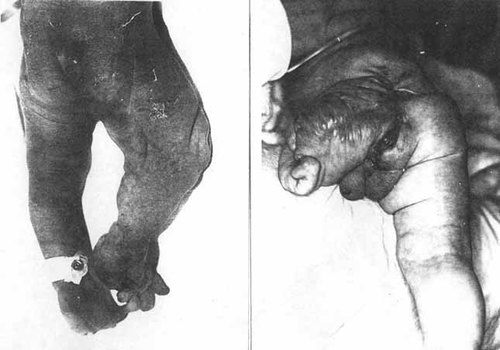
a, b. Dysplasia-malformation sequence of highly asymmetrical nature due to apparent infiltration of developing body parts with teratomatous tissue, increasing bulk of right leg in patient 1 of Durkin-Stamm et al. [1978], and clearly visible in the posterior thigh of the severely malformed left lower limb of their patient 2. Reproduced with the kind permission of the authors, the American Journal of Medical Genetics and its publishers, Wiley-Liss, Inc.
MUTATIONAL ASYMMETRIES
There is no more spectacular example of (presumed) mutational asymmetry affecting development than the Proteus syndrome, epitomized by the “Elephant Man”, and reviewed at this conference by Cohen [2001]. Indeed, it is a truism that none of the autosomal dominant dysplasia syndromes whether von Recklinghausen “disease” (NF1), the tuberous scleroses, or the nevoid basal cell carcinoma syndrome show symmetrical involvement presumably because of the stochastic nature of and irregular timing of their clonal origin and tumor distribution.
The complex mutational nature of the Wiedemann-Beckwith syndrome was reviewed by Li et al. [1998], its equally complex pathogenetic nature was studied earlier at the University of Wisconsin [Kosseff et al., 1972, 1976; Herrmann et al., 1977; Herrmann and Opitz, 1977; Opitz and Herrmann, 1977]. Hemihyperplasia, quarter-hyperplasia, uni- or bilateral macroglossia and the asymmetrical distribution of modular adrenal hyperplasia or tumor formation presumably reflects the clonal nature of the mutant cell lines in mosaic or non-mosaic individuals.
VASCULAR ASYMMETRIES
The vascular asymmetries of body structure as in the Sturge-Weber or Klippel-Trenaunay-Weber syndromes usually are of sporadic origin; pathogenetically they impress as a more or less severe exaggeration of the natural asymmetry in vascular development, most pronounced in the lymphatic system, slightly less so in the venous part of the circulation, and least in the arterial system. Impressive examples of such morphogenetic vascular asymmetry were described by Way et al. [1974, patient 1] in a 7-year-old boy with a form of cutis marmorata telangiectatica congenita (CMTC), enlarged right leg, nightly leg cramps, large vessel anomalies and recurring phlebectasias. Their case 2, also a boy, was studied at 13 months for hemihypertrophy involving the entire left side of the body and venous and capillary involvement in CMTC covering “most areas of the body”, but beginning to regress. Case 3, a 9-year-old boy with left leg pain since infancy, an increasing occurrence of varicose veins on buttocks and left leg and thigh, had experienced recurrent but evanescent crops of angiomata since age 3 years. When the left leg was raised its circumference was as normal as that of the right. Angiography showed venous dilatation and plexus formation of the left leg. Detailed histological and electronmicroscopic data were reported.
MOSAIC ASYMMETRIES
…are most easily observed and demonstrated in heterozygotes of X-linked conditions as a consequence of Lyonization. No two carriers of Fabry disease [von Gemmingen et al., 1965; Opitz et al., 1965] have the same pattern of corneal dystrophy in both eyes, or equal involvement with angiokeratomata on right or left body halves. The same is true of the fundus involvement in various types of retinitis pigmentosa, ocular albinism or choroideremia; indeed, it is to be expected that no manifesting carrier of an X-linked disorder will be symmetrically affected. Those conditions also affecting skin as in incontinentia pigmenti usually follow a pattern of Blaschko's lines, however, others as in calico cats, manifest mutant and non-mutant patches of variable size. There is one X-linked condition which, in hetero- and in hemizygotes, shows predominant unilateral involvement, namely the CHILD syndrome [Grange et al., 2000; König et al., 2000; Opitz, 2000] due to mutations of the NSDHL gene. The mechanism whereby one-half of the body is unaffected in a hemizygote with an X-linked dominant, male lethal disorder is presently unknown; according to R. I. Kelley (personal communication, 2000) the normal half of the body is as mutant as the abnormal side.
ANEUPLOID MOSAICISM
Hypo- and, in some cases hypermelanosis of Ito has become almost synonymous with aneuploid (or polyploid) mosaicism, the Blaschkolinear pigment patterns presumably indicating the distribution of the major cell lines. It has been seen in numerous disorders including mosaicism with trisomy 18, deletion 19p, partial trisomy 10, trisomy 13, functional disomy Xp, ring 22, trisomy 22, ring 10, X/autosome translocations, diploid/triploid mosaicism, and most lately trisomy 7 mosaicism [Magenis et al., 1999]. An early experience was with the Pallister mosaic aneuploidy syndrome first studied in 2 severely mentally retarded adults in Montana and one in Wisconsin with multiple congenital anomalies, hypertelorism, cataracts, Blaschkolinear pigmentary dysplasia, polymastia and seizures [Pallister et al., 1977]. This, not uncommon condition, later called Pallister-Killian syndrome, was found to be due to an isochromosome 12p in a cell line with rapid disappearance after birth from blood, and variable distribution in skin and buccal smear cells [Reynolds et al., 1987].
In 1977 also Marçallo et al. reported on a 10-year-old girl with a mental age of 7–8 years, normal height and head circumference, several minor anomalies and entire left side of body uniformly smaller than the right (Fig. 6a,b). The smaller side was considered the abnormal side and her condition interpreted as hemihypotrophy on the basis of mosaicism with a t(13q;7p) translocation, all lymphocytes showing both translocation chromosomes, but fibroblasts only one, hence monosomic for 13p and most of 13q and a terminal portion of 7p. In some cases, as in the boy reported by Magenis et al. [1999], the asymmetric mosaic nature of the skin pigmentary dysplasia may not be apparent until a later developmental age, with or without sun exposure. In the original cases of Pallister et al. [1977] the faint skin changes became especially noticeable with use of a Wood's light.
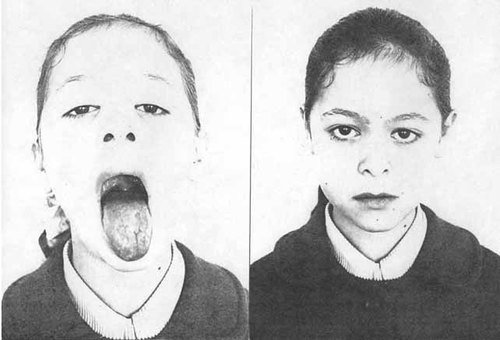
a, b. Ten-year-old girl with borderline intelligence, normal growth, multiple minor anomalies, and left hemihypotrophy of the entire body due to mosaicism with 2 cell lines, one with a balanced translocation between 13q and 7p, the other lacking the small translocation chromosome consisting of the tip of 7p, 13p, centromere and proximal part of 13q. From Marçallo et al., 1977. Reproduced with the kind permission of the authors, the European Journal of Pediatrics and its publishers, Springer-Verlag.
DYSOSTOSES AND LIMB MALFORMATIONS
As a rule, skeletal dysplasias have a far more symmetrical pattern of involvement than the dysostoses even if the latter are regularly inherited. Indeed, all dysostoses, especially limb malformations, must be regarded as asymmetrical until proven otherwise. Only a few examples will be shown.
In sporadic fibular a/hypoplasia, the commonest malformation of the long bones, only ¼ or so of cases are bilaterally affected, involving the right side in 62%, and the left in 38% of cases, males in 56%, females in 44% of cases; heterogeneity easily identifies this as a field defect [Lewin and Opitz, 1986].
A similar example is PFFD (proximal focal femoral deficiency, Fig. 7a,b [Sorge et al., 1995]). Again most cases are of sporadic occurrence; they vary from simple hypoplasia to total absence of the femur.
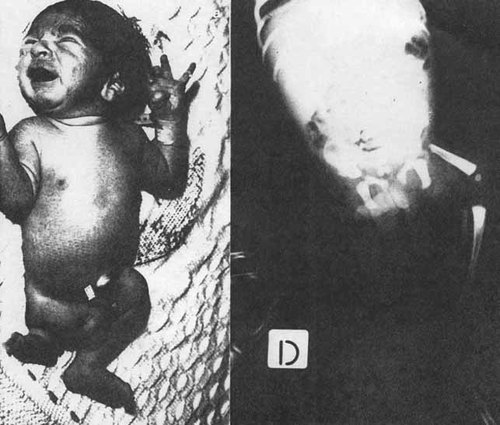
a, b. Patient 4 with PFFD (proximal focal femoral deficiency) of Sorge et al. [1995]. Reproduced with the kind permission of the authors, the American Journal of Medical Genetics, and its publishers, Wiley-Liss, Inc.
Since a large proximal limb element is defective, it would be surprising if the distal ones had been able to maintain their morphogenetic integrity. Indeed, in the FFU complex the ulna and fibula are also defective; in the femoral-facial complex that may be associated with maternal gestational diabetes, there may also be fibular and corresponding toe deficiencies. Ulnar ray defects are an uncommon anomaly of the long bones and are a phenotypically variable and genetically heterogeneous group of defects that may occur per se, usually sporadically, or as part of hereditary syndromes. Thus, they are field defects and pleiotropic components. In the report by Richieri-Costa and Opitz [1986] an instructive patient, a 4-year-old girl, had hypoplasia of both forearms with flexion contracture at the left elbow, and bilateral monodactylous ectrodactyly. She also had severe hypoplasia of the right leg and tibial deviation of the right foot with 5 normal toes. Radiographs showed absence of left ulna, and marked hypoplasia of the right ulna with monodactylous ectrodactyly, hypoplasia of the distal half of the right tibia and fibula and proximal and lateral displacement of the fibula with normal IVP. Her case is of especial interest in that she was born with a normal monozygotic (MZ) twin sister.
Striking asymmetry of (generally bilateral) limb involvement is also seen in the F-syndrome (F-form of acro-pectoro-vertebral “dysplasia”, [Grosse et al., 1969]) and in the Vordingborg form of synpolydactyly [Opitz, 1996; Kjaer et al., 2001] (Fig. 8).
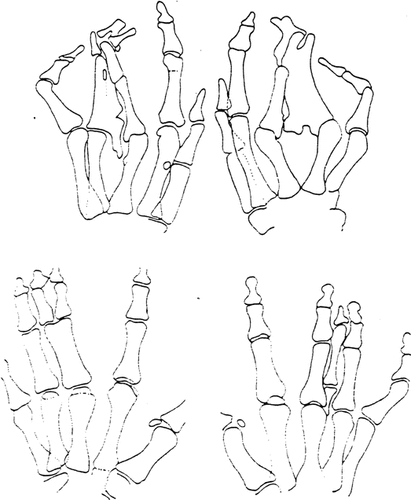
Strikingly asymmetric involvement of hands in Danish synpolydactyly, Vordingborg type, recently restudied with Danish colleagues with documentation of a HOX-D13 mutation as cause. Since the right and left hands of a patient can be considered monozygotic twins, this asymmetry likely is not due to the mutation, but local, stochastic, epigenetic factors.
However, it is the almost invariant unilateral involvement in tibial deficiency, frequently associated with a high degree of preaxial polydactyly, even in dominantly inherited cases, that caused Wiedemann and Opitz [1983] to propose a mechanism of “developmental resistance” to explain the non-concordance of the anomaly in right and left limbs of the same patient, limbs that can be regarded MZ twins, paralleling the frequent non-concordance in MZ twins with limb anomalies, as in the case of Richieri-Costa and Opitz [1986]. In retrospect, a different, or perhaps complementary mechanism for such non-concordance may be invoked, reviewed recently in Utkus et al. [2001] and supporting the epigenetic view of development, whereby the outcome of all gross morphogenetic processes is the result of complex interactions between genetic and environmental factors that confer, under specific circumstances a specific probability of a specific ontogenetic event to happen (or not to happen). The best model illustrating this concept was worked out over 70 years ago by Sewall Wright [1984] in his guinea pig work, specifically the over 30-year breeding experiments leading to the establishment of the pure-bred lines producing under specific circumstances of selection (of parental lines) and inbreeding, with a specific probability varying from line to line, a specific anomaly, namely otocephaly, to happen (or not to happen). The important point that emerged being that the genetic predisposition to otocephaly conferred only a limited potential of occurrence, and since the affected and unaffected littermates had the identical genetic constitution, the actual presence of the anomaly, to any degree of severity, must have been the result of purely random, stochastic epigenetic events early during embryogenesis. This is probably still a highly pertinent model to consider in any case of lateral non-concordance.
CANALIZATION AND FLUCTUATING ASYMMETRY
Wright's work and conclusions have a direct bearing on the related question of the stability of developmental systems and their “resistance” to epigenetic perturbances, such as the probability of otocephaly not happening. Earlier developmental geneticists such as Schmalhausen [1946] and Waddington [1957] emphasized the “amazing stability of complex developmental processes” [Gibson and Wagner, 2000]. Developmental canalization [Waddington, 1940] is generally defined as the property of developmental pathways of achieving a standard phenotype (i.e., one with a low variance about the mean) inspite of genetic and/or environmental disturbance. A synonym for this property is developmental homeostasis. Thus, canalization represents a morphogenetic buffering system reducing potential variation that may act at the gene level or on variation due to either genotype or environment [Rieger et al., 1968]. This concept has extremely important phylogenetic consequences since any process that reduces the level of expressed variation of a trait reduces the capacity for evolution of the trait since the rate of evolution under natural selection is proportional to the amount of additive genetic variance [Fisher, 1930]. A detailed discussion of canalization and buffering, aspects of development e.g., responsible for the symmetry of paired appendages, is beyond the scope of this brief review, however has direct pertinence to the concept of fluctuating asymmetry.
Traditionally morphological asymmetry has been categorized into three types: fluctuating asymmetry, directional asymmetry, and antisymmetry [Van Valen, 1962].
Directional asymmetry describes normal distribution with a mean that is significantly different from zero, antisymmetry describes a bimodal distribution about a mean of zero, and fluctuating asymmetry a normal distribution, a mean of zero, and is quantified by the variance [Goldberg, 1997].
Size discrepancies in normally symmetrical traits are thought to result from an inability of the individual to buffer environmental and/or genomic stress [Livshits and Kobyliansky, 1991; Clarke, 1992; Parsons, 1992] during development. If these stresses play a role in the cause of disorders of developmental origin, then high levels of fluctuating asymmetry may serve as a risk marker for developmental disorders [Naugler and Ludman, 1996]. Increased fluctuating asymmetry has been reported in Down syndrome [Garn et al., 1970; Townsend, 1983], the fetal alcohol syndrome [Wilber et al., 1993], cleft lip [Woolf and Gianas, 1976], and fragile X syndrome [Peretz et al., 1988]. Increased fluctuating asymmetry relative to controls has also been demonstrated in schizophrenia [Markow and Wandler, 1986; Markow and Gottesman, 1989; Mellor, 1992] and mental retardation [Barden, 1980; Malina and Buschang, 1984], disorders in which fluctuating asymmetry could potentially aid in diagnosis. In humans, the effect of environmental stress is perhaps best exemplified by the finding of increased asymmetry in children exposed to alcohol in utero [Kieser, 1992; Wilber et al., 1993].
The fact that the same level of stress produces different degrees of fluctuating asymmetry in different individuals seems to indicate that the phenotypic outcome is modified by a variable ability to buffer the effects of environmental stresses. There are two explanations why the same level of environmental stress produces different fluctuating asymmetries in different individuals—existence of overdominance [Clarke, 1993; Zouros, 1993], or the expression of an increased number of deleterious recessive alleles [Parsons, 1992; Clarke, 1993].
The mechanism of the so-called out-breeding depression may also explain the decreased symmetry seen in disorders such as Down syndrome [Shapiro, 1983] and fragile X syndrome [Peretz et al., 1988].
The potential implications of clinical significance of increased fluctuating asymmetry is that it has been noted not only in some affected individuals, but also in relatives of affected individuals [Livshits, 1988; Livshits, 1988]. So, there is a higher heritability of fluctuating asymmetry in families with a history of genetic or developmental diseases. It shows that fluctuating asymmetry would be present even in the absence of any environmental stress and fluctuating asymmetry may have implications in genetic counseling.
Of course, clinicians and researchers who want to use fluctuating asymmetry in diagnosis or research must decide which traits will be measured. The most commonly used traits in studies of human populations are dermatoglyphics and odontometrics. However, no character has proven ideal.
There are many problems which can discredit analysis of fluctuating asymmetry: measurement error, problems with interpreting fluctuating asymmetry if traits have a long developmental time (e.g., ear length, foot breadth, palm breadth, facial asymmetry etc.), or short developmental time (e.g., dermatoglyphics) reflecting events occurring in early development. It is also unclear if fluctuating asymmetry in specific anthropometric traits remains constant as the individual grows, although it may in other species [Chippindale and Palmer, 1993].
In spite of the fact that increased fluctuating asymmetry has been shown in a number of disorders, the full potential of fluctuating asymmetry as a risk marker for disorders of developmental origin has yet to be determined [Naugler and Ludman, 1996].
CONCLUSION
Variability of body symmetry is so common that it generally is accepted as a normal phenomenon. The exquisitely sensitive (but culturally adapted) ability of humankind to perform facial phenotype or pattern analysis may well be responsible for some mate selection favoring facial asymmetry as an aesthetic value. However, in the clinic gross degrees of asymmetric development of paired body parts are likely to present as an abnormality begging for causal and pathogenetic explanations. In this brief review we have alluded to only a few of the biological phenomena that may present as developmental asymmetry including defects of pattern formation or induction of laterality, absence of one-half of the body or gross abnormality of several parts of the body largely confined to one-half of the body, the results of a defect of earliest blastogenesis; “associations” with frequently asymmetrical expression of multiple polytopic field defects; dysplastic “disruptions” of blasto- or organogenesis; mutational asymmetries; mosaic/chimeric asymmetries; vascular asymmetries; and dysmorphogenetic limb asymmetries.
The phenomenon of “developmental resistance”, i.e., non-concordance of right and left halves of the body in gross limb malformations, was revisited on hand of Wright's robust and highly pertinent work on the stochastic aspects of potential anomalies in animals of identical genetic constitution, and as a necessary prolegomenon to a discussion of the concepts of developmental canalization (the process) and -buffering (the result).
Nothing can occur in development that evolution has not made possible; and while “permanent” racial and species differences in structure are the result of evolutionary processes, they are also their beginning since differences in variance of somatic and sexual traits govern the rate of evolution under natural selection. Modern morphology has yet to develop an effective method of analyzing the developmental nature, natural history and causes of fluctuating asymmetry, a phenomenon that may well offer a litmus test for environmental, mutational and inbreeding vulnerability.
Acknowledgements
We are most grateful to the Foundation of the Primary Children's Medical Center for a generous grant in support of the International Clinical Genetics Research and Consultation Program, Division of Medical Genetics, Department of Pediatrics, University of Utah, and to Mrs. Sandie Ramos for secretarial collaboration.




