Ab initio study of the reaction of CHO+ with H2O and NH3
Abstract
An MP4(full,SDTQ)/6-311++G(d,p)//MP2(full)/6-311++G(d,p) ab initio study was performed of the reactions of formyl and isoformyl cations with H2O and NH3, which play an important role in flame and interstellar chemistries. Two different confluent channels were located leading to CO+H3O+/NH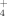 . The first one corresponds to the approach of the neutral molecule to the carbon atom of the cations. The second one leads to the direct proton transfer from the cations to the neutrals. At 900 K the separate products CO+H3O+/NH
. The first one corresponds to the approach of the neutral molecule to the carbon atom of the cations. The second one leads to the direct proton transfer from the cations to the neutrals. At 900 K the separate products CO+H3O+/NH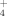 are the most stable species along the Gibbs energy profiles for the processes. For the reaction with H2O the reaction channel leading to HC(OH)
are the most stable species along the Gibbs energy profiles for the processes. For the reaction with H2O the reaction channel leading to HC(OH)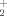 (protonated formic acid) is disfavored with respect to the two CO+H3O+ channels in agreement with the experimental evidence that H3O+ is the major ion observed in hydrocarbon flames. According to our calculations, NH
(protonated formic acid) is disfavored with respect to the two CO+H3O+ channels in agreement with the experimental evidence that H3O+ is the major ion observed in hydrocarbon flames. According to our calculations, NH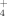 +H2O are considerably more stable in Gibbs energy than NH3+H3O+;NH
+H2O are considerably more stable in Gibbs energy than NH3+H3O+;NH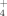 will predominate in the reaction zone when ammonia is added to CH4+Ar diffusion flame, as experimentally observed. At 100 K the most stable structures are the intermediate complexes CO…HOH
will predominate in the reaction zone when ammonia is added to CH4+Ar diffusion flame, as experimentally observed. At 100 K the most stable structures are the intermediate complexes CO…HOH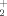 /HNH
/HNH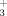 . Particularly the CO…HOH
. Particularly the CO…HOH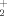 complex has a lifetime large enough to be detected and, therefore, could play a certain role in interstellar chemistry. ©1999 John Wiley & Sons, Inc. J Comput Chem 20: 1432–1443, 1999
complex has a lifetime large enough to be detected and, therefore, could play a certain role in interstellar chemistry. ©1999 John Wiley & Sons, Inc. J Comput Chem 20: 1432–1443, 1999




