Expression of nitric oxide synthase and soluble guanylyl cyclase in the developing olfactory system of Manduca sexta
Abstract
The gaseous messenger nitric oxide (NO), with its ability to mediate both intercellular and intracellular communication, can play important roles in mediating cellular communication in both the development and the function of the nervous system. The authors investigated the possible role of NO signaling in the developing olfactory system (antennal lobe) of the moth Manduca sexta. NO synthase (NOS), the enzyme that generates NO, was localized by using immunocytochemistry, in situ hybridization, and nicotinamide adenine dinucleotide phosphate-diaphorase (NADPH-d) histochemistry. Although NADPH-d staining appears to be a poor indicator of the presence of NOS in this system, immunocytochemistry and in situ hybridization reveal that NOS is expressed in the axons of olfactory receptor neurons throughout development and in the perineurial sheath that covers the brain early in development. NOS is present in axon terminals as they form protoglomeruli, raising the possibility that NO mediates cell-cell interactions during antennal lobe development. NO-sensitive soluble guanylyl cyclase (sGC), one of the best characterized targets of NO, was localized in the developing olfactory system by using in situ hybridization and immunocytochemistry for the Manduca sexta sGCα1 subunit. The ability of the developing olfactory system to respond to exogenous NO also was examined by using cyclic guanosine monophosphate immunocytochemistry. sGC is expressed in mechanosensory neurons in the developing antenna and in many antennal lobe neurons in both the medial and lateral cell body clusters. Thus, NOS and sGC are expressed in a pattern that suggests that this signaling pathway may mediate intercellular communication during development of the olfactory system in Manduca sexta. J. Comp. Neurol. 422:191–205, 2000. © 2000 Wiley-Liss, Inc.
The gaseous messenger nitric oxide (NO) plays many different roles in mediating cellular communication in the nervous system. Its ability to act as either a retrograde transmitter or an orthograde transmitter and its ability to cross cell membranes to coordinate communication between groups of cells have led to the suggestion of its involvement in a wide variety of neuronal processes (Bredt and Snyder, 1992). For example, this pathway appears to be involved in neuronal plasticity, including long-term potentiation and long-term depression (Gage et al., 1997), and the processing of olfactory information in both vertebrates (Kendrick et al., 1997) and invertebrates (Nighorn et al., 1998). Its importance was underscored further by a recent study (De Vente et al., 1998) that demonstrated elevated cyclic guanosine monophosphate (cGMP) levels in neuronal subsystems in virtually every part of the rat brain in response to incubation with NO.
NO-mediated neuronal communication also is important for the development of the nervous system (Matsumoto et al., 1993a). It has been implicated in refinement of retinotectal and retinogeniculate projections (Wu et al., 1994; Cramer et al., 1996) and in both promotion and suppression of neurite outgrowth (Hess et al., 1993; Rentería and Constantine-Paton, 1996; Hindley et al., 1997). In insects, NO signaling has been shown to be important for the development of the Drosophila visual system (Gibbs and Truman, 1998), and it has been suggested to play a role in synapse maturation in a variety of motor neurons in the locust (Truman et al., 1996; Ball and Truman, 1998). In the olfactory system, work in vertebrates suggests that NO plays an important role in development, acting to synchronize differentiation of olfactory receptor neuron (ORN) precursor cells, to modulate synaptic stabilization at ORN axon terminals, and in the regeneration of ORN axons (Roskams et al., 1994; Arnhold et al., 1997).
The goal of the current study was to examine the role of NO signaling in the development of the antennal lobe of the hawkmoth, Manduca sexta. The antennal lobe is the first synaptic neuropil of the olfactory system, in which ORN axons synapse with processes of antennal lobe interneurons and projection neurons in spherical neuropil structures called glomeruli (the signature neuropil structure of almost all chemosensory systems; Hildebrand, 1995). During the development of this system, ORN axons project to the site of the future antennal lobe, where their terminal arborizations (called protoglomeruli) induce glial cell migration and are joined by processes of antennal lobe neurons (Oland et al., 1990; Oland and Tolbert, 1996). Because the afferent axon terminals are essential for establishment of the glomerular architecture of the antennal lobe, NO-mediated communication between these afferents and the neurons and glia of the antennal lobe may be important to development in Manduca.
One way to investigate the potential role of NO in mediating cellular communication events during development is to determine the localization of the enzyme that generates NO, nitric oxide synthase (NOS), and the best characterized target of NO, soluble guanylyl cyclase (sGC). A number of different methods have been devised to do this. The presence of NOS has been inferred in many systems by using a histochemical stain for nicotinamide adenine dinucleotide phosphate-diaphorase (NADPH-d), an activity that frequently colocalizes with NOS (Schmidt et al., 1992). NOS mRNA also can be localized by using in situ hybridization, and NOS protein can be detected immunocytochemically with commercially available universal NOS antibodies.
NO-sensitive sGC is a heme-containing heterodimer consisting of an α subunit and a β subunit. The sGC mRNA and protein products have been localized by using in situ hybridization and immunocytochemistry with specific antisera (Elphick and Jones, 1998). Another popular strategy has been to stimulate artificially the tissue in question with NO and use an antiserum generated against cGMP (DeVente and Steinbusch, 1997) to identify the cells that can respond to NO with an increase in cGMP.
We recently cloned and characterized cDNAs for Manduca sexta NOS (MsNOS) and the α and β isoforms of sGC (MsGCα1 and MsGCβ1) (Nighorn et al., 1998). Here, we report on the pattern of expression of NOS and sGC during the development of the olfactory system of Manduca sexta. NOS is expressed strongly by perineurial cells through pupal stage 7 and in the ingrowing axons of ORNs throughout development and in the adult. The presence of NOS at ingrowing axon terminals raises the possibility that NO plays a role in formation or stabilization of glomerular structure in the developing antennal lobe. sGC is expressed in the dendrites of some antennal lobe neurons, indicating a mechanism by which receptor axons may communicate with or influence the development of antennal lobe neurons through an NO-cGMP pathway. cGMP immunocytochemistry also indicates a possible role for NO/sGC-mediated communication in antennal mechanosensory neurons.
MATERIALS AND METHODS
Animals
Male Manduca sexta (Lepidoptera: Sphingidae) were reared on an artificial diet, as described previously (Sanes and Hildebrand, 1976; Prescott et al., 1977). Animals were staged according to external morphology, as described by Tolbert et al. (1983).
RNA isolation
Antennae and antennal lobes were dissected from male pupae. Total RNA was isolated by using Trizol reagent (Life Technologies, Gaithersburg, MD). Poly (A+) RNA was obtained from antennal total RNA by using Oligotex beads (Qiagen, Valencia, CA).
ANTI-MSGC1 ANTIBODY
Antiserum was generated in chickens by Alpha Diagnostics International (San Antonio, TX). The antigen was a peptide with the sequence SAPKKPEFRSRTSSVHLTGPE. This sequence was found to be unique to the MsGCα1 isoform (Nighorn et al., 1998).
Northern blot analysis
Antennal poly (A+) RNA (2 μg/lane) and antennal lobe total RNA (10 μg/lane) from a range of pupal stages were separated on formaldehyde-1% agarose gels and blotted onto Zetaprobe membranes (BioRad, Hercules, CA). Blots were hybridized overnight at 42°C in hybridization buffer containing 50% formamide, 5× sodium-saline-phosphate ethylenediamine tetraacetic acid (EDTA) buffer (SSPE), 5× Denhardt's solution, 1% sodium dodecylsulfate (SDS), 10% dextran sulfate, 100 μg/ml sheared salmon sperm DNA, and 106 cpm/ml 32P-labeled, random-primed probe made from gel-purified cDNA clones by using a Decaprime labeling kit (Ambion).
NOS Western blot analysis
For each lane, one stage 5 brain was harvested and homogenized in homogenization buffer (50 mM Tris-HCl, pH 7.4; 0.1 mM EDTA; 0.1 mM ethylene glycol-bis (β-aminoethyl ether) N,N,N′,N′-tetraacetic acid EGTA; 12 mM β-mercaptoethanol; 1 μM leupeptin; 1 μM pepstatin A; 1 mM 4-(2-aminoethyl) benzenesulfonyl fluoride AEBSF; all from Sigma, St. Louis, MO). The samples were then separated by SDS-polyacrylamide gel electrophoresis and were transferred to Immobilon-P membrane (Millipore, Bedford, MA). The blots were probed with a polyclonal universal NOS antibody (uNOS; Oncogene Research Products, Cambridge, MA) made in rabbit against the peptide sequence KRYHEDIFG. This sequence is almost identical to the Manduca sexta NOS sequence, NRYHEDIFG. The primary antisera were used at a dilution of 1:1,000, and the secondary antibody (goat anti-rabbit AP; Jackson Laboratories, West Grove, PA) was used at a dilution of 1:10,000. The blot was developed by using an Opti-4CN substrate kit (Bio-Rad).
NOS immunocytochemistry
Animals were anesthetized on ice. Antennae for cross sections were fixed in situ in 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4, for 20 minutes; cut into ten-segment lengths, fixed for an additional 3 hours at room temperature, and embedded in 5% agarose.
Antennae for fillets were dissected into insect saline adjusted to 360 mOsm with mannitol, cut lengthwise with iridectomy scissors, pinned out, fixed overnight in 4% paraformaldehyde, then handled as for sections. Brains were dissected into insect saline and fixed overnight at 4°C in 4% paraformaldehyde. Both brains and antennae were washed in phosphate-buffered saline (PBS), embedded in 5% agarose, and sectioned on a Vibratome at 100 μm. Sections were soaked in PBS with 0.5% Triton X-100 (PBST) for varying periods of time, then blocked in PBST containing 5% normal goat serum at room temperature for 2 hours. Primary antiserum (uNOS; Oncogene Research Products) was added at a dilution of 1:300 (1:200 for antennae). After incubation overnight at 4°C, sections were washed, blocked, and incubated for 2 hours at room temperature with goat anti-rabbit antibody conjugated to Alexa 546 or 488 dyes (Molecular Probes, Eugene, OR) at 1:250. Sections that also were labeled with propidium iodide (Sigma) were treated first with 100 μg/ml RNAse (Sigma) for 5–15 minutes at room temperature, washed, then incubated in 25 μg/ml propidium iodide for 15 minutes at room temperature, then washed. Sections were mounted in PBS with 80% glycerol and viewed on a Nikon PCM 2000 laser-scanning confocal microscope (Nikon, Tokyo, Japan) on an E800 microscope using Simple 32 software (Compix Inc., Cranberry Township, PA). Controls in which no primary antibody was used gave no discernible image at the confocal microscope settings used for the experimental images.
GC immunocytochemistry
Animals were anesthetized on ice. Brains were dissected into insect saline and fixed and sectioned as described above. Sections were blocked in PBST containing 10% normal goat serum at room temperature for 2 hours. Primary antiserum (anti-MsGCα1; made in chick) was added at a dilution of 1:5,000. After incubation overnight at 23°C, sections were washed, blocked, and incubated for 2 hours at room temperature with goat anti-chick antibody conjugated to Alexa 546 dye (Molecular Probes) at 1:250. Sections were mounted and viewed as described above. Controls in which preimmune serum was used gave no discernible image at the confocal microscope settings used for the experimental images.
cGMP immunocytochemistry
Brains and antennae were dissected as described above then incubated for 60 minutes in insect saline at room temperature followed by incubation for 20 minutes in insect saline alone or in insect saline containing 1 mM sodium nitroprusside (SNP; Sigma) plus 10 mM 3-isobutyl-1-methylxannthine (IBMX; Sigma), IBMX alone, or SNP alone, then rinsed, and fixed in 4% paraformaldehyde for 2 hours at room temperature. After fixation, brains were washed in PBS, embedded in 5% agarose, soaked in PBST (0.5% Triton X-100) at 4°C overnight, and sectioned on a Vibratome at 200 μm. Sections were blocked in PBST containing 5% normal donkey serum at room temperature for 2 hours. Sheep polyclonal anti-cGMP antisera (H. Steinbusch, Maastricht, The Netherlands) was added at a dilution of 1:10,000, and sections were incubated overnight at 4°C. Sections were then washed, blocked, and incubated for 2 hours at room temperature with donkey anti-sheep antibody conjugated to indocarbocyanine dye (Jackson Laboratories, West Grove, PA) at a dilution of 1:250. Sections were then processed and viewed as described above. Controls in which no primary antibody was used gave no discernible image at the confocal microscope settings used for the experimental images.
NADPH-d staining
Brains were dissected in saline and fixed in 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4, for 2 hours at 4°C. Frozen sections (20 μm) were cut and mounted onto subbed slides. After a rinse in 50 mM Tris-HCl, pH 7.5, sections were incubated in 50 mM Tris-HCl, pH 7.5, containing 0.1% Triton X-100, 0.1 mM nitro blue tetrazolium NBT, and 0.1 mM β-NADPH (all from Sigma) for 2 hours at room temperature.
Diaminofluorescein labeling
Stage 5 antennae (with attached brains) were dissected in insect saline and then filleted along part of their length to increase contact of the luminal surface with the medium. Tissue was transferred to insect saline containing 3 μl/ml of the NO indicator diaminofluorescein (DAF-2 DA; Calbiochem, San Diego, CA) and 3 μl/ml Pluronic F-127 (Molecular Probes) and incubated at room temperature in the dark for 4 hours. The tissue was incubated in insect saline for 1 hour, then fixed overnight in 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4, at 4°C. The tissue was then viewed on the Nikon laser-scanning confocal microscope using appropriate filters.
In situ hybridization
In situ hybridization was performed as described in Nighorn et al. (1998). Briefly, brains were dissected and fixed in 4% paraformaldehyde in PBS overnight at 4°C. Frozen sections (20 μm) were then cut and incubated overnight at 42°C. After pretreatment, the slides were hybridized overnight at 42°C in 50% formamide, 10% dextran sulfate, 1× Denhardt's, 4 × SSPE, 500 μg/ml yeast tRNA, 250 μg/ml sonicated salmon sperm DNA, and ≈5 ng of digoxigenin-labeled riboprobe. Equal amounts of a sense riboprobe were used as a negative control. After washing, the probes were visualized by using alkaline phosphatase-labeled sheep antidigoxigenin antibody (Boehringer Mannheim), and the staining was developed by using a 5-Bromo-4-chloro-3-indoyl phosphate BCIP/NBT solution. Slides were then viewed with a Nikon E600 microscope with a Spot CCD camera (Diagnostic Instruments, Sterling Heights, MI).
Image processing
All of the confocal images and CCD camera images above were imported into Corel Photopaint software, in which false color was applied to the confocal images, and the contrast was adjusted for maximum clarity. The images were then put together into figures in Corel Draw, and annotations were added.
RESULTS
Manduca sexta olfactory system expresses both NOS and sGC during development
Northern blot analyses using total RNA isolated from antennal lobes at different stages of pupal development were performed to determine whether MsNOS and NO-sensitive sGC (MsGCα1 and MsGCβ1) were expressed in the developing antennal lobe. Pupal development is divided into 18 stages, each lasting approximately 1 day (Tolbert et al., 1983). The following stages were examined: stage 2, just prior to the major developmental events initiated by the ingrowth of ORN axons; stages 3 and 4, when ORN axons first enter the developing antennal lobe but just before protoglomeruli start to form; stage 6, after projection neuron dendrites have entered protoglomeruli and the glomeruli have begun to stabilize; stage 7, whenglomeruli are stabilized and waves of synaptogenesis are occurring; stage 12, when the organization of the antennal lobe is established; and stage 18, just prior to adult emergence (Oland and Tolbert, 1996). MsGCα1 mRNA remained constant, whereas MsGCβ1 mRNA increased somewhat throughout development (Fig. 1A). The MsNOS message was developmentally regulated very strongly: Amounts were high at developmental stages 2–4, declined sharply at stages 6 and 7, and were undetectable at stages 12 and 18. The peak of MsNOS expression was at stage 4, just prior to the time protoglomeruli form and when interactions between ORN axons and antennal lobe neuronal and glial processes are likely to be very important. The expression of MsNOS, MsGCα1, and MsGCβ1 also were examined in the developing antennae and were found to be present throughout development. A Northern blot showing their expression at stages 5 and 7 is shown in Figure 1B.
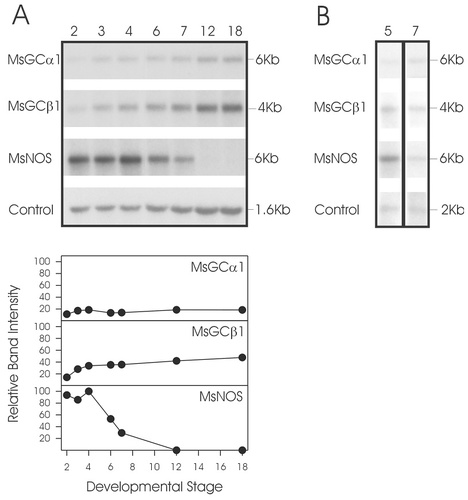
Northern blot analyses of Manduca sexta nitric oxide synthase (MsNOS) and guanylyl cyclase (MsGC) mRNA expression. A: Northern blot of total RNA showing the developmental regulation of expression of MsNOS and of the MsGC α1 and β1 subunits (MsGCα1 and MsGCβ1, respectively) in the antennal lobe. The stage of development is noted at the top of each lane. The size of each of the bands is noted on the right in Kb. Manduca eukaryotic elongation factor (EEF) was used as a control for loading. Relative band intensities, which were normalized to the EEF bands for the same stage, are shown below. B: Northern blot analysis of poly-A+ RNA showing the expression patterns of MsNOS, MsGCα1, and MsGCβ1 in the antenna at pupal stages 5 and 7. Manduca ATP synthase was used as a control for loading and efficiency of poly-A+ selection.
MsNOS is expressed in perineurial cells and ORN axons
Having established that the genes for Manduca NOS and sGC are expressed in the developing antennal lobe, we sought to identify the cells that were expressing NOS during development by using NOS immunocytochemistry, NADPH-d histochemistry, and in situ hybridization. Pupal stage 5 was chosen as a starting point, because this was close to the peak of the developmental expression of NOS in both the antennae and the antennal lobes. It also is a stage in which the interactions between the olfactory receptor axons and the antennal lobe glial cells and neurons are important for the formation of glomeruli.
The commercially available NOS antipeptide antiserum (uNOS antibody) was checked for specificity on a Western blot of stage 5 pupal brain homogenates and produced a strong band of approximately 138 kD with weak staining of two higher molecular weight bands around 225 kD (Fig. 2). The measured size of 138 kD is a good match to the predicted size of 137 kD for MsNOS (Nighorn et al., 1988) and suggests that this antiserum can be used to specifically localize MsNOS.
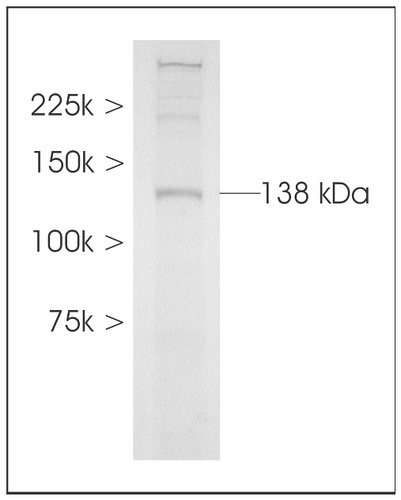
Western Blot analysis of universal nitric oxide synthase antibody (uNOS) antisera in Manduca sexta brain. A band of 138 kDA is labeled strongly along with two weak bands of higher molecular weight. The predicted size of Manduca NOS is 137 kDa.
NOS immunocytochemistry revealed strong NOS expression in the perineurium of stage 5 antennal lobes (Fig. 3A). This staining was not restricted to the antennal lobe area but was present in perineurium covering all regions of the brain. There also was strong NOS immunoreactivity in a subset of ORN axons that could be seen traveling together in the antennal nerve. Two ordinary protoglomeruli near the entrance region of the antennal nerve also were labeled strongly, whereas several adjacent protoglomeruli that were just beginning to form showed weak staining (Fig. 3A). Protoglomeruli near the nerve are known to be the first to develop, with those farther away from the nerve forming later in relation to their distance (Malun et al., 1994). Although it is not seen in the image in Figure 3, similar sections have revealed that protoglomeruli of the macroglomerular complex (Hildebrand, 1995) also are NOS immunoreactive at this stage, but the staining is very diffuse, perhaps reflecting a more diffuse arborization of the axon terminals. In addition to the strong staining of antennal nerve axon terminals, there was very faint staining of neuronal nuclei surrounding the antennal lobe neuropil. This staining of the nuclei was relatively constant throughout antennal lobe development, even though the levels of mRNA message for NOS was not. Thus, we suspect that this staining reflects background, especially in view of its nuclear location. The problem of faint background staining with this antibody has been described previously in lobster by Scholz et al. (1998). It is possibly due to the fact that the epitope recognized by the antibody corresponds to the NADPH-binding region of NOS and, thus, may cross react with other enzymes that use NADPH.
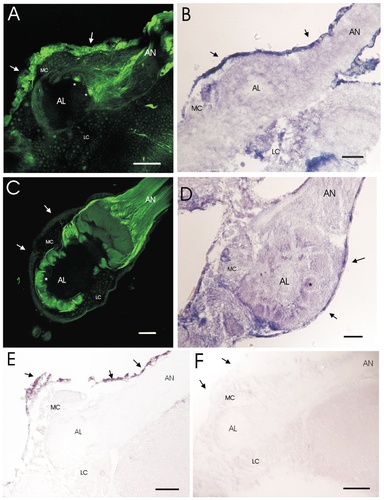
Localization of MsNOS in the developing antennal lobe. In A–F, the antennal lobe (AL), antennal nerve (AN), medial cell body cluster (MC), and lateral cell body cluster (LC) are labeled. A: Nitric oxide synthase (NOS) immunocytochemistry in a stage 5 antennal lobe. Staining of the perineurial sheath (arrows) and antennal nerve are shown. Note the staining in protoglomeruli (asterisks). B: Nicotinamide adenine dinucleotide phosphate-diaphorase (NADPH-d) staining in stage 5 antennal lobe. Note the staining in perineurial sheath (arrows) and both the LC and the MC cell body clusters. C: NOS immunocytochemistry in a stage 18 (pharate adult) antennal lobe. Note the lack of staining in the perineurial sheath (arrows) and the lateral and medial cell body clusters. Strong staining can be seen in the antennal nerve and in the caps of various glomeruli. A representative glomerulus is denoted by an asterisk. D: NADPH-d staining of a stage 18 (pharate adult) antennal lobe. Note the staining in basal portions of glomeruli (a representative glomerulus is denoted by an asterisk), the faint staining of the perineurial sheath (arrows), and strong staining of some cell bodies in the medial cell body cluster. E: In situ hybridization of MsNOS mRNA in a stage 5 antennal lobe. Note the strong staining of the perineurial sheath (arrows). F: In situ hybridization of a stage 5 antennal lobe with MsNOS sense control probe. Note the lack of staining of the perineurial sheath (arrows). Scale bars = 100 μm.
Staining of antennal lobes at later stages of development revealed that, by stage 6, all protoglomeruli were NOS immunoreactive and that, by the end of stage 7, the perineurium had lost its NOS immunoreactivity (not shown). At stage 18, just prior to adult eclosion, uNOS staining revealed that NOS was present in the axon terminal portion of all of the glomeruli (Fig. 3C), where it presumably plays a role in modulation of odor detection in the adult (Nighorn et al, 1998). At this stage, the macroglomerular complex can be seen to be NOS immunoreactive, although the intensity increases from the cumulus, to toroid 1, to toroid 2 (Homberg et al., 1995), the last showing an intensity similar to that of the ordinary glomeruli.
NADPH-d staining was used as another way to localize NOS in the antennal lobe. NADPH-d, like NOS immunoreactivity, labeled the perineurial cells strongly at stage 5. However, it also stained some antennal lobe neuronal cell bodies and produced faint staining around the edges of the developing antennal lobe neuropil (Fig. 3B). There was no detectable staining of the antennal nerve. Thus, whereas both NOS immunocytochemistry and NADPH-d staining methods labeled the perineurial cells, only the NADPH-d method labeled antennal lobe neurons, whereas only the NOS antibody labeled the axons in antennal nerve. The difference between the two staining methods was even more striking at stage 18 (Fig. 3C,D). The two methods were most similar in the perineurial sheath, which was completely unlabeled using NOS immunocytochemistry and only weakly labeled with NADPH-d staining. Unlike NOS immunocytochemistry, however, NADPH-d failed to label ORN axons either in the antennal nerve or in the apical regions of the glomeruli. Instead, we saw strong staining of a few antennal lobe neuronal cell bodies and faint staining of the bases of the glomeruli.
In situ hybridization was used to investigate the localization of MsNOS mRNA. At stage 5, the NOS in situ probe labeled the perineurial cells strongly (Fig. 3E,F), whereas no other cell bodies in the antennal lobe were strongly positive for NOS mRNA. There were one or two cells that were very weakly positive, but these were difficult to discern from background. At stage 18, no NOS mRNA expression can be detected in antennal-lobe neurons (Nighorn et al., 1998). Overall, the pattern was much more consistent with the NOS immunocytochemical staining pattern than the NADPH-d staining pattern.
To summarize, all of the methods used, including Northern blot analyses, showed that NOS is expressed in a transient fashion in cells of the developing antennal lobes. NOS immunocytochemistry and in situ hybridization suggest that this localization is limited to the perineurium, whereas NADPH-d staining suggests strong labeling of antennal lobe neuronal cell bodies as well. In the antennal nerve, NOS immunocytochemistry showed strong labeling of the ORC axons, whereas NADPH-d staining was absent.
Because NADPH-d measures enzymatic activity and NOS immunocytochemistry measures only the presence of the protein, we used the NO-sensitive dye DAF-2 (Kojima et al., 1998) in dissected stage 5 antennae-plus-brain primary olfactory system preparations as another measure of NOS activity. In the antennal lobe, penetration problems prevented the use of this dye. In the antennae, however, the dye penetrated the tissue well and responded to the presence of NO. This method clearly showed the presence of NO in ORNs in the antennae (Fig. 4). We detected high levels of NO in the ORN axons and low levels of NO in the cell bodies and dendrites. This result, which suggests that NOS protein is present and can generate NO in the ORN axons, supports the immunocytochemical localization of NOS.
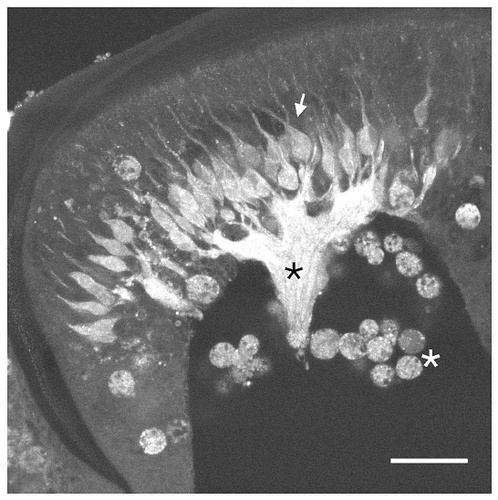
Localization of NO in the developing antenna. DAF-2 labeling of a sagittal section from stage 5 antennae showing strong staining of the antennal nerve rootlet (black asterisk) and olfactory receptor neuron (ORN) cell bodies (arrow). The small, round cells near the white asterisk are hemocytes. Scale bar = 50 μm.
MsGCα1 and MsGCβ1 are expressed in antennal lobe neurons
Because NOS is expressed transiently in the perineurium and continuously at afferent axon terminals, identification of target cells should help to identify NO-mediated developmental processes. Although NO can have many different targets, one of the best characterized is sGC, which mediates the formation of cGMP. Upon binding heme, the activity of the sGC heterodimer can be stimulated strongly by NO, resulting in an increase in cGMP. Therefore, the ability of NO to stimulate an increase in cGMP levels in the developing antennal lobe was examined by using cGMP immunocytochemistry.
At stage 5, cGMP was found to be elevated in a subset of lateral cell body cluster neurons; the number of stained cells was increased only slightly by incubation in SNP, an NO donor (not shown). Attempts to potentiate the response by incubating the brain in IBMX, which is a phosphodiesterase inhibitor, produced many stained cells in both the lateral and medial cell body packets, with no visible staining of antennal lobe glia (Fig. 5A). Incubation with SNP, however, did not increase these already high levels of cGMP (not shown). These results suggest either that there are other, NO-insensitive GCs mediating this effect or that there is endogenous stimulation with NO coupled with active phosphodiesterase activity. An NO-mediated increase in cGMP in developing antennal lobe neurons in response to dissection or stimulation of the antennae has been reported recently in some lateral cell body cluster neurons (Schachtner et al., 1998, Schachtner et al., 1999). Although labeling of the lateral cell body neurons was extensive and consistent at stage 5, we found extensive labeling of the medial cell body neurons ≈50% of the time. This result most likely reflects small variations in the extent of development, because stages earlier than midstage 5 did not display medial cell body neuron staining (not shown). At stage 7, the cGMP staining pattern was very similar, with strong, consistent staining of both the medial cell body cluster and the lateral cell body cluster. At this later developmental stage, processes from some of these cells could be seen entering developing glomeruli (Fig. 5B). Again, however, this staining could not be potentiated significantly by incubation in SNP.
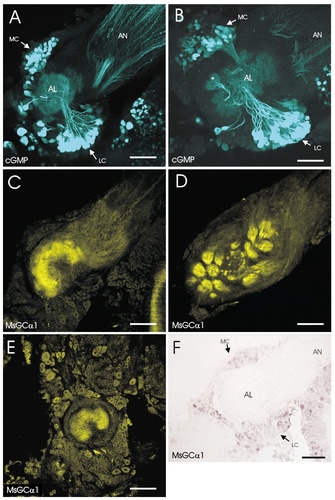
Localization of MsGC in the developing antennal lobe. In A, B, and F, the antennal lobe (AL), antennal nerve (AN), medial cell body cluster (MC), and lateral cell body cluster (LC) are labeled. A: Cyclic guanosine monophosphate (cGMP) immunocytochemistry of stage 5 antennal lobe in the presence of sodium nitroprusside (SNP) and 3-isobutyl-1-methylxannthine (IBMX). Note the strong staining of cell bodies in both the medial and lateral cell body clusters. Weak staining of some mechanosensory axons in the antennal nerve can also be seen. B: cGMP immunocytochemistry of stage 7 antennal lobe in the presence of SNP and IBMX. Note the strong staining of a large subset of cell bodies in both the lateral and the medial cell body clusters. Weak staining of axons in the antennal nerve can be seen. Staining of processes in developing glomeruli (asterisk) also can be seen. C: MsGCα1 immunocytochemistry of stage 5 antennal lobe. Orientation is identical to that in A. Note that only the antennal lobe neuron dendrites are stained. Nascent glomeruli can be seen around the periphery of the neuropil. D: MsGCα1 immunocytochemistry of stage 7 antennal lobe. A number of glomeruli, including the macroglomerular complex (MGC), are clearly visible at this stage. E: MsGCα1 immunocytochemistry of stage 3 antennal lobe. At this stage, many cell bodies are stained in addition to the central coarse neuropil. F: In situ hybridization of stage 5 antennal lobe with MsGCα1 probe. Note the staining of a subset of neurons in both the medial and the lateral cell body clusters. Scale bars = 100 μm.
Because of the possibility that high levels of endogenous NO stimulation of sGC may obscure the interpretation of the exogenous NO experiments, and because NOS is present in both afferent axon terminals and perineurial cells, the results described above do not provide sufficient information to localize potentially important NO-sGC interactions. In addition, in the presence of IBMX, cGMP in stimulated neurons accumulates and diffuses throughout the length of the neuron, obscuring the location of the initial interaction of NO with sGC. To help localize these interactions, we generated a polyclonal antiserum directed against a unique region of MsGCα1. By using this antiserum at midstage 5, clear staining of antennal lobe neuron dendrites is evident (Fig. 5C), although no cell bodies or output tracts are stained. Because lateral cell body neurons do not extend dendrites into protoglomeruli at this stage, the staining of protoglomeruli indicates that medial cell body projection neurons are MsGCα1-positive. By stage 7, staining clearly shows individual glomeruli, including the macroglomerular complex (Fig. 5D). This pattern is present as early as stage 3 (Fig. 5E), although, at the earlier stages, cell body staining also is apparent. Comparison with earlier neuroanatomic studies (Oland et al., 1990; Malun et al., 1994) suggests that the MsGCα1-positive dendrites belong to cells from both groups of neurons (Lynne Oland, personal communication). The localization of MsGCα1 to the dendrites of antennal lobe neurons positions it favorably for interactions with NO produced by the sensory axon terminals.
The presence of NO-sensitive sGC in the developing antennal lobe also was investigated by using in situ hybridization to localize both the α and β subunits. Subsets of stage 5 antennal lobe neurons from both the lateral and medial cell body packets were found to be positive for MsGCα1 message (Fig. 5F). The pattern was similar for MsGCβ1 (not shown), although fewer cells were detectably positive for the MsGCβ1 message. This localization pattern supports the idea that NO can interact with sGC expressed in both populations of antennal lobe neurons.
NOS and sGC also are expressed in the antennae
The expression of NOS in ORN axons led us to investigate the localization of NOS and sGC in the antenna itself by using NOS immunocytochemistry and NO-stimulated cGMP immunocytochemistry. At stage 5, NOS immunoreactivity was present in a subset of ORN axons all along the length of the antennal nerve (Fig. 6A,B) and in rootlets from each antennal segment as they joined the nerve. By comparing the fillet (Fig. 6B) with a cross section (Fig. 6C), it becomes clear that the subset of antennal nerve axons that are NOS-positive are those that have been added by the joining of nerve rootlets. In addition, a double label of NOS and propidium iodide (to label glial cell nuclei) shows that only the subset of axons in contact with glial cells is NOS-immunoreactive (Fig. 6D). Thus, there is a correlation between NOS-positive axons and migrating glial cells. Glial cells have been shown recently to migrate down antennal nerve rootlets to the nerves during pupal stages 3 and 4, although the cause of their migration is not known (Rössler et al., 1999). Finally, large numbers of NOS-positive cells also can be seen in the nonolfactory epithelium (scalar side of the antenna) at stage 5 (Fig. 6C). The significance of this staining is unknown.
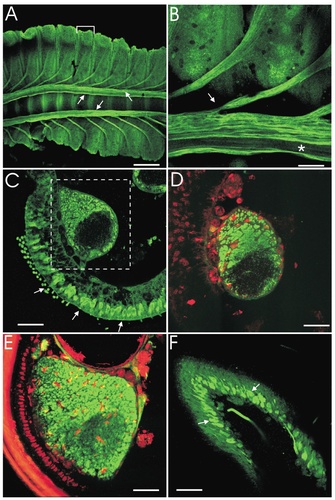
Localization of MsNOS in the developing antenna. NOS immunoreactivity is shown in green, and propidium iodide labeling is shown in red. A: Fillet of stage 5 antenna showing NOS immunoreactivity in the antennal nerve (arrows). The bracket outlines the extent of one antennal segment. B: Magnification of the fillet from A. NOS-immunoreactive axons of the rootlet (arrow) can be seen entering the antennal nerve. Note the portion of the antennal nerve that is lacking NOS immunoreactivity (asterisk). C: Cross section of stage 5 antenna showing NOS immunoreactivity in a subset of the antennal nerve (dashed box) and in the cell bodies on the scale side of the antennae (arrows). D: Cross section of stage 5 antennal nerve. The ORN axons are labeled with the uNOS antibody (green), and the glial cell nuclei are labeled with propidium iodide (red). Note the lack of glial cells in the NOS-negative portion of the antennal nerve. E: Cross section of stage 7 antennal nerve double labeled with the uNOS antibody and propidium iodide. Note that the central core of the nerve, which previously was negative for NOS, is becoming NOS positive while simultaneously showing the presence of glia. F: Cross section through the sensillar portion of a stage 7 antenna showing NOS immunoreactivity in cell bodies (arrows). Scale bars = 500 μm in A; 100 μm in B; 50 μm in C–F.
By stage 7, a cross section of the antennal nerve stained with propidium iodide and the uNOS antibody reveals that both glial cells and NOS immunoreactivity invest the full width of the antennal nerve (Fig. 6E). At this stage, there also is strong staining of cell bodies throughout the olfactory sensillar portion of the epithelium (Fig. 6F). This portion of the epithelium contains the cell bodies of the ORNs and related support cells. The staining of the cell bodies in the nonolfactory epithelium is now lost (Fig. 6E). By stage 18, none of the cell bodies in the epithelium is NOS immunoreactive (not shown), although the antennal nerve remains strongly NOS positive (Fig. 3C). Thus, NOS is present in ORN axons throughout development, but it is present in ORN cell bodies only transiently, indicating that there is developmental regulation of NOS localization within these cells, as has been demonstrated in vertebrates (Matsumoto et al., 1993a).
The expression of NOS by different cell types in the antennae raises the possibility that NO affects antennal cells through stimulation of sGC. This possibility was investigated by using NO-stimulated cGMP immunocytochemistry. In stage 5 antennae, a small number of cells, including six or seven large neurons in each antennal segment, stained intensely in the presence of SNP and IBMX (Fig. 7A,D,F). These neurons are in a location similar to, and are likely to be, the receptor neurons of the mechanosensory sensilla chaetica (Lee and Strausfeld, 1990). These neurons do not stain for cGMP when the antennae are treated with IBMX only (Fig. 7G) and, thus, appear to contain NO-sensitive sGC. The other cGMP-positive cells stain equally well in the presence or absence of SNP (Fig. 7D,G). These cells, which include the ORNs, either must contain other types of NO-insensitive GCs or must have been stimulated maximally by endogenous NO. Also identified was a new class of neurons with cell bodies that reside in the lumen of the antenna and with processes that extend within the antennal nerve (Fig 7A–C,E). These cells are likely to be the same as the cGMP-positive luminal cells seen in the adult (Monika Stengl, personal communication). It is noteworthy that, when we examined cGMP staining at stages 6-7 in the presence of IBMX and SNP, we found strong labeling of the ORNs in addition to the cells that were labeled at stage 5 (Fig. 7H,I). This staining, unlike the faint staining seen at stage 5, is dependent on SNP addition. It is intriguing that MsNOS also is expressed highly in ORN cell bodies at this stage (Fig. 6F). The relation between the MsNOS expression and the increase in cGMP levels will have to be investigated further with the use of NOS and sGC inhibitors.
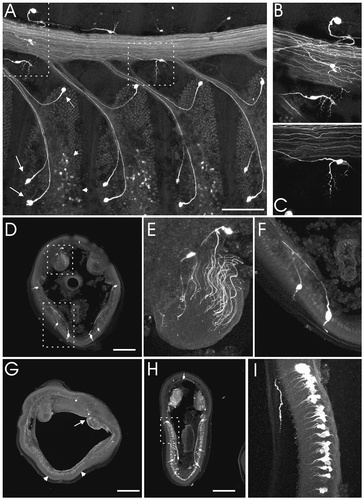
Localization of cGMP in the developing antennae (cGMP immunoreactivity is shown in white). A: Fillet of a stage 5 antennae treated with SNP and IBMX. One half of the fillet is shown, demonstrating the segmental arrangement of the cGMP staining. Four antennal segments are shown: Each is defined by the bundle of chemosensory axons joining the antennal nerve. Within each segment, there are three classes of cells stained; the mechanosensory cells (examples are denoted by arrows), the ORNs and supporting cells (examples are denoted by arrowheads), and the antennal nerve associated luminal cells (examples are outlined by dashed rectangles). B: Close-up view of two lumenal cells (behind the antennal nerve and below it) and a presumptive sensilla chaetica from the scale side of the antenna (cell and process above the antennal nerve). C: Close-up view of the lumenal cell outlined in A. D: Cross section of stage 5 antenna treated with IBMX and SNP. Note the staining of the six sensilla chaetica (large cells) and the cell bodies and axons in and around the antennal nerve. E: Close-up view of the antennal nerve and two lumenal cells outlined by the small rectangle in D. Note the axons traveling through the breadth of the nerve. F: Close-up view of the two mechanosensory cells outlined by the large rectangle in D. Note the strong staining of both the axons and the dendrites of these cells. Faint staining of ORNs and/or support cells can be seen between the two brightly staining mechanosensory cells. G: Cross section of stage 5 antenna treated with IBMX only. Note the staining of the cell bodies and axons of the lumenal cells (arrow) and the faint staining of the ORN cell bodies (arrowheads). H: Cross section of a stage 6 antenna treated with SNP and IBMX. Note strong staining of the mechanosensory cell axons, somata, and dendrites as well as luminal cell and ORN staining. I: Close-up view of the ORNs from the area outlined by the dashed rectangle in H. Note the relatively strong staining of these cells compared with F. Scale bars = 200 μm.
DISCUSSION
The ability of NO to cross cell membranes and interact with targets in neighboring cells independent of synaptic machinery has made it a molecular candidate in many different processes in which coordinated communication between cells is important. These types of interactions are especially important in the developing brain, in which intercellular communication is a vital part of the cascade of events necessary to form a functional unit out of hundreds of individual neurons. In this study, the role of NO in mediating developmental events in the olfactory system of Manduca was examined by localizing the distribution of NOS, the enzyme that generates NO, and sGC, an enzyme that is a well-characterized target of NO. Particular attention was given to pupal stages 5 and 7, because they account for much of the period during which interactions between incoming sensory afferents, antennal lobe glial cells, and antennal lobe neurons are critical to the formation of the glomerular organization of the neuropil (Oland and Tolbert, 1996).
NADPH-d staining is a poor measure of NOS expression
Much of the work investigating NOS expression in insects has focused on adults and has relied on NADPH-d staining as an indirect indicator of NOS activity (Müller, 1994; Müller and Bicker, 1994; Elphick et al., 1995; Müller and Hildebrandt, 1995; Bicker et al., 1996). Although attempts were made to show that biochemical NOS activity was present in tissues that exhibited NADPH-d, the two activities could not be colocalized at the cellular level in most of these studies. Reliance on this technique seemed justified, however, given the frequent convergence of NADPH-d staining and NOS protein expression in vertebrates. Here, too, there has long been controversy (Matsumoto et al., 1993b; Buwalda et al., 1995; Spessert and Claassen, 1998). Recently, Ott and Burrows (1999) addressed this problem in insect histochemistry, arguing that NADPH-d activity actually can arise from overfixation of tissue.
The current results show clearly that NADPH-d staining is a poor measure of NOS expression in the developing Manduca olfactory system. Because NADPH-d is believed to measure the ability of an aldehyde-fixed enzyme to reduce NADPH, NOS should account for at least a portion of NADPH-d activity. In this system, however, NOS staining and NADPH-d staining appear to be independent. Although both methods stained the perineurial sheath cells, the antennal lobe neurons were positive for NADPH-d but negative for NOS immunoreactivity. This may be explained by the idea that NOS is only a subset of NADPH-d activity. The antennal nerve, however, stained strongly with the NOS antibody yet was negative for NADPH-d activity. Moreover, the presence of NOS in the antennal nerve was confirmed by the DAF-2 staining. In situ hybridization results at stage 5, combined with the Northern blot analyses for antennal lobes throughout development, support the conclusion that NOS is expressed only in perineurial cells in the antennal lobe. Thus, we conclude that NADPH-d staining does not reflect accurately the distribution of NOS in this system and that NADPH-d should be used with great caution as a quick measure of NOS in insects.
Developmental role of NOS
The current study demonstrates that NOS is expressed in ORN axons and in the perineurial cells. The NOS in the perineurium is regulated developmentally and is present in these cells around the entire brain. The timing of its decline at pupal stage 7 is intriguing, because that is a time at which levels of the steroid hormone 20-OH ecdysone peak (Weeks and Truman, 1986), and it suggests that this phenomenon may be regulated hormonally. The developmental significance of this expression, however, is unknown. Although perineurial NO production may act to maintain sGC-positive antennal lobe neurons in a developmentally necessary state until stage 7, the localization of the MsGCα1 protein in neuron dendrites suggests that they respond primarily to NO released from axon terminals. It is possible that the presence of migrating glia in the perineurium (Rössler et al., 1999) is an important factor, as it appears to be in the antennal nerve (Fig. 6D,E).
The NOS that is expressed in the axon terminals of the ORNs is expressed in an ideal location to mediate developmental events in the antennal lobe. At the beginning of stage 5, ORN axon terminals form protoglomeruli, which are entered almost immediately by antennal lobe projection neuron processes (Oland et al., 1990; Malun et al., 1994). Glial cells then proliferate and migrate to surround the protoglomeruli, after which antennal lobe interneurons extend processes into the protoglomeruli at stage 6. Stage 6 sees the stabilization of the glomeruli and the beginning of a wave of synaptogenesis (Oland et al., 1990). NOS is present in ORN axon terminals at all of these stages and, thus, may be involved in any or all of these events. Initiation and cessation of antennal lobe neuron dendrite outgrowth, stimulation of glial cell migration, and stimulation of synaptogenesis are all processes with counterparts in vertebrates that have been shown to be modulated by NO (Hess et al., 1993; Duman et al, 1993; Meffert et al., 1994; Meulemans et al., 1995; Rentería and Constantine-Paton, 1996; Hindley et al., 1997).
Developmental role of NO-stimulated sGC
One likely target of NO in axon terminals is sGC. The current study demonstrated by using multiple techniques that MsGC is present in neurons of both the lateral and medial cell bodies during development. Immunocytochemistry demonstrates that MsGCα1 is present in the processes of both lateral and medial cell body neurons as early as stage 3 and clearly is present at stages 5 and 7. However, the presence of MsGCα1 may be necessary but insufficient to confer NO responsiveness to a particular neuron. At stage 5, for example, we find that medial cell body neurons are cGMP positive in the presence of exogenous NO plus IBMX in only about 50% of the preparations, whereas neurons from both cell body clusters appear to be positive for MsGCα1. This suggests that the sensitivity to NO may be regulated developmentally, because these cells are cGMP positive in the presence of NO/IBMX at all of the later stages examined. A developmentally regulated sensitivity to stimulation by NO has been shown in locust motor neurons (Truman et al., 1996). More work will be needed to understand the mechanisms by which the sensitivity of a neuron to NO may be regulated in this system. It is important to note that our results differ from those reported by Schachtner et al. (1998, Schachtner et al. 1999), who found only lateral cell body neurons stained after phosphodiesterase inhibitor treatment. Because the Schachtner et al. studies relied solely on cGMP immunocytochemistry, their results may reflect a threshold effect of the sensitivity of the antibody, they may reflect the influence of as yet unidentified environmental factors, or they simply may be the result of a difference in the effectiveness of the phosphodiesterase inhibitor treatment used in the two different sets of experiments.
In our experiments, the inability of SNP to potentiate the cGMP staining in the presence of IBMX implies either that NO-insensitive GCs are mediating the increased cGMP levels in these cells or that endogenous NO production already stimulates sGC to maximal detectable levels. The current results cannot rule out the possible contribution of active, NO-insensitive GCs. Six NO-insensitive GCs are expressed in the nervous system of Manduca sexta (A.N., unpublished data), although their expression and activity during development have not yet been characterized.
Although the relative contributions of sGC and NO-insensitive GCs are unknown, the current results show clearly that sGC is present in antennal lobe neurons and is in a location that is optimal for stimulation by NO released by ORC axons. Sensory axons and antennal lobe uniglomerular projection neurons are known to have little direct synaptic contact (Christensen et al., 1993; Malun et al., 1994); the NO/sGC pathway may permit developmentally important, direct signaling between these cell types to stabilize neurite arbors while awaiting synaptogenesis, which commences with the arrival of local interneuron dendrites (Oland et al., 1990). The NO/sGC pathway also may be important for the initiation of synaptogenesis in the antennal lobe, as has been suggested for motor neurons in the embryonic development of Locusta migratoria (Truman et al., 1996; Ball and Truman, 1998).
NO/sGC signaling also may have a role in the development of the antenna. At stage 5, and at every stage examined including the adult (M. Stengl, personal communication), an NO-sensitive GC is present in the mechanosensory cell bodies and axons (Fig. 7A,D,F,H,I). NO may be produced by the ORN axons and interact with the sGC in these cells. The increase in cGMP staining in ORN cell bodies at stage 7 is intriguing, because synaptogenesis occurs in the antennal lobe at stage 7, the same stage at which ecdysteroid levels are at their highest. This may represent a hormonally regulated signal to alter gene expression to allow for increased synapse formation or to begin the process of maturation of the peripheral components of the signal-transduction cascade in the ORNs. The NO-dependence of this effect and its developmental consequences need to be examined further.
The upstream and downstream events surrounding a possible NOS/sGC signaling mechanism have yet to be determined in the antennae and antennal lobe of Manduca. Previous work has shown that MsNOS is likely to be sensitive to activation by Ca2+/calmodulin (Nighorn et al., 1998). Visualization of the Ca2+ currents present in developing ORNs may yield important insights into the regulation of the production of NO in this system. Cyclic nucleotide-gated channels are possible downstream targets of cGMP. Although such channels have not yet been identified in Manduca sexta, they are known to be present in the olfactory bulb of vertebrates (Kingston et al., 1999) and in the antennae of Drosophila (Baumann et al., 1994), and they may be well placed to modulate the electrical properties of developing neurons in response to changes in cGMP levels. The effect of increased cGMP also may be mediated by the activation of the cGMP-dependent protein kinase. Finally, in the antennal nerve and its rootlets, the correlation between the presence of migrating glial cells and NOS immunoreactivity of ORN axons raises interesting questions of cause and effect that will be pursued in future work.
Developmental role of non-sGC-mediated effects of NO
The NO-sensitive sGC almost certainly is not the only target of the NO produced in the developing olfactory system, especially given the relatively restricted expression of sGC. NO is known to activate directly cyclic nucleotide-gated channels (Broillet and Firestein, 1996) and thereby may cause the release of neurotransmitters from adjacent axon terminals, mimicking an odorant-induced activity that would not occur naturally until approximately stage 16 (Schweitzer et al., 1976). Furthermore, if it is acting on the channels of antennal lobe neurons and glia, NO could cause activity-dependent changes by promoting Ca2+ influx (Kossel et al., 1997). NO also has been shown to affect neurite outgrowth, cell migration, synaptogenesis, and synaptic activity by altering acylation, ADP-ribosylation, and nitrosylation of critical proteins (Hess et al., 1993, Hess et al., 1999; Meffert et al., 1994; Schuman et al., 1994; Stamler et al, 1997).
The presence of NOS clearly is not equivalent to the generation of NO, because a number of conditions, particularly high intracellular calcium levels, must be met to activate this enzyme in Manduca (Nighorn et al., 1998). Future work will focus on determining when and where NO actually is generated during development as well as the signaling pathways by which it achieves its actions on a given cell type. This will include investigating enzymes downstream of cGMP, such as protein kinase G and cyclic nucleotide gated channels, as well as the sGC-independent pathways mentioned above. Preliminary experiments with the NOS inhibitor L-NAME during metamorphosis have revealed pronounced effects on glial migration and neurite outgrowth in the antennal lobe (Gibson et al., 1999).
In summary, MsNOS has been shown to be present in or near the antennal lobe at times and at places that would allow it to influence antennal lobe development. Specifically, MsNOS is present at the terminals of ingrowing ORN axons as they innervate the developing antennal lobes, and it also is expressed highly by perineurial cells until stage 7. One possible target of NO generated in the antennal lobe is the NO-sensitive MsGC expressed in the dendrites of both medial and lateral cell body cluster neurons.
Acknowledgements
The authors thank Drs. Leslie Tolbert, Lynne Oland, and John Hildebrand for numerous helpful discussions and insights. They also thank Patricia Jansma and Dr. Norm Davis for sharing their knowledge of immunocytochemistry and confocal microscopy. This work was supported by National Institutes of Health grant P01-NS28495 (Project 5) to J.G.H. and by National Science Foundation grant IBN9604536 to A.N.




