Theoretical study of a vanadate peptide complex
Corresponding Author
Michael Bühl
Organisch-Chemisches Institut, Universität Zürich, Winterthurerstrasse 190, CH-8057 Zürich, Switzerland
Organisch-Chemisches Institut, Universität Zürich, Winterthurerstrasse 190, CH-8057 Zürich, SwitzerlandSearch for more papers by this authorCorresponding Author
Michael Bühl
Organisch-Chemisches Institut, Universität Zürich, Winterthurerstrasse 190, CH-8057 Zürich, Switzerland
Organisch-Chemisches Institut, Universität Zürich, Winterthurerstrasse 190, CH-8057 Zürich, SwitzerlandSearch for more papers by this authorAbstract
Optimized geometries (BP86/I) and 51V-NMR chemical shifts (GIAO-B3LYP/I) are reported for selected [VO(OH)(OH2)(glygly′)] isomers, deprotonated forms thereof, and [VO(OH)(glygly′)] (glygly′=H2NCH2CONCH2COO). The δ(51V) values are quite sensitive to structural details in the first and second coordination spheres of the vanadium center. In the deprotonated forms, the water molecule is not bound to vanadium, suggesting that the coordination geometry about vanadium can be sensitive to the pH value. ©1999 John Wiley & Sons, Inc. J Comput Chem 20: 1254–1261, 1999
References
- 1 H. Sigel; A. Sigel, Eds.; Metal Ions in Biological Systems; Marcel Dekker: New York, 1995; Vol. 31.
- 2
(a)
N. D. Chasteen, Ed.;
Vanadium in Biological Systems;
Kluwer Academic Publishers:
Amsterdam,
1990.
10.1007/978-94-009-2023-1 Google Scholar(b) A. S. Tracey; D. C. Crans, Eds.; Vanadium Compounds—Chemistry, Biochemistry, and Therapeutic Applications, ACS Symposium Series Washington, DC Vol. 711, 1998.
- 3 Rehder, D. Angew Chem Int Ed Engl 1991, 30, 148.
- 4 (a) Vilter, H. In Metal Ions in Biological Systems; H. Sigel; A. Sigel, Eds.; Marcel Dekker: New York, 1995; Vol. 31, p 325; X-ray structure: (b) Messerschmidt, A.; Wever, R. Proc Natl Acad Sci USA 1996, 93, 392.
- 5 (a) Eady, R. R. In Metal Ions in Biological Systems; H. Sigel; A. Sigel, Eds.; Marcel Dekker: New York, 1995; Vol. 31, p 363; (b) Eady, R. R. Polyhedron 1989, 8, 1695.
- 6 Butler, A.; Carrano, C. J. Coord Chem Rev 1991, 109, 61.
- 7 (a) Orvig, C.; Thompson, K. H.; Battell, M.; McNeill, J. H. In Metal Ions in Biological Systems; H. Sigel; A. Sigel, Eds.; Marcel Dekker: New York, 1995; Vol. 31, p 575; (b) A. K. Srivastava; J.-L. Chiasson, Eds. Molecular and Cellular Biochemistry; Kluwer Academic Publishers: Boston, 1995; Vol. 153.
- 8 (a) Rehder, D. Inorg Chem 1988, 27, 4312; (b) Crans, D. C.; Holst, H.; Keramidas, A. D.; Rehder, D. Inorg Chem 1995, 34, 2524; (c) Fritzsche, M.; Elvingston, K.; Rehder, D.; Pettersson, L. Acta Chem Scand 1997, 51, 483.
- 9 (a) Jaswal, J. S.; Tracey, A. S. Can J Chem 1991, 69, 1600; (b) Jaswal, J. S.; Tracey, A. S. J Am Chem Soc 1993, 115, 5600; (c) Einstein, F. W. B.; Batchelor, R. J.; Angus–Dunne, S. J.; Tracey, A. S. Inorg Chem 1996, 35, 1680.
- 10 Keramidas, A. D.; Miller, S. M.; Anderson, O. P.; Crans, D. C. J Am Chem Soc 1997, 119, 8915.
- 11 Tasiopoulos, A. J.; Deligiannakis, Y. G.; Woollins, J. D.; Slawin, A. M.; Kabanos, T. A. J Chem Soc Chem Commun 1998, 569.
- 12 (a) Horwath, O. W. Prog NMR Spectrosc 1990, 22, 453; (b) Rehder, D. In Transition Metal Nuclear Magnetic Resonance; P. S. Pregosin, Ed.; Elsevier: Amsterdam, 1991; p 1.
- 13 Even the binding to large proteins such as the transferrins can be studied with 51V NMR: Saponja, J. A.; Vogel, H. J. J Inorg Biochem, 1996, 62, 253.
- 14 Malkin, V. G.; Malkina, O. M.; Casida, M. E.; Salahub, D. R. J Am Chem Soc 1994, 116, 5898.
- 15 (a) Schreckenbach, G.; Ziegler, T. Theor Chem Acc 1998, 2, 71; (b) Bühl, M.; Kaupp, M.; Malkin, V. G.; Malkina, O. L. J Comput Chem, 1999, 20, 91.
- 16 Bühl, M.; Hamprecht, F. A. J Comput Chem 1998, 19, 113.
- 17 (a) Cremer, D.; Olsson, L.; Reichel, F.; Kraka, E. Isr J Chem 1993, 33, 369; (b) Bühl, M. In Encyclopedia of Computational Chemistry; P. v. R. Schleyer, Ed.; Wiley: New York, 1998; p 1835.
- 18 McMahon, M. T.; deDios, A. C.; Godbout, N.; Salzmann, R.; Laws, D. D.; Le, H.; Havlin, R. H.; Oldfield, E. J Am Chem Soc 1998, 120, 4784.
- 19 Becke, A. D. Phys Rev A 1988, 38, 3098.
- 20 (a) Perdew, J. P. Phys Rev B 1986, 33, 8822; (b) Perdew, J. P. Phys Rev B 1986, 34, 7046.
- 21 (a) Wachters, A. J. H. J Chem Phys 1970, 52, 1033; (b) Hay, P. J. J Chem Phys 1977, 66, 4377.
- 22 (a) Gonzales, C.; Schlegel, H. B. J Chem Phys 1989, 90, 2154; (b) Gonzales, C.; Schlegel, H. B. J Phys Chem 1990, 94, 5523.
- 23 Ziegler, T. Can J Chem 1995, 73, 743.
- 24 Cheeseman, J. R.; Trucks, G. W.; Keith, T. A.; Frisch, M. J. J Chem Phys 1996, 104, 5497.
- 25 Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Gill, P. M. W.; Johnson, B. G.; Robb, M. A.; Cheeseman, J. R.; Keith, T.; Petersson, G. A.; Montgomery, J. A.; Raghavachari, K.; Al-Laham, M. A.; Zakrzewski, V. G.; Ortiz, J. V.; Foresman, J. B.; Peng, C. Y.; Ayala, P. Y.; Chen, W.; Wong, M. W.; Andres, J. L.; Replogle, E. S.; Gomperts, R.; Martin, R. L.; Fox, D. J.; Binkley, J. S.; DeFrees, D. J.; Baker, J.; Stewart, J. J. P.; Head–Gordon, M.; Gonzales, C.; Pople, J. A. Gaussian 94; Gaussian Inc.: Pittsburgh, PA, 1995.
- 26 Becke, A. D. J Chem Phys 1993, 98, 5648.
- 27 Lee, C.; Yang, W.; Parr, R. G. Phys Rev B 1988, 37, 785.
- 28 Bühl, M. Chem Phys Lett 1997, 267, 251.
- 29
(a)
Bader, R. W. F.
Atoms In Molecules. A Quantum Theory;
Clarendon Press:
Oxford, UK,
1990;
10.1093/oso/9780198551683.001.0001 Google Scholar(b) Bader, R. W. F. Chem Rev 1991, 91, 893.
- 30 Popelier, P. L. A. Comput Phys Commun 1996, 93, 212.
- 31 Clark, T.; Chandrasekhar, J.; Spitznagel, G. W.; Schleyer, P. v. R. J Comput Chem 1983, 4, 294.
- 32 Bashirpoor, M.; Schmidt, H.; Schulzke, C.; Rehder, D. Chem Ber/Recueil 1997, 130, 651.
- 33 In a related Schiff-base complex with 2-butanol as the donor ligand, somewhat larger V–O separations are encountered up to 2.328(8) Å: Fulwood, R.; Schmidt, H.; Rehder, D. J Chem Soc Chem Commun 1995, 1443.
- 34 Rehder, D. In Metal Ions in Biological Systems; H. Sigel; A. Sigel, Eds.; Marcel Dekker: New York, 1995; Vol. 31, p 1.
- 35 Griffith, W. P.; Wickins, T. D. J Chem Soc A 1966, 1087.
- 36 Harnung, S. E.; Larsen, E.; Pedersen, E. J. Acta Chem Scand 1993, 47, 674.
- 37
Only at lower pH with formation of VO
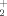 does the coordination number increase to six:
Cruywagen, J. J.;
Heyns, J. B. B.;
Westra, A. N.
Inorg Chem,
1996,
35, 1556.
does the coordination number increase to six:
Cruywagen, J. J.;
Heyns, J. B. B.;
Westra, A. N.
Inorg Chem,
1996,
35, 1556.
- 38 The same was previously found at the self-consistent field level employing small basis sets: Ribeiro–Claro, P. J. A.; Amado, A. M.; Teixeira–Dias, J. J. C. J Comput Chem, 1996, 17, 1183>.
- 39 In order to probe if a pentacoordinate form might be stabilized by medium effects due to the highly polar solvent, an optimization was also started in a spherical, polarizable continuum [(a) Kirkwood, J. G. J Chem Phys, 1934 2, 351; (b) Onsager, L. J Am Chem Soc 1936, 58, 1486; implementation in the Gaussian program: (c) Wong, M. W.; Frisch, M. J.; Wiberg, K. B. J Am Chem Soc 1991, 113, 4776], employing the dielectric constant of water, which also resulted in dissociation of the water molecule from vanadium.
- 40 A referee pointed out that DFT usually underestimates weak donor–acceptor bonds and that MP2 would do a better job. However, at the MP2/I level 6a also does not exist and optimizes to 5⋅ H2O.
- 41 Krauss, M.; Basch, H. J Am Chem Soc 1992, 114, 3630.
- 42 Ultimately, molecular dynamics simulations should be performed for a sampling of the configuration space on the free energy surface. Polycyclic H-bonded motifs as in 5⋅H2O and 5⋅2H2O are probably disfavored entropically.
- 43 Removal of the water molecule from 7a affords [V(O)2(glygly)′]− (i.e., deprotonated 4) with a computed δ(51V)=−610; the deshielding effect of a single solvent molecule is thus much smaller in this case than for 5.
- 44 (a) Eckert, H.; Wachs, I. E. J Phys Chem 1989, 93, 6796; (b) Das, N.; Eckert, H.; Hu, H.; Wachs, I. E.; Walzer, J. F.; Feher, F. J. J Phys Chem 1993, 97, 8240; (c) Crans, D. C.; Felry, R. A.; Chen, H.; Eckert, H.; Das, N. Inorg Chem 1994, 33, 2427; (d) Lee, M.-H.; Heo, N. H.; Hayashi, S. Polyhedron 1998, 17, 55.
- 45 Direct assessment of theoretical 51V shift tensors is difficult because of the lack of suitable experimental reference data. For the solid reaction product of vanadate with tert-butanol, presumably VO(OtBu)3, components δii=−555, −575, and −875 were reported (ref. 44c), which is in fair accord with the GIAO-B3LYP/I data for model compound VO(OMe)3, δii=−637, −637, and −835.




