Candidate region for Gilles de la Tourette syndrome at 7q31
Abstract
Gilles de la Tourette Syndrome (GTS) is a complex neuropsychiatric disorder characterized by motor and vocal tics. The cause of this syndrome is unknown, although based on family studies there is evidence of a strong genetic component. We report on a 13-year-old boy with GTS, minor physical anomalies, and a de novo partial duplication of chromosome 7q [dup(7)(q22.1–q31.1)]. The distal breakpoint in our patient is similar to the breakpoint of an apparently balanced familial translocation t(7;18) segregating with GTS. Together, these cases provide evidence that a gene located in the breakpoint region at 7q31 can be involved in the formation of GTS. © 2001 Wiley-Liss, Inc.
INTRODUCTION
Gilles de la Tourette syndrome (GTS) is a chronic neuropsychiatric disorder characterized by multiple motor and vocal tics. Onset is almost always in childhood or adolescence with an estimated prevalence of 1/1,000 in boys and 1/10,000 in girls [Burd et al., 1986]. Its cause is unknown, but there is evidence of a strong genetic component. It is likely that GTS is inherited as an autosomal dominant trait with reduced penetrance [Carter et al., 1994]. However, no candidate genes have been identified as yet, despite reported chromosome anomalies and several genome-wide linkage studies.
Boghosian-Sell et al. [1996] reported on a family where GTS was segregating with an apparently balanced 7;18 translocation in several affected patients. The breakpoint on chromosome 7 was described to be within genomic markers D7S515 and D7S522, which are mapped to chromosomal bands 7q22 and 7q31, respectively.
In this communication, we describe a de novo dup(7)(q22.1–q31.1) observed in a 13-year-old boy with GTS with moderate mental retardation and minor physical anomalies. Both breakpoints are within or close to the breakpoint region in 7q of the 7;18 translocation mentioned above. A detailed study of previously reported cases with 7q duplications did not show any signs of GTS, which supports evidence for the causal involvement of gene or genes at the breakpoints rather than genes within the duplicated region.
CLINICAL REPORT
The propositus was born at the 38th week of gestation as a second child of healthy unrelated parents (maternal age 27 years and paternal age 29 years). Birth weight was 2,580 g and length was 40 cm. He started to walk and speak with 15 month, which was about 3–4 months later than his healthy sister. In 1989 and 1992, he underwent an ophthalmologic surgery because of strabismus convergens and also received surgical treatment of a stenosis of his left meatus acusticus. At an age of 11 years and 6 months, he was treated at the Department of Pediatrics because of stuttering, tics, and a moderately reduced learning capacity. He showed an overall slight delay in his neurological development. His left ear is smaller than his right, protrudes, and has a grooved helix and a poorly developed antihelix. Middle ear ossicles on his left side are malformed and non-functional with consecutive unilateral neurosensory deafness. In addition, mild micrognathia, high nasal bridge, thick lips, low posterior hair line, and bilateral transverse palmar crease were observed. Based on the neurological findings, in particular the frequent occurrence of motor and vocal tics and also episodes of involuntary coprolalia, he was diagnosed with GTS in full accordance with the criteria from the American Psychiatric Association, Diagnostic and Statistical Manual of Mental Disorders, revised edition 3 (DSM-III-R). An electroencephalogram and MR of the brain were normal, as were all standard laboratory parameters. There are no records of GTS or any other neuropsychiatric disorders in his family history. Upon physical re-examination at 13 years of age, his height was 175 cm and his weight was 80 kg (both > 97 centile). Gynecomastia was noticed but his pubertal development, currently Tanner P3-4, G 3, is only slightly delayed. He attends a school for the mentally disabled but has rather good records in language, whereas mathematical skills and motor performance are more severely affected. A relatively successful pharmaceutical treatment with the drug Clonidin reduced the occurrence of tics.
CYTOGENETICS
Cytogenetic analysis of GTG-banded peripheral blood lymphocytes was performed by using standard protocols. A total of 200 metaphases were examined and all showed an abnormal male karyotype that contained a chromosome 7 with an elongated q arm. By using high-resolution chromosome banding, the following karyotype could be described: 46,XY,dup(7)(q22.1–q31.1) (Fig. 1). Parental chromosomes were normal.
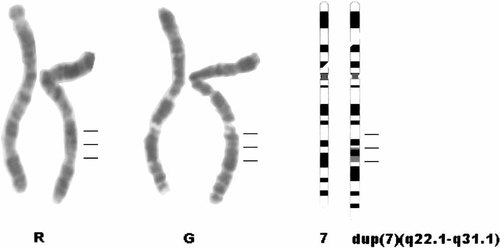
Partial RBG (R) and GBG (G) banded karyotype and ideogram showing the normal and derivative chromosome 7 from the propositus. The breakpoints in 7q are indicated by horizontal lines.
Fluorescence in situ hybridization (FISH) analysis with a wcp7 probe (Vysis) indicated that the der(7) was composed entirely of chromosome 7 material (data not shown).
DISCUSSION
In this patient with a duplication of 7q22–q31.1, clinical findings characteristic for a duplication in this chromosomal region were present, including dysmorphic ears and mental retardation [Schinzel, 1984]. However, at the age of 9 years, additional uncommon behavioral problems with involuntary vocal and motor tics were noted. A neuropsychiatric examination lead to the diagnosis of GTS.
The distal breakpoint was shown to be within an already reported breakpoint region of a t(7;18)(q22–q31,q22.3) in a familial case with GTS [Boghosian-Sell et al., 1996]. However, because of one report of a patient with an 18q deletion and GTS [Donnai, 1987], a gene in 18q22.3 was suspected as a possible candidate for its involvement in this neuropsychiatric disease.
A fragile site (FRA7G) mapped to 7q31 could be responsible for more frequent chromosomal rearrangements that occurred in this chromosomal band. Interestingly, the same band is also well known as a candidate region for other neuropsychiatric disorders like autism and speech-language disorder [Folstein and Mankoski, 2000], where an occasional co-morbidity with GTS is already confirmed [Baron-Cohen et al., 1999].
Coincidental occurrence of the behavioral anomalies and the duplication or breakpoints in the patient reported here could not be excluded formally, but given the previously described familial 7;18 translocation segregating with GTS, the chromosomal band 7q31 becomes a strong candidate region for GTS.




