1-Amino-2-Methyl-3-Buten-2-ol
Abstract
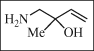
InChI = 1S/C5H11NO/c1-3-5(2,7)4-6/h3,7H,1,4,6H2,2H3
InChIKey = HIZLKTYBQGWVMQ-UHFFFAOYSA-N
([3,3]-Sigmatropic rearrangement1-3 and other reactions4)
- Physical Data: boiling point 72–75 °C (17 mmHg),1 75–77 °C (12 mmHg),4 50–54 °C (4.5–6 mmHg).5 .4
- Solubility: soluble in benzene, toluene.
- Preparative Methods: the title compound was prepared from the halogenation of isoprene using hypochlorous acid5 or N-bromosuccinimide under acidic conditions4 to give the corresponding chloro- or bromohydrin derivatives. The halohydrins were next treated with sodium or potassium hydroxide to afford 2-methyl-2-vinyloxirane. Upon reaction with aqueous ammonia (28%), the amino-allyl alcohol was obtained.4, 5 An alternative method involves the treatment of 1-bromo-2-methylbut-3-en-2-ol with aqueous ammonia, leading to the in situ generated 2-methyl-2-vinyloxirane, affording the title compound.1



