Synthese, Struktur und Hydrolyse von Tetrakis(tetrahydropyran)strontium-bis[bis(dimethylisopropylsilyl)phosphanid]
Abstract
deDie Metallierung von Bis(dimethylisopropylsilyl)phosphan in Tetrahydropyran mit Strontium-bis[bis(trimethylsilyl)amid] ergibt nahezu quantitativ Tetrakis(tetrahydropyran)strontium-bis[bis(dimethylisopropylsilyl)phosphanid], das in der monoklinen Raumgruppe C2/c kristallisiert (a = 2340,71(1), b = 1028,74(1), c = 2186,02(1) pm, β = 91,03(1)°, Z = 4, wR2 = 0,076). Mit einem P–Sr–P-Bindungswinkel von 168,5° sind die Phosphanid-Substituenten trans-ständig angeordnet. Die partielle Hydrolyse dieser Verbindung liefert neben Bis(dimethylsilylisopropylsilyl)phosphan eine Verbindung der Formel Sr4O[P(SiMe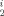 Pr)2]6 mit einem zentralen, tetradrisch von vier Strontiumatomen umgebenen Sauerstoffatom (monoklin, C2/c, a = 2265,83(6), b = 1702,11(5), c = 2462,46(9) pm, β = 91,34(1)°, Z = 4, wR2 = 0,106). Die Kanten des Strontiumtetraeders sind von den Phosphanid-Liganden überbrückt, während die Metallatome trigonal planar von je drei Phosphoratomen umgeben sind.
Pr)2]6 mit einem zentralen, tetradrisch von vier Strontiumatomen umgebenen Sauerstoffatom (monoklin, C2/c, a = 2265,83(6), b = 1702,11(5), c = 2462,46(9) pm, β = 91,34(1)°, Z = 4, wR2 = 0,106). Die Kanten des Strontiumtetraeders sind von den Phosphanid-Liganden überbrückt, während die Metallatome trigonal planar von je drei Phosphoratomen umgeben sind.
Abstract
enSynthesis, Structures, and Hydrolysis of Tetrakis(tetrahydropyran)strontium Bis[bis(dimethylisopropylsilyl)phosphanide]
The metalation of bis(dimethylisopropylsilyl)phosphane in tetrahydropyran with strontium bis[bis(trimethylsilyl)amide] yields almost quantitatively tetrakis(tetrahydropyran)strontium bis[bis(dimethylisopropylsilyl)phosphanide], which crystallizes in the monoclinic space group C2/c (a = 2340.71(1), b = 1028.74(1), c = 2186.02(1) pm, β = 91.03(1)°, Z = 4, wR2 = 0.0759). The phosphanide ligands are in trans-positions and the P–Sr–P bond angle is found to be 168.5°. Partial hydrolysis of this compound leads to the formation of bis(dimethylsilylisopropylsilyl)phosphane and Sr4O[P(SiMe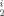 Pr)2]6 with a central oxygen atom surrounded tetrahedrally by four alkaline earth metal atoms (monoclinic, space group C2/c, C2/c, a = 2265.83(6), b = 1702.11(5), c = 2462.46(9) pm, β = 91.34(1)°, Z = 4, wR2 = 0.1057). The edges of the strontium tetrahedron are bridged by phosphanide ligands. The metal atoms are coordinated trigonal planarily by three phosphanide groups.
Pr)2]6 with a central oxygen atom surrounded tetrahedrally by four alkaline earth metal atoms (monoclinic, space group C2/c, C2/c, a = 2265.83(6), b = 1702.11(5), c = 2462.46(9) pm, β = 91.34(1)°, Z = 4, wR2 = 0.1057). The edges of the strontium tetrahedron are bridged by phosphanide ligands. The metal atoms are coordinated trigonal planarily by three phosphanide groups.




