Allyl Phenyl Sulfone
Richard K. Haynes
Hong Kong University of Science and Technology, Kowloon, Hong Kong
Search for more papers by this authorSimone C. Vonwiller
The University of Sydney, Sydney, New South Wales, Australia
Search for more papers by this authorVikas Sikervar
Arekere Mico-layout Bangalore, KA., Bangalore, India
Search for more papers by this authorRichard K. Haynes
Hong Kong University of Science and Technology, Kowloon, Hong Kong
Search for more papers by this authorSimone C. Vonwiller
The University of Sydney, Sydney, New South Wales, Australia
Search for more papers by this authorVikas Sikervar
Arekere Mico-layout Bangalore, KA., Bangalore, India
Search for more papers by this authorAbstract
[16212-05-8] C9H10O2S (MW 182.24)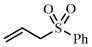
InChI = 1S/C9H10O2S/c1-2-8-12(10,11)9-6-4-3-5-7-9/h2-7H,1,8H2
InChIKey = KYPIULIVYSQNNT-UHFFFAOYSA-N
(metalated derivatives serve as ambident nucleophiles; reacts as a 1,1-allylic dianion or 1,1-allylic dipole equivalent1)
Alternate Name: 1-phenylsulfonyl-2-propene.
Physical Data: bp 111–113 °C/0.5 mmHg; d 1.189 g cm−3.
Solubility: insol water; sol most organic solvents.
Form Supplied in: colorless liquid with purity >98% as obtained commercially.
Preparative Method: from Allyl Bromide and benzenesulfinate in ethanol according to the original procedure2 is most straightforward and avoids the use of strong-smelling thiols.
Purification: distillation in vacuo prior to use.
Handling, Storage, and Precautions: the sulfone is a stable compound and is handled without special precaution.
Bibliography
- 1(a) Magnus, P. D., Tetrahedron 1977, 33, 2019. (b) Block, E. Reactions of Organosulfur Compounds; Academic Press: New York, 1978. (c) Biellmann, J.-F.; Ducep, J.-B., Org. React. 1982, 27, 1.
- 2
Otto, R.,
Liebigs Ann. Chem.
1894,
283,
18;
10.1002/jlac.18942830115 Google ScholarCope, A. C.; Morrison, D. E.; Field, L., J. Am. Chem. Soc. 1950, 72, 59. Vennstra, G. E.; Zwaneburg, B., Synthesis 1975, 519.
- 3 Gais, H.-J.; Vollhardt, J.; Lindner, H. J., Angew. Chem. Int. Ed. Engl. 1986, 939. Boche, G., Angew. Chem. Int. Ed. Engl. 1989, 277 and references cited.
- 4 Cuvigny, T.; Herve du Penhoat, C.; Julia, M., Tetrahedron 1986, 42, 5329.
- 5 Kraus, G. A.; Frazier, K., Synth. Commun. 1978, 8, 483.
- 6(a) Hirama, M., Tetrahedron Lett. 1981, 22, 1905. (b) Vasil'eva, L. L.; Mel'nikova, V. I.; Gainullina, É. T.; Pivnitskii, K. K., J. Org. Chem. USSR (Engl. Transl.) 1983, 19, 835. (c) Binns, M. R.; Haynes, R. K.; Katsifis, A. G.; Schober, P. A.; Vonwiller, S. C., J. Org. Chem. 1989, 54, 1960.
- 7
Miyaoka, H.;
Kajiwara, M.,
Chem. Pharm. Bull.
1985,
33,
2531.
10.1248/cpb.33.2531 Google Scholar
- 8 Masaki, Y.; Sakuma, K.; Kaji, K., J. Chem. Soc., Chem. Commun. 1980, 434.
- 9 Trost, B. M.; Schmuff, N. R.; Miller, M. J., J. Am. Chem. Soc. 1980, 102, 5979.
- 10(a) Ueno, Y.; Aoki, S.; Okawara, M., J. Am. Chem. Soc. 1979, 101, 5414. (b) Ueno, Y.; Aoki, S.; Okawara, M., J. Chem. Soc., Chem. Commun. 1980, 683. (c) Giese, B.; Erdmann, P.; Göbel, T.; Springer, R., Tetrahedron Lett. 1992, 33, 4545.
- 11 Savoia, D.; Trombini, C.; Umani-Ronchi, A., J. Chem. Soc., Perkin Trans. 1 1977, 123.
- 12 Sataty, I.; Meyers, C. Y., Tetrahedron Lett. 1974, 4161.
- 13 Julia, M.; Arnould, D., Bull. Soc. Claim. Fr., Part 2 1973, 746. Manchand, P. S.; Rosenberger, M.; Saucy, G.; Wehrli, P. A.; Wong, H.; Chambers, L.; Ferro, M. P.; Jackson, W., Helv. Chim. Acta 1976, 59, 387. Fischli, A.; Mayer, H.; Simon, W.; Stoller, H.-J., Helv. Chim. Acta 1976, 59, 397.
- 14 Grieco, P. A.; Masaki, Y., J. Org. Chem. 1974, 39, 2135. Olson, G. L.; Cheung, H.-C.; Morgan, K. D.; Neukom, C.; Saucy, G., J. Org. Chem. 1976, 41, 3287.
- 15(a) Trost, B. M.; Ghadiri, M. R., J. Am. Chem. Soc. 1984, 106, 7260. (b) Adams, J. P.; Bowler, J.; Collins, M. A.; Jones, D. N.; Swallow, S., Tetrahedron Lett. 1990, 31, 4355.
- 16 Martel, J.; Huynh, C., Bull. Soc. Chem. Fr. Part 2 1967, 985. Schatz, P. F., J. Chem. Educ. 1978, 55, 468.
- 17 Campbell, R. V. M.; Crombie, L.; Findley, D. A. R.; King, R. W.; Pattenden, G.; Whiting, D. A., J. Chem. Soc., Perkin Trans. 1 1975, 897.
- 18 Charreau, P.; Julia, M.; Verpeaux, J. N., Bull. Soc. Chem. Fr. Part 2 1990, 275.
- 19 Trost, B. M.; Merlic, C. A., J. Org. Chem. 1990, 55, 1127.
- 20 Liu, Y.; Jacobs, H. K.; Gopalan, A. S., J. Org. Chem. 2009, 74, 782.
- 21 Merino, P.; Mannucci, V.; Tejero, T., Tetrahedron 2005, 61, 3335.
- 22 Jankowski, P.; Wicha, J., J. Chem. Soc., Chem. Commun. 1992, 802.
- 23 Funk, R. L.; Umstead-Daggett, J.; Brummond, K. M., Tetrahedron Lett. 1993, 34, 2867.
- 24(a) Kumareswaran, R.; Balasubramanian, T.; Hassner, A., Tetrahedron Lett. 2000, 41, 8157. (b) Kumareswaran, R.; Hassner, A., Tetrahedron: Asymmetry 2001, 12, 3409.
- 25 Guijarro, D.; Yus, M., Tetrahedron Lett. 1994, 35, 2965.
- 26 Clayden, J.; Julia, M., J. Chem. Soc., Chem. Commun. 1994, 1905.
- 27 Chatgilialoglu, C.; Ballestri, M.; Vecchi, D., Tetrahedron Lett. 1996, 37, 6383.
- 28 Chatgilialoglu, C.; Alberti, A.; Ballestri, M.; Macciantelli, D., Tetrahedron Lett. 1996, 37, 6391.
- 29 Ponten, F.; Magnusson, G., J. Org. Chem. Soc. 1996, 61, 7463.
- 30 Kang, S.; Kim, S.; Lee, J., Tetrahedron: Asymmetry 1992, 3, 1139.
- 31 Hou, X.; Hong, D.; Rong, G.; Guo, Y. Dai, L., Tetrahedron Lett. 1993, 34, 8513.
- 32 Nanno, T.; Sakai, N.; Nozaki, K.; Takaya, H., Tetrahedron: Asymmetry 1995, 6, 2583.
- 33 Wrobel, Z., Eur. J. Org. Chem. 2000, 521.
- 34 Ek, F.; Wistrand, L.; Frejd, T., J. Org. Chem. 2003, 68, 1911.
- 35 Pilarski, L. T.; Janson, P. G.; Szabo, K. J., J. Org. Chem. 2011, 76, 1503.
- 36 Alam, R.; Pilarski, L. T.; Pershagen, E.; Szabo, K. J., J. Am. Chem. Soc. 2012, 134, 8778.



