2-Aminobenzaldehyde†
Abstract
[529-23-7] C7H7NO (MW 121)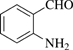
InChI = 1S/C7H7NO/c8-7-4-2-1-3-6(7)5-9/h1-5H,8H2
InChIKey = FXWFZIRWWNPPOV-UHFFFAOYSA-N
(Friedländer synthon; quinoline synthesis; o-amino aldehyde)
Alternate Names: 2-formylaniline; 1-amino-2-formylbenzene.
Physical Data: mp 38–39 °C.
Solubility: sol ether, alcohol.
Preparative Methods: the reagent is best when freshly prepared. At one time it was commercially available but samples more than a few months old were found to be essentially useless due to oligomerization. The propensity of the reagent to self-react and the resulting oligomer structures have been extensively discussed.1a A useful preparative method involves Iron(II) Sulfate reduction of 2-nitrobenzaldehyde.2 The key to success is limiting the reaction time to 8–10 min and immediate, rapid steam distillation of the reaction product. The precursor is commercially available from a variety of sources and its preparation has also been reported.3 A supposedly simplified procedure has been suggested but it does not differ significantly from the earlier method.4
Purification: can be effected by steam distillation from saturated NaCl solution.
Handling, Storage, and Precautions: the reagent can be stored for periods of up to several months under an inert atmosphere at 0 °C in the dark. The mp is a good general indicator of purity and the NMR spectrum should show an aldehyde proton integrating 1∶4 against the aromatic protons. Use in a fume hood.



