High-frequency occurrence of chromosome translocation in a mutant strain of Candida albicans by a suppressor mutation of ploidy shift
Abstract
Significant occurrence of high-ploidy cells is commonly observed among many Candida albicans strains. We isolated two isogenic strains, STN21 and STN22, each from a half sector of a colony obtained after mild UV-irradiation of a Arg− derivative of CBS5736. The two strains were different from each other in ploidy states and chromosome organization. Although cells of STN22 were homogeneous in size and had a single nucleus, high-ploidy cells, with either a single large nucleus or several nuclei, were present together with apparently normal cells with a single nucleus in the cell population of STN21. Flow cytometry showed that STN22 was a stable diploid; however, STN21 seemed to be the mixture of different ploidy states, including diploid and tetraploid. The phenotype of STN21 containing high-ploidy cells is referred to here as the Sps− phenotype (suppressor of ploidy shift). STN22 showed a typical electrophoretic karyotype similar to strain 1006 in C. albicans. However, an extra chromosomal band appeared in some clones of STN21 at high frequency. By assignment of several DNA probes, this extra chromosome was shown to be a translocation of the 7F–7G portion of chromosome 7 with the 470 kb DNA segment containing H SfiI fragment from chromosome 4. Thus, this extra chromosome is a hybrid of 4H and 7F–7G. Since the isogenic Sps+ strain STN22 exhibited no extra chromosome bands, a correlation is suggested between the Sps− phenotype and the occurrence of chromosome translocations. Copyright © 2000 John Wiley & Sons, Ltd.
INTRODUCTION
Candida albicans is an asexual fungus and a commensal in the healthy human body. However, it confers a life-threatening infection as a opportunistic pathogen in an immunocompromised host. Electrophoretic karyotype studies showed that C. albicans has extensive chromosome length polymorphism among clinical isolates (Iwaguchi et al., 1990). The typical electrophoretic karyotype of C. albicans consists of seven to eight chromosome bands, ranging in size from 1.0 to 4.2 Mb. Each chromosome band seems to comprise two homologous chromosomes. However, approximately 40% of isolates show significant variation in their karyotypes (Iwaguchi et al., 1990). Chromosomes containing the rDNA cluster are most variable in size. The size change of this chromosome should not be counted as karyotype variation, because such changes can occur during vegetative growth due to simple mitotic recombinations (Iwaguchi et al., 1992a). Extra chromosome bands which do not contain rDNA also appear in some strains. Such extra bands are often detected in strains which show changes in colony morphology (Suzuki et al., 1989; Rustchenko-Bulgac et al., 1990; Rustchenko-Bulgac, 1991). Karyotype variation might provide genetic variation, sometimes leading to a strain which is more resistant to host defence mechanisms. By assignment of DNA probes to electrophoretically separated chromosomal DNA bands, some of these atypical karyotypes are concluded to be derived from the size variation between homologous chromosomes (Iwaguchi et al., 1990). The others seem to be created by chromosome translocation. Several groups have described chromosome translocation as the cause of karyotype variation (Barton and Gull, 1992; Thrash-Bingham and Gorman, 1992; Chu et al., 1993; Navarro-Garcia et al., 1995). Chu et al. (1993) constructed a macro restriction map of C. albicans strain WO-1, which has extra chromosomes, with the restriction enzyme SfiI. They proved that three reciprocal translocations occurred in strain WO-1 by comparing its restriction map with that of a strain 1006 of C. albicans. All translocation events seem to have occurred at or near the SfiI site.
C. albicans usually exists in a diploid state and a haploid state has not yet been found (Scherer and Magee, 1990). This means that a recessive mutation in a given gene appears in the phenotype only when the homozygous state of the mutation is created by mitotic recombination or mutation. Although most C. albicans strains show diploidy, some strains of C. albicans produce a proportion of the cell population with a higher ploidy than the normal diploid state (Suzuki et al., 1986, 1989). A ploidy shift was observed between two different ploidy states in such Pld− strains: diploid (or lower ploidy state) cells in a population of the strain enter G2 arrest and then bypass the M phase, resulting in an upshift of the ploidy state; tetraploid (or higher ploidy state) cells engage in reductional nuclear division during the downshift of the ploidy state, producing diploid (or lower ploidy state) daughter cells (Suzuki et al., 1986, 1989). This phenotype of mixed ploidy states was named as Pld− and the phenotype of stable ploidy that all the cells of a population maintain a certain level of ploidy was referred to as Pld+ (Suzuki et al., 1994). Here, we are proposing a new name, Sps (suppressor of ploidy shift) instead of Pld. Suzuki et al. (1994) also described a possible correlation between the auxotrophic segregation and the polyploid nature in C. albicans strains. Micromanipulated high ploidy cells of Sps− strain SGF7–2 gave rise to auxotrophic segregant colonies at high frequencies. This phenomenon was assumed to be due to the occurrence of either mitotic recombination or chromosome loss. However, it has remained unclarified whether the Sps− phenotype has a direct correlation with a high degree of karyotype variation.
We isolated two isogenic strains, STN21 and STN22, which differed in their ploidy states and the degree of chromosome reorganization; STN22 is a diploid showing a stable karyotype, while STN21 contained high-ploidy cells at a certain frequency in the cell population showing a chromosome translocation between chromosomes 4 and 7 at high frequencies. This is the first report that describes the correlation between the Sps− phenotype and the high frequency of chromosome translocation.
MATERIALS AND METHODS
Strains and plasmids
The Arg− auxotrophic Candida albicans strain STN14 used in this study as a parent was a derivative of CBS5736 (Suzuki et al., 1986). Saccharomyces cerevisiae strain X2180–2D (diploid) was used as a reference standard for ploidy determinations (Mortimer and Schild, 1985). Plasmid pARG–SK (H. Chibana, University of Minnesota, St. Paul, unpublished), which contains the C. albicans ARG4 gene (Hoyer et al., 1994) cloned in the NaeI site of pBluescript II-SK+ (Stratagene), was used for the complementation of the arginine auxotrophs. Transformation experiments were performed as described by Magee (1994). The plasmids used as probes for Southern hybridization are listed in Table 1.
| Probe | Plasmid | Chromosome (SfiI fragment) | DNA fragment | Source |
|---|---|---|---|---|
| ALD1 | p1808 | 7 (F) | 4 kb EcoRI | B. B. Magee |
| CDC34 | p1888 | 7 (F) | 1.5 kb HindIII | B. B. Magee |
| YCF1 | p306 | 7 (F) | 6.5 kb HindIII | B. B. Magee |
| INP53 | p1810 | 7 (C) | 4.2 kb EcoRI | B. B. Magee |
| YPL12 | p282 | 7 (A) | 4 kb HindIII | B. B. Magee |
| p212 | 7 (A) | 1.7 kb HindIII | B. B. Magee | |
| CHS1 | 7 (G) | 0.4 kb | *PCR product | |
| p52–11 | 5 (I) | 2 kb EcoRI, HindIII | S.-I. Iwaguchi | |
| S9G4 | 6 (C) | 3.5 kb NotI | B. B. Magee | |
| CHS2 | R (B2) | 0.5 kb | *PCR product | |
| p356 | 4 (H) | 5 kb HindIII | B. B. Magee | |
| HIS5 | pHIS5-2 | 4 (N) | 2.3 kb HindIII | B. B. Magee |
- * PCR product; primers for PCR were referred from Candida albicans information server (http://alces.med.umn.edu/candida/primers.html).
Growth conditions and media
Yeast cells were grown in YPD medium (containing, per litre, 10 g Bacto-peptone (Difco), 10 g yeast extract (Difco), and 20 g glucose) with shaking (120 strokes/min) at 28°C. Minimal medium (MIN) contained, per litre, 6.7 g Yeast Nitrogen Base without amino acids (Difco) and 20 g glucose. For solid medium, 2% (w/v) agar was added to YPD or MIN. MIN agar plates were supplemented, when appropriate, with auxotrophic requirements at the concentrations specified by Sherman et al. (1979).
Mild UV irradiation
Mitotic recombination was induced by mild UV irradiation as described previously (Suzuki et al., 1994). A Toshiba bactericidal 15 W UV lamp (254 nm) was used to irradiate plates spread with an appropriate dilution of yeast cells at a distance of 80 cm with a 25 s exposure with 6×10−5 J mm. This irradiation usually gave 80–90% survival rates in C. albicans.
Visualization of nuclear DNA by fluorescence microscopy
Yeast cells were grown for 8 h at 28°C in YPD broth. The cells were washed once with distilled water, and resuspended in 50% (v/v) ethanol and incubated for at least 30 min at 22°C for fixation. After washing with STE buffer (10 mM Tris–HCl, pH 7.5, 1 mM EDTA, 50 mM NaCl), cellular RNA was removed by treatment with RNase A (1 mg/ml) for 90 min at 37°C. Then, the fixed cells were stained with propidium iodide at a concentration of 10 µg/ml and examined with an epifluorescence microscope (BH-2-RFL; Olympus).
Flow cytometory of ploidy state
Intensities of propidium iodide fluorescence of the fixed individual yeast cells were measured using a flow cytometer (Epics, Coulter). Usually more than 50 000 cells of a clonal population of the strains STN21, STN22 and X2180–2D were examined. Ploidy states of STN21 and STN22 were estimated by comparing their fluorescence with the diploid strain of S. cerevisiae.
Pulsed field gel electrophoresis (PFGE)
Yeast chromosomal DNA for PFGE was prepared as described previously (Iwaguchi et al., 1990). PFGE was carried out by the contour-clamped homogeneous electric field method using a Pulsaphor system with a hexagonal electrode array (Pharmacia-LKB) or CHEF DRII system (Bio-rad) as described previously (Iwaguchi et al., 1990). Chromosomes of S. cerevisiae (YPH149) (Guthrie and Fink, 1991) were used as molecular size reference markers.
Densitometric analysis
The gel pictures were incorporated into the computer (Power PC 8100/ 80AV, Apple Computer) by scanner (GT-9600, EPSON). Densitometric analysis was performed on a Macintosh computer using the public domain NIH Image programme (written by Wayne Rasband at the US National Institutes of Health).
Southern hybridization
Probes were labelled by digoxigenin, using the DIG DNA labelling kit (Boehringer-Mannheim). Detection with the digoxigenin probe was carried out using the DIG luminescent detection kit for nucleic acids (Boehringer-Mannheim). Hybridization signals were detected on X-ray film (Fuji film).
RESULTS
Isolation of a Sps− variant from CBS5736
Van der Walt and Pitout (1969) reported that a derivative of C. albicans strain CBS5736 showed a sexual process in which presumed haplophase yeast cells developed into diplophase cells after karyogamy, and mycelial formation occurred in the diplophase. However, this description has not been reproduced by other investigators; one paper demonstrated that all the cells in the described cycle were diploid (Magee et al., 1983). Suzuki et al. (1986) described another phenomenon, named ploidy shift, in a clinical isolate of this organism: the population of the isolate contained both diploid and tetraploid (or higher ploidy) cells, and a shift occurred between a diploid and a higher ploidy state. We assumed that the description by Van der Walt and Pitout would have indicated the phenomenon of ploidy shift, no more than asexual process in this organism. Natural auxotrophic mutations are known to exist as a heterozygous state in diploid C. albicans isolates (Whelan et al., 1980). A low dose of UV irradiation is used to induce mitotic recombination, leading to variant strains with an auxotrophic mutation in a homozygous state. In some cases such auxotrophic variant could be isolated as sectored colonies and no similar auxotroph could be detected from the other sector of the colony, which indicated the occurrence of segregation of the auxotrophic mutation after mitotic recombination (Whelan et al., 1980). We also presumed that, in strain CBS5736, some defective gene(s) may exist as a heterozygous state and if the given gene(s) were in a homozygous state, such a variant would show the ploidy shift. If so, we could isolate the variant from the strain after inducing mitotic recombination by mild UV-irradiation.
The variant was presumed to be defective, maintaining a monoploid state, and to have the Sps− phenotype as described above. The formation of small colonies and the yielding of colonies of various sizes after restreaking was one of the characteristics of the strains with ploidy shift (Suzuki et al., 1986, 1994). Therefore, we used these characteristics as the index for screening for Sps− variants. On the hypothesis that the gene(s) causing the Sps− phenotype exists in the heterozygous state in CBS5736, mild UV irradiation giving 80% survival was first performed on the wild-type strain. Among 3758 survival colonies, 20 small colonies with semi-rough surfaces were picked up and streaked on to YPD medium to examine the mode of colony formation. One showed a mixture of various-sized colonies, even after three rounds of subculturing. Subcultures of the variant clone showed the presence of high-ploidy cells in its cell population by fluorescence microscopy, suggesting that the variant was Sps−. Also, one Arg− auxotroph with normal colony morphology was isolated among the UV survivals. This Arg− variant was a normal in ploidy state by fluorescent microscopy and was named STN14. Next, we tried to isolate Sps− variants from STN14. Among 5730 UV-survival colonies of the subculture of STN14, we found four small full colonies with semi-rough morphology and one half-sectored colony, one of the sectors showing slow growth with a semi-rough morphology and the other apparently normal growth with a smooth phenotype. From the normal growth sector, no semi-rough colony with the Sps− phenotype was isolated among 11 898 UV-survival colonies, differing significantly from STN14 in this respect (p<0.05). This behaviour is expected if the normal growth sector is homozygous for the dominant allele that suppresses the Sps− phenotype. The subculture obtained from the Sps− sector was referred to as STN21, and that from the Sps+ one as STN22. The nuclear DNAs were stained using propidium iodide (PI) and examined by fluorescent microscopy. As shown in Figure 1A, cells of STN22 had a single nucleus. However, in the case of STN21, the size of cells was variable and the cells contained much larger nuclei and multi-nucleate cells were also observed at a low frequency (Figure 1B). Further characterization of the ploidy state of STN21 was performed by flow cytometry. The intensities of DNA fluorescence of clones from STN21 and STN22 were compared with that of a diploid strain of S. cerevisiae (X2180–2D) (Figure 2). The diploid strain X2180–2D contained two peaks, corresponding to 2N and 4N; G1 DNA content and G2-M DNA content, respectively (Figure 2A). In five clones of STN22 that we examined, the fluorescent intensity profile was almost the same as that in the diploid S. cerevisiae strain X2180–2D (Figure 2B). On the other hand, clones from STN21 showed a mixture of different ploidy levels (Figure 2C, D and E). The profiles of fluorescence intensity were classified into several types. The first type of clone of STN21 consisted of three peaks (Figure 2C). Those were estimated to be 2N, 4N and 8N, by comparison with the profile of the diploid S. cerevisiae strain X2180–2D. Thus tetraploid cells seemed to be a small fraction of the cell population of this clone. The second type exhibited four peaks, corresponding to 2N, 4N, 6N and 8N (Figure 2D). This type of clone seemed to contain tetraploid cells and aneuploid cells in a small fraction of the cell population. The third type showed two peaks, corresponding to the 4N and 8N of stable tetraploid cells (Figure 2E). Subculture of clones of the third type revealed the second type. We concluded that the ploidy shift occurs in STN21, but the proportions of high-ploidy cells were different from clone to clone.
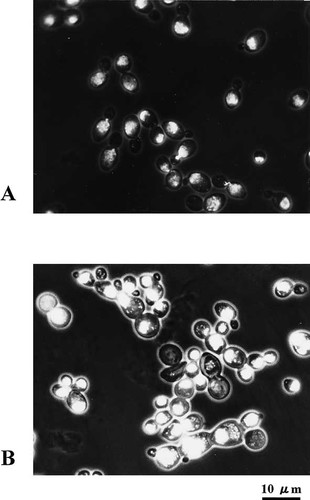
Visualization of nucleus by propidium iodide staining. The cells of STN22 (A) and STN21 (B) were stained with propidium iodide and observed with an epifluorescence microscope. Bar=10µm.
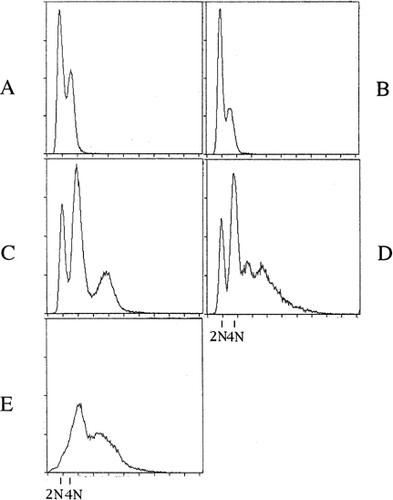
Flow cytometer analysis of ploidy state. The intensity of propidium iodide was measured in about 50 000 cells in S. cerevisiae, X2180–2D (A), C. albicans, STN22 (B) and STN21 (C, D and E) with a flow cytometer (Epics, Coulter). The biphasic peaks of X2180–2D (A) represent cells with G1 (2N) and G2/M (4N) DNA content.
Chromosome translocation occurred on chromosome 7
Previous study has shown that some colony morphological variants of C. albicans NUM961 showed a mixture of different ploidy states and also an extra chromosomal band compared to its parental isolate (Suzuki et al., 1989). Thus, the mixture of different ploidy states might have a correlation with chromosome reorganization. It was clarified from flow cytometry analyses that polyploid cells exist in the population of the C. albicans derivative STN21. To investigate the possibility that the ploidy shift in this derivative was associated with the changes of chromosome organization, we analysed the karyotypes of STN21 and STN22 by PFGE. All 20 clones of STN22 showed the standard C. albicans karyotype, containing chromosomes 3, 4, 5, 6 and 7 in pairs (Figure 3, lanes 1, 2 and 3). They had no extra chromosome bands. Some clones (lanes 4, 5 and 6) of STN21 showed the same karyotype as STN22. However, an extra chromosomal DNA band of about 1.2 Mb in size appeared between chromosomes 5 and 6 in the other clones of STN21 (lanes 7, 8 and 9). We named this extra band chromosome 5.5. This chromosome 5.5 was also observed in 10% of subcultures of clones shown as lanes 4–6 (data not shown). Some clones of STN21 also showed an additional new band, which migrated slower than chromosome 5 (Figure 4, lane 6, for example). This chromosome was identified as a homologue of chromosome 5 by Southern hybridization (data not shown). To compare the densities of bands corresponding to chromosome 5.5 in STN21, densitometric analysis was carried out in some clones of Figure 3A. The intensity of chromosome 5.5 of STN21 was similar to that of chromosome 7 (Figure 3, lane 8) and the two new bands and chromosome 7 were about half of chromosome 5 and 6. The intensity of chromosome 5.5 in lane 7 and 9 was almost similar to those of chromosome 6 and 7. This might be due to the difference of the proportion of cells having chromosome 5.5 in the population because the proportion of polyploid cells was different among clones of STN21 (Figure 2, C, D and E). We anticipated that the extra chromosome band was derived from one homologue of chromosome 7. Chromosome 7 consists of four SfiI fragments; 7A, 7C, 7F and 7G in strains 1006 and WO-1 of C. albicans (Chu et al., 1993; Chibana et al., 1998). In strain WO-1, one of the chromosome 7 homologues was involved in a chromosome translocation event with chromosome 4. To examine the possibility of chromosome translocation in chromosome 5.5 in STN21, we hybridized DNA probes from the four SfiI fragments of chromosome 7. Figure 4 shows the hybridization of the probe, YCF1, on the 7F SfiI fragment. This probe hybridized to the smallest band in STN22 (lanes 1) and some clones of STN21 (lanes 2, 3, 4 and 5) and to two bands including chromosome 5.5 in other clones of STN21 (lanes 6, 7, 8 and 9). Other DNA probes on 7F and 7G SfiI fragments showed the same hybridization profiles. However, the probe INP53 on 7C the SfiI fragment was only assigned to the smallest band of chromosome 7 in all cases (Figure 5). Other DNA probes on 7A and 7C SfiI fragments also failed to hybridize to chromosome 5.5 in clones of STN21 (lanes 5, 6 and 7). Therefore, we assume that chromosome 5.5 contains 7F and 7G SfiI fragments but not the 7A and 7C regions. Figure 6 shows a diagram of the results of hybridization experiments. Chromosome 5.5 was larger than the sum of 7F (340 kb) and 7G (390 kb) SfiI fragments in size. A fragment of 470 kb from some other chromosome(s) seems to be joined to the 7F and 7G regions. We could imagine several candidates for the origin of the 470 kb fragment added to chromosome 5.5, based on the known size of SfiI fragments in the strain 1006: the possible SfiI fragments were 5I of 480 kb, 4H of 430 kb, rB2–rD1 of 405 kb and 4F2 of 380 kb. Although the 7G region was also regarded as a candidate, the possibility of the duplication of the 7G region was eliminated because the intensity of hybridization to the two chromosomal bands recognized by the 7G probe was almost similar (Figure 4). Among these candidates examined, only the probe p356 (Table 1) from the 4H SfiI fragment hybridized to chromosome 5.5 and chromosome 4 (Figure 7, lane 3). We therefore deduced that chromosome 5.5 was composed of 4H, 7F and 7G. In the clones having chromosome 5.5, the 7A and 7C region exists only on the original sized chromosome 7. Thus, chromosome 5.5 seems to have been created by a non-reciprocal chromosome translocation between chromosomes 4 and 7. Chromosome 4 consists of four SfiI fragments; 4H, 4F2, 4N and 4BB. One copy of the 4H fragment was concerned to create chromosome 5.5 with 7F and 7G. We looked for the other components of chromosome 4, 4BB, 4N and 4F2, on the genome of STN21. When the 4N probe, pHIS5–2, was hybridized to STN21 chromosomes, the signal only appeared on chromosome 4, not in the region corresponding to the size of 4BB–4N–4F2 (data not shown). Figure 7D summarizes the putative chromosome translocation event in STN21.
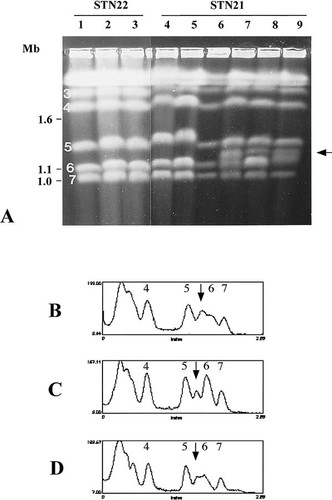
Electrophoretic karyotypes of STN21 and STN22 by pulsed field gel electrophoresis (PFGE) and densitometric analysis of chromosome bands of STN21. (A) showed the chromosomes in a sample plug separated by PFGE in a 0.8% agarose gel under the following conditions: 120 s switch time for 18 h, followed by 180 s switch time for 12 h at 180 V (panel A). The samples were prepared from STN22 (lanes 1, 2 and 3), STN21 (lanes 4, 5, 6, 7, 8 and 9). The numbers 3, 4, 5, 6 and 7 in lane 1 indicate the chromosome number. The figures on the left indicate chromosomal DNA molecular size markers of S. cerevisiae (Mb). The arrow on the right indicates the extra chromosomal DNA band in lane 9. Image (A) was incorporated into the computer and then analysed by NIH-Image programme. The histograms (B, C and D) correspond to lanes 7, 8 and 9 in panel A, respectively. Figures in each histogram show the positions of chromosome 4, 5, 6 and 7. The downward arrow indicates the position of chromosome 5.5.
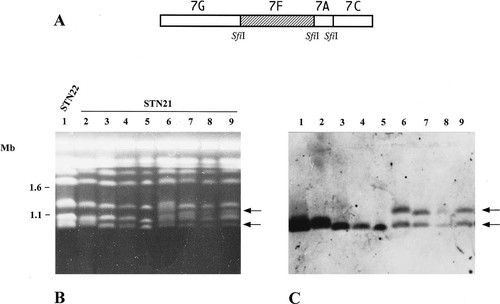
Hybridization profile by DNA probes on 7F SfiI fragment. Chromosomal DNAs were separated as described in Figure 3. (A) indicates the SfiI map of chromosome 7. The map is based on that of Chu et al. (1993). The hatched box indicates the region 7F, where the YCF1 probe is assigned. (B) shows the karyotype of STN22 (lane 1) and STN21 (lanes 2, 3, 4, 5, 6, 7, 8 and 9) stained by ethidium bromide. (C) shows the hybridization profile of the YCF1 probe. Arrows indicate the bands from lane 9 that hybridized to YCF1. The numbers on the left indicate chromosomal DNA molecular size markers from S. cerevisiae (Mb).
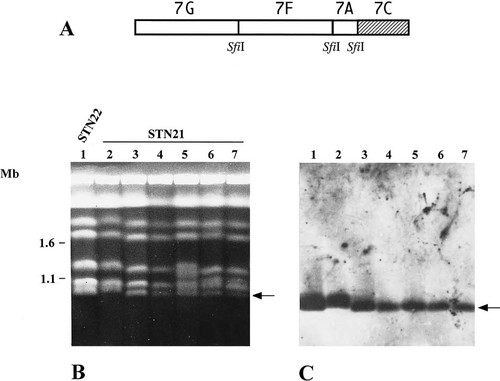
Hybridization profile by DNA probes on 7C SfiI fragments. Chromosomal DNAs were separated under the same conditions as in Figure 3. (A) indicates the SfiI map of chromosome 7. The hatched box indicates the region 7C where the INP53 probe is assigned. (B) shows the karyotype of STN22 (lane 1) and STN21 (lanes 2, 3, 4, 5, 6 and 7) stained by ethidium bromide. (C) shows the hybridization profile of the INP53 probe. The arrow indicates the band from lane 7 that hybridized to INP53. The numbers on the left indicate chromosomal DNA molecular size markers from S. cerevisiae (Mb).
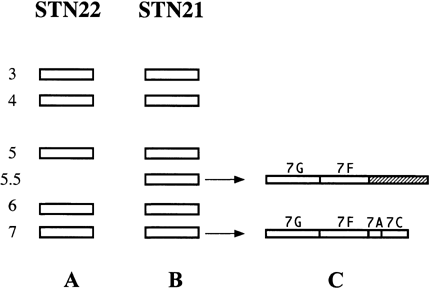
Scheme of chromosome band profiles and hybridization pattern by the DNAs on chromosome 7 in strains STN21 and STN22. The ethidium bromide-stained chromosome bands of STN22 (A) and STN21 (B) are represented as bars. The numbers on the left indicate the chromosome number. (C) indicates the SfiI map of chromosome 5.5 and 7. The hatched box on chromosome 5.5 represents the region where the DNAs on chromosome 7 were not assigned.
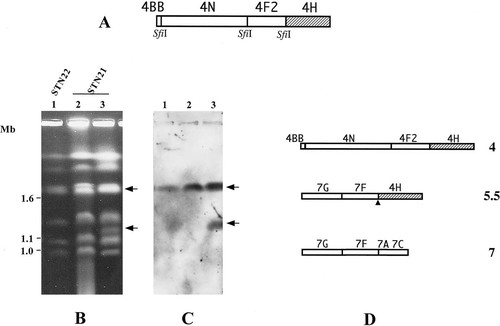
Determination of the origin of the extra chromosome band and the SfiI map of chromosome 4, 5.5 and 7 of STN21. Chromosomal DNAs were separated by PFGE in a 0.7% agarose gel under the following condition: 100 s switch time for 15 h at 180V, followed by 300 s switch time for 20 h at 140 V. Panel (A) indicates the SfiI map of chromosome 4. The hatched box indicates the region 4H, where the p356 probe is assigned. Panel (B) shows the karyotype of STN22 (lane 1) and STN21 (lane 2 and 3) stained with ethidium bromide. Panel (C) shows the hybridization profile of the p356 probe derived from the 4H SfiI fragment. The arrows indicate the band from lane 3 that hybridized to p356. The figures on the left indicate chromosomal DNA molecular size marker from S. cerevisiae (Mb). Panel (D) shows the SfiI map of chromosomes 4, 5.5 and 7 of STN21. The map is constructed from both Chu et al. (1993) and the hybridization results in this study. The figures on the right indicate the chromosome number. The filled triangles indicate the putative breakpoint of chromosome translocation.
DISCUSSION
There are several possible explanations for the Sps− phenotypes in STN21: one is that the pleiotropic phenotype is due to a single gene mutation; the second is that there is a synthetic effect of multiple mutations; and the third is that the individual aspects of the phenotype are due to different mutations. Although we can not exclude the possibility that more than one gene may participate in controlling the state of ploidy, it seems to be appropriate as a working hypothesis that a single gene mutation causes the pleiotropic phenotype. According to this hypothesis, the genotypes of STN14, STN21 and STN22 are defined as follows: The parent strain STN14 seems to have a recessive sps gene in a heterozygotic state (SPS/sps). By mild UV treatment, mitotic recombination was induced in this strain. As a result, a strain STN21 has the recessive allele sps in a homozygous state (sps/sps) and its isogenic strain STN22 carries a wild-type SPS in a homozygotic one (SPS/SPS). STN14 is also the parent of the strains 981 and 1006 which are the standard strains used for karyotype analysis and genomic mapping. These strains do not show any Sps− phenotypes. However, the SPS gene in these strains might exist in the heterozygous state (SPS/sps). It is possible to predict that many clinical isolates might also carry a recessive sps in a heterozygotic state (SPS/sps). Such strains might become homozygous at the gene by mitotic recombination or a mutation at the locus. Once the recessive sps becomes homozygous, the ploidy would become unstable and chromosome translocation could be induced, as in the case of STN21. The ratio of polyploid cells in the population varied from clone to clone in the Sps− strain STN21 (Figure 2). This may depend on how many cells underwent G2 arrest and M-phase skip in the population of individual clones. The function of the SPS gene product is considered to be the exclusive supervision and repression of the ploidy shift. Thus, it appears that the loss of function of the gene induces the increase in the percentage of polyploid cells in the population. Some factors other than the SPS gene seem to regulate the process of reductional nuclear division during the downshift of the ploidy state, producing diploid daughter cells. As a result, a tetraploid population (Figure 2E) had switched to the reduced ploidy state (Figure 2D).
The SPS gene product seems to relate to chromosome organization. Mutation of this gene can lead to a shift in the ploidy of the cell and can also lead to chromosome translocation. It was found in S. cerevisiae that mutation of several genes can lead to an increase in ploidy. Of these genes, CDC31 (Schild et al., 1981), NDC1 (Thomas and Botstein, 1986), MPS1 and MPS2 (Winey et al., 1991) are known to be essential genes for the proper function of the spindle pole body. SPA1 (Snyder and Davis, 1988) and KAR1 (Rose and Fink, 1987; Vallen et al., 1992) are components of the spindle pole body. Defects in the spindle pole body exhibit asymmetric DNA segregation. Such a missegregation yields one daughter cell having increased ploidy and one aneuploid daughter. IPL2 (Chan and Botstein, 1993) was also isolated as a gene which affects an increase in ploidy, and this gene is allelic to BEM2 (Bender and Pringle, 1991) which is required for normal bud growth. The processes of DNA repair and DNA replication are also considered to be important for maintaining genomic stability (Palmer et al., 1990). Of the genes which have been found to be involved in maintaining stable ploidy in S. cerevisiae, CDC31, MPS1 and BEM2 have been found in the C. albicans sequencing project. These could thus be disrupted and the mutant phenotypes to be tested for the Sps− phenotype. STN22 has normal ploidy and does not exhibit any aberrant karyotype and also has the same genetic background as the Sps− STN21. The comparison between the two strains will help us to isolate the putative SPS gene. The isolation of the gene is in progress.
Electrophoretic karyotype variations in C. albicans were shown by PFGE in several studies (Magee and Magee, 1987; Merz et al., 1988; Iwaguchi et al., 1990). By assignment of several DNA probes to chromosome DNA bands on PFGE, it was demonstrated that these variations of electrophoretic karyotype were mainly due to wide size heterogeneity in one of the homologous chromosomes (Iwaguchi et al., 1990). Chu et al. (1993) and Navarro-Garcia et al. (1995) have described chromosome translocation as the cause of karyotype variation. We succeeded in showing another example of chromosome translocation in this paper. The translocation observed on our study occurred between chromosomes 4 and 7, but the translocation was not reciprocal. One copy of the SfiI fragments 7A and 7C seemed to be lost from the genome after translocation. Although the translocation occurred between chromosomes 4 and 7 in both WO-1 and STN21, the breakpoints of chromosome translocation were different in the two strains; the translocation in WO-1 seems to have occurred at or near the SfiI sites located between 7F and 7G, and between 4F2 and 4N (Chu et al., 1993), but in STN21 it seems to have occurred at or near SfiI sites located between 7F and 7A and between 4F2 and 4H (Figure 7). Therefore, chromosomes 4 and 7 both have at least two regions in which chromosome translocation can occur. The loss of one copy of 7A and 7C SfiI fragments observed in clones of STN21 seems to have been brought about by a chromosome translocation event between chromosomes 4 and 7. It is possible that the probe we used for the 7A–7C region did not recognize the translocated region because of rearrangements, although this seems unlikely. However, we can test the prediction that this region is present in only one copy by mutagenizing and looking for Leu− mutants. The LEU2 gene is found on fragment 7C. As shown with WO-2, mutagenesis of a strain with only one copy of the LEU2 gene yields auxotrophic mutants at a frequency much higher than found in the wild-type diploid (Magee and Magee, 1997). The polyploid strain NUM961 contained an extra chromosome and a strain SGF7–2 producing polyploid cells had lost one complete homologue of chromosome R (Suzuki et al., 1989, 1994). However, chromosome translocation has not been proved in these strains. Chromosome translocation and a loss of part of chromosome 7 were only observed in the Sps− strain STN21 but not in the Sps+ strain STN22. Ploidy shift in a strain WO-1 has not been observed. Chromosome translocation observed in strains WO-1 and 1001 was reciprocal, but that in the STN21 was non-reciprocal. The mechanism of chromosome rearrangements in the former strains may be different from that of STN21. However, the possibility is not excluded that ploidy shift occurred transiently in these strains. Some phenomenon of ploidy shift might induce chromosome translocation. Rustchenko-Bulgac et al. (1994) reported that a laboratory strain, 3153A, of C. albicans spontaneously produced mutants which acquired the ability to assimilate particular sugars. Sorbose and D-arabinose-positive mutants were especially associated with chromosome rearrangements. Their observations suggested the possibility that chromosomal alternations in C. albicans are involved in phenotypic change. As ploidy shift is thought to be involved in chromosome rearrangements, it might induce genetic variations of fundamental function of C. albicans in some cases. We are now examining the correlation between ploidy shift followed by chromosome rearrangements and phenotypic changes in C. albicans.
Our data and that of Chu et al. (1993) suggested that both ends of the SfiI fragment F on chromosome 7 are active for recombination. It seems that the regions at or near the SfiI site on both ends of 7F fragment can be breakpoints for chromosome translocation. Chibana et al. (1998) showed that the major repeat sequence (MRS) is located on both ends of the 7F SfiI fragment from the fosmid contig map of chromosome 7; one is between SfiI fragments A and F and one is between F and G. The MRS contains the repeated sequence RPS (Iwaguchi et al., 1992b; Chibana et al., 1994), HOK and RB2 (Chindamporn et al., 1995, 1998). Of these, RPS contains multiple SfiI sites and is located as a cluster on all but one chromosome (Iwaguchi et al., 1992b). Therefore, the MRS seems to play an important role as the template in chromosome translocation on chromosome 7, and RPS is presumed to serve as a preferred site for chromosome breakage. As the MRS exists in part of every C. albicans chromosome, it might also serve as a hot spot for chromosome translocation in this species. In our data, chromosome translocation was only observed in the Sps− strain, STN21, but not in the Sps+ strain, STN22. The putative SPS gene may also function to control or suppress recombination between the MRSs on C. albicans chromosomes.
Acknowledgements
We thank B. B. Magee (University of Minnesota, USA) for providing plasmids for DNA probes and H. Chibana (University of Minnesota, USA) for providing plasmid pARG-SK. This work is supported in part by a Grant-in-aid (No. 06640865 and No. 07857019) for scientific research from the Ministry of Education, Science, Sports and Culture of Japan. The work in PTM's laboratory is supported by USPHS Grant 1R01AI16567.




