μ-opioid receptors are present in vagal afferents and their dendritic targets in the medial nucleus tractus solitarius
Abstract
Ligands of the μ-opiate receptor (MOR) are known to influence many functions that involve vagal afferent input to the nucleus tractus solitarius (NTS), including cardiopulmonary responses, gastrointestinal activity, and cortical arousal. The current study sought to determine whether a cellular substrate exists for direct modulation of vagal afferents and/or their neuronal targets in the NTS by ligands of the MOR. Anterograde tracing of vagal afferents arising from the nodose ganglion was achieved with biotinylated dextran amine (BDA), and the MOR was detected by using antipeptide MOR antiserum. The medial subdivision of the intermediate NTS was examined by electron microscopy for the presence of peroxidase-labeled, BDA-containing vagal afferents and immunogold MOR labeling. MOR was present in both presynaptic axon terminals and at postsynaptic sites, primarily dendrites. In dendrites, MOR immunogold particles usually were located along extrasynaptic portions of the plasma membrane. Of 173 observed BDA-labeled vagal afferent axon terminals, 33% contained immunogold labeling for MOR within the axon terminal. Many of these BDA-labeled terminals formed asymmetric, excitatory-type synapses with dendrites, some of which contained MOR immunogold labeling. MORs were present in 19% of the dendrites contacted by BDA-labeled terminals but were present rarely in both the vagal afferent and its dendritic target. Together, these results suggest that MOR ligands modulate either the presynaptic release from or the postsynaptic responses to largely separate populations of vagal afferents in the intermediate NTS. These results provide a cellular substrate for direct actions of MOR ligands on primary visceral afferents and their second-order neuronal targets in NTS. J. Comp. Neurol. 422:181–190, 2000. © 2000 Wiley-Liss, Inc.
The vagus nerve contains visceral afferents from the heart, lungs, and abdominal viscera that terminate in the nucleus of the solitary tract (NTS; Kalia and Mesulam, 1980a,b; Kalia and Sullivan, 1982; Lawrence and Jarrott, 1996). Activation of vagal afferents can influence numerous physiologic responses, including cardiopulmonary reflexes, respiration, swallowing, gastrointestinal motility, and feeding (Paintal, 1973; Jänig, 1996), and also has been shown to produce antinociception and sleep (Lewis et al., 1987; Randich et al., 1992; Reinoso-Barbero and De Andrés, 1995). Opioid ligands acting at the μ-opioid receptor (MOR) modulate many of these responses (Hassen et al., 1982; Willette and Sapru, 1982; Ruegg et al., 1994).
MORs also have been implicated in both acute and chronic changes in cardiovascular function, respiratory depression, gastrointestinal motility, and decreased arousal to exogenously administered opioids (Lawrence and Jarrott, 1996). Chronic opioid administration alters vagal reflexes in the intact rat (Napier et al., 1998) and also reduces the responses to both opioid and nonopioid ligands in the NTS slice (Malanga et al., 1997). Many exogenously applied MOR ligands, such as morphine, can cross the blood-brain barrier and potentially have access to both the presynaptic and the postsynaptic receptive sites on vagal afferents and their NTS targets (Hassen et al., 1982; Oley et al., 1982; Willette and Sapru, 1982; Sessle and Henry, 1985; Feldman et al., 1996).
In the NTS slice preparation, MOR ligands have been shown to directly depolarize cells, presumably by acting at a postsynaptic site (Rhim et al., 1993). However, MOR ligands also reduced spontaneous excitatory postsynaptic potentials as well as excitatory responses evoked by stimulation of the solitary tract in the slice preparation (Rhim et al., 1993). In other experiments, MOR ligands produced disinhibition of some NTS neurons through a γ-aminobutyric acidergic (GABAergic) mechanism (Malanga et al., 1997). Thus, MOR ligands influence both presynaptic and postsynaptic responses in the NTS, but the specific receptor sites of action for the production of these responses to opioids are not known.
The nodose ganglion, which contains the cell bodies of vagal afferents, has MOR binding sites (Atweh et al., 1978; Ding et al., 1998) as well as extremely high levels of MOR messenger RNA and protein (Nomura et al., 1996; Búzás and Cox, 1997). These receptors may be transported to presynaptic sites in the NTS where they may alter vagal afferent function. Within the vagus, there is evidence of axonal transport of MORs along the vagus nerve both toward the brain and toward the periphery (Li et al., 1996). Because vagotomy can reduce MOR binding and receptor density in the NTS (Dashwood et al., 1988; Lawrence and Jarrott, 1996; Nomura et al., 1996), it has been suggested that many of the MOR-containing axon terminals in the NTS are likely to be vagal afferents. MORs have been demonstrated in the NTS with immunocytochemical localization of sequence-specific antipeptide antisera (Mansour et al., 1995; Cheng et al., 1996a,b; Nomura et al., 1996), and the NTS also contains mRNA for the MOR (Mansour et al., 1994). Ultrastructurally, MORs are present in both presynaptic and postsynaptic neuronal profiles in the intermediate NTS at the level of the area postrema (Cheng et al., 1996a). In this region, MORs often are seen in axon terminals resembling vagal afferents (Cheng et al., 1996a). Thus, we sought to determine whether MORs in the NTS are located on vagal afferents and/or their neuronal targets. We combined immunogold detection of MOR with peroxidase detection of anterogradely transported biotinylated dextran amine (BDA) after injection of this tracer into the nodose ganglion. Our results show presynaptic and postsynaptic distributions of MOR in subsets of neurons in the region of the NTS subserving primarily cardiopulmonary functions (Ciriello, 1983; Loewy, 1990).
MATERIALS AND METHODS
Injections
Male Sprague-Dawley rats (weight, 300–400 g; n = 8 rats) were anesthetized with chloral hydrate (450 mg/kg, i.p.), and the left nodose ganglion was exposed and isolated from surrounding tissues. A small piece of Parafilm was placed under the ganglion and the cervical vagus to prevent leakage of the tracer into surrounding tissues. A glass micropipette (20–40 μm tip diameter) containing the anterograde tracer BDA [10% in 0.1 M phosphate buffer, pH 7.4 (PB); Molecular Probes, Eugene, OR] was inserted under the sheath of the left cervical vagus and into the center of the nodose ganglion. The tracer was pressure-injected (Picospritzer; General Valve, Fairfield, NJ) directly into three or more sites within the ganglion (total volume, 1–2 μl) to allow maximal filling of the entire structure. Diffusion of BDA was visualized by a small amount of Fast Green dye (<1%) that was added to the solution. After the final injection, the pipette was removed, and the surrounding area was rinsed with warm saline. The incision was sutured, and the animal was monitored during recovery from anesthesia and then returned to the animal colony in an individual cage. The procedures used in these studies were approved by the Institutional Animal Care and Use Committee of Weill Medical College. All efforts were made to reduce animal suffering and to use the minimum number of animals needed to make sound scientific conclusions.
Perfusion and tissue processing
Ten days after injection of BDA into the nodose ganglion, rats were deeply anesthetized with sodium pentobarbital (150 mg/kg, i.p.) and perfused through the ascending aorta with 3.8% acrolein in 2% paraformaldehyde (50 ml) followed by 2% paraformaldehyde (in 0.1 M PB; 200 ml). The medulla oblongata was removed, placed in 2% paraformaldehyde for 30 minutes, then sectioned (40 μm) on a vibrating microtome (Leica, Rockleigh, NJ), and collected into 0.1 M PB. Anterogradely transported BDA was visualized by incubating sections in avidin-biotin complex (ABC) solution (Elite Kit; Vector Laboratories, Burlingame, CA) for 2 hours at room temperature prior to the 3,3′-diaminobenzidine tetrahydrochloride (DAB)-peroxide reaction (Aicher et al., 1996). Tissue sections were then processed for immunogold-silver localization of MOR (Chan et al., 1990). A few adjacent sections were processed for only BDA or for immunoperoxidase localization of MOR and were mounted onto gelatin-coated glass slides for light microscopic examination. The tissue was dehydrated through a series of ethanols and coverslipped with DPX.
Antisera.
A rabbit antipeptide antibody recognizing MOR was used for these studies. This antibody has been characterized in previous studies, and its distribution is similar to that of other antibodies to the MOR (Chen et al., 1996; Cheng et al., 1996a,b). The rabbit polyclonal antiserum (a gift from Dr. Liu-Chen, Temple University School of Medicine) was raised against a synthetic peptide with the sequence CTNHQLENLEAETAPLP, which corresponds to the last 16 amino acids of the C-terminal domain of the μ opioid receptor with an added cysteine residue for conjugation to keyhole limpet hemocyanin or bovine serum albumin (BSA). In an immunoblot analysis, this antiserum did not cross react with several other MOR peptides or peptides of the cloned δ or κ opioid receptors (Cheng et al., 1996b). In addition, preabsorption of the antiserum with the cognate peptide abolished immunoreactivity (Cheng et al., 1996b). The MOR antibody was used at a dilution of 1:5,000 for immunogold-silver labeling (Chan et al., 1990). The gold-conjugated anti-rabbit immunoglobulin G (Amersham, Arlington Heights, IL) secondary antibody was raised in goats and was used at a dilution of 1:50.
Dual labeling for BDA and MOR.
Details of the methodsfor combined localization of BDA peroxidase labeling and immunogold labeling have been published previously (Aicher et al., 1995, 1996, 1999). The tissue was cryoprotected in a solution containing 12.5% sucrose and 1% glycerol for 15 minutes and then was rapidly frozen in liquid nitrogen to increase membrane permeability prior to antibody incubations. Tissue sections were incubated in 1% sodium borohydride (30 minutes) to enhance antigenicity and then placed in ABC solution for 2 hours at room temperature. BDA was visualized by using the DAB-peroxidase method (Aicher et al., 1996). Tissue sections were then incubated for 30 minutes in 0.5% BSA in 0.1 M Tris-buffered saline, pH 7.6, to reduce nonspecific binding and were placed in the primary antibody solution (see above) for 48 hours at 4°C. The MOR antiserum was then visualized by using immunogold detection and silver intensification (IntensEM kit; Amersham) of the secondary antisera (Chan et al., 1990).
Electron microscopy
For electron microscopy, tissue was rinsed in PB, placed in 2.0% osmium tetroxide for 1 hour, dehydrated, and embedded in Epon 812 between two sheets of Aclar plastic. Ultrathin sections (50 nm) were collected through the intermediate NTS at the level of the area postrema ipsilateral to the injected nodose ganglion. These sections were mounted onto copper grids and counterstained with uranyl acetate and Reynold's lead citrate. Sections from the Epon/tissue interface that contained both BDA peroxidase and MOR immunogold labeling within the same field were viewed and photographed with a Philip's CM10 electron microscope.
Thin sections from the surface of 12 Vibratome sections from the 6 most successful cases (i.e., those with the greatest density of anterogradely labeled fibers and the best preservation of ultrastructural detail) were analyzed under the electron microscope (approximately 2.0 mm2 were examined). Labeled structures were classified as perikarya, dendrites, axons, axon terminals, or glia based on morphologic information available in the plane of section viewed (Peters et al., 1991). Axon terminals were defined by the presence of small clear vesicles (scvs). These terminals were characterized with regard to their size, content of dense core vesicles (dcvs), type of contact with neuronal targets (symmetric or asymmetric synapse or apposition; Peters et al., 1991), and type of target (perikarya, dendrite, axon terminal). Synapses were defined by parallel membranes separated by a widened cleft and membrane specializations. Asymmetric synapses were defined by a prominent density on the postsynaptic side of the contact, whereas symmetric synapses showed equivalent densities on either side of the contact (Peters et al., 1991). Appositions were defined as parallel membranes between two elements that lacked dense membrane specializations. Profiles smaller than 0.3 μm in minimum cross-sectional diameter that contained neurotubules and occasional vesicles but were devoid of myelination and lacked synaptic input were classified as unmyelinated axons. An additional criterion to distinguish unmyelinated axons from small dendrites was that they usually were found in bundles with other small axons. Profiles without synaptic vesicles that received synaptic input were classified as dendrites, and these were categorized as either small (<1.5 μm in diameter) or large (>1.5 μm in diameter and lacking a nucleus). Astrocytic processes were classified by their amorphous shape and lack of vesicles or synaptic contacts and by the occasional presence of glial microfilaments.
Immunogold-silver labeling of MOR was characterized as either plasmalemmal (within 20 nm of the plasma membrane) or cytoplasmic. The former would reflect potential functional sites accessible to extracellular ligands, whereas the latter may reflect internalized or newly synthesized receptor protein (Boudin et al., 1998). Plasmalemmal labeling was characterized further as either synaptic (in contact with or within 80 nm of the postsynaptic density) or extrasynaptic (>80 nm away from the synaptic density). Stray immunogold-silver particles were minimal in this study, and most gold particles were localized to the plasma membrane or membranous cytoplasmic organelles. In the current study, at least two particles were required for positive identification of immunogold-labeled profiles. Even the use of one gold particle as a criterion has been shown to be sufficiently stringent to yield a statistically consistent distribution of antigenic labeling among different classes of profiles as long as the gold particle is localized to the plasma membrane (Garzón et al., 1999). In addition, these labeling criteria for the immunogold method have been proven to yield distributions of receptors similar to those observed with the immunoperoxidase method when the same antibodies were used (Wang et al., 1999; Aicher et al., 2000; Huang et al., 2000). In the current study, by using the criterion of at least two gold particles for the identification of MOR-labeled profiles, we most likely have underestimated the number of small axons compared with larger dendrites and axon terminals.
Preparation of figures
The electron photomicrographs used for the figures were scanned from photomicrographs on a Power Macintosh 8500/150 Computer (Apple Computers, Inc., Cupertino, CA) with an AGFA Arcus II scanner (Agfa-Gevaert NV, Montsel, Belgium) using Fotolook (Agfa-Gevaert NV) and Adobe Photoshop (version 4.0; Adobe Systems Inc., Mountain View, CA) software. The photomicrographs were adjusted only for saturation levels, sharpness, and contrast. Composite illustrations were composed and labeled with QuarkXPress (version 3.32; Quark, Inc., Denver, CO) and Adobe Illustrator (version 6.0; Adobe Systems Inc.) software.
RESULTS
Intense anterograde labeling (but no retrograde labeling) was seen with light microscopy throughout all subnuclei of the NTS at the level of the area postrema after injections of BDA into the nodose ganglion (Fig. 1A). The BDA labeling was more dense in varicose, axonal-type processes on the side ipsilateral to the BDA injection, particularly in the area extending between the area postrema and the solitary tract. This was the region that was examined ultrastructurally. MOR-immunoperoxidase labeling showed an overlapping distribution with the anterogradely labeled axons in the medial NTS at the level of the area postrema (Fig. 1B). In this region, electron microscopy confirmed the presence of BDA exclusively in axons and axon terminals (Figs. 2, 4, 5). BDA-labeled axons (n = 123 axons) were primarily unmyelinated (94%) or occasionally myelinated (6%; Fig. 5A). Anterogradely labeled terminals (n = 173 terminals) contained many small clear vesicles (scvs) and a few dense core vesicles (dcvs; Figs. 2C, 4). Many of these vagal afferents (24% of axons and 33% of axon terminals) also contained immunogold labeling for MOR (Fig. 2; see below). BDA-labeled terminals apposed many other neuronal processes, including other axons and dendrites (Figs. 2, 4, 5), and formed either asymmetric synapses or appositions with dendrites, some of which also contain MOR (Fig. 5; see below).
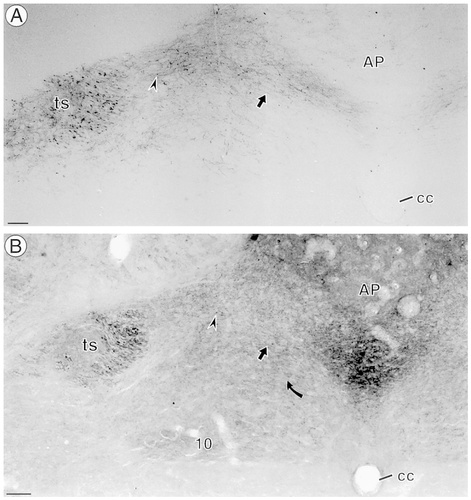
Biotinylated dextran amine (BDA) anterograde transport and μ-opioid receptor (MOR) immunoperoxidase-labeling are found within the medial nucleus tractus solitarius (NTS). A: BDA peroxidase labeling was seen in most nuclei of the NTS at the level of the area postrema (AP). Many labeled axons were seen within the solitary tract (ts), and labeled axons (arrow) and puncta (arrowhead) were seen throughout the NTS, especially on the left side, which was ipsilateral to the nodose injection. B: MOR-immunoperoxidase labeling was distributed into regions of the NTS and the dorsal motor nucleus of the vagus (10) but was virtually absent in regions ventral to the central canal (cc). MOR immunolabeling was seen the same regions as BDA transport and was found in profiles resembling axons (straight arrow) as well as in puncta (arrowhead). MOR immunolabeling also was seen in profiles resembling somata (curved arrow). Scale bars = 0.3 mm.
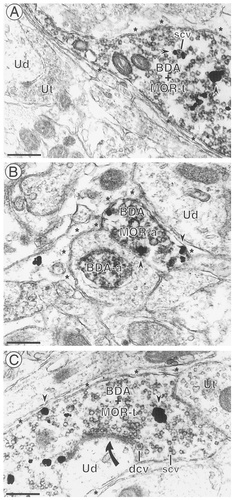
BDA-labeled axons and axon terminals often contained immunogold labeling for MOR. A: An axon terminal with immunoperoxidase labeling for BDA and immunogold labeling for MOR (arrowheads) (BDA + MOR-t) contains small clear vesicles (scv). The immunogold labeling is associated with vesicles in the varicosity. The labeled profile does not contact any other neurons in this field and is apposed to astrocytic glial processes (asterisks). Unlabeled axon terminals (Ut) and an unlabeled dendrite (Ud) are seen in this field. B: Both a single-labeled axon (BDA-a) and a dual-labeled axon (BDA + MOR-a) are seen in this photomicrograph. The immunogold particles in the dual-labeled axon are associated with regions of the plasma membrane (arrowheads) apposed to astrocytic glial processes (asterisks) as well as cytoplasmic organelles. An unlabeled dendrite (Ud) also is seen in this field. C: A dual-labeled axon terminal (BDA + MOR-t) contains both small clear vesicles (scv) and dense core vesicles (dcv). The terminal forms an asymmetric synapse (curved arrow) with an unlabeled dendrite (Ud) and is apposed to an unlabeled terminal (Ut). The remainder of the axon terminal and the dendrite are surrounded by astrocytic glial processes (asterisks). Scale bars = 0.50 μm.
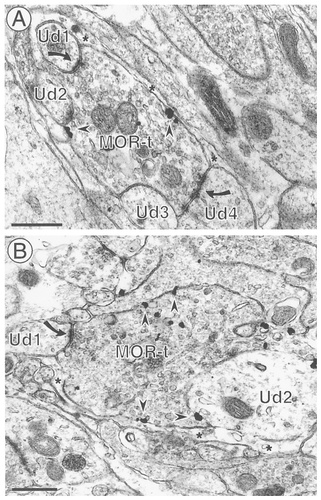
MOR-labeled axon terminals contact multiple targets. A: An axon terminal containing immunogold labeling for MOR (MOR-t) forms asymmetric synapses with several dendrites (Ud1, Ud2, and Ud4) and is apposed to another (Ud3) in this field. Immunogold particles are associated with intracellular organelles as well as nonsynaptic portions of the plasma membrane (arrowheads). Astrocytic glial processes (*) also cover much of the surface of this terminal. B: A large, immunogold-labeled axon terminal (MOR-t) contacts two unlabeled dendrites (Ud1 and Ud2), forming an asymmetric synapse (curved arrow) with Ud1. The immunogold particles are associated with vesicles as well as with nonsynaptic portions of the plasma membrane (arrowheads). Scale bars = 0.5 μm.
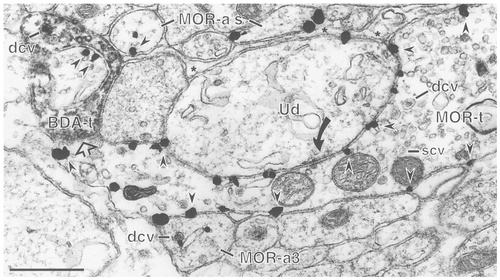
Axoaxonal appositions between BDA- and MOR-labeled profiles. An immunogold-labeled MOR terminal (MOR-t) forms an asymmetric synapse (solid curved arrow) with an unlabeled dendrite (Ud) and also is apposed (open straight arrow, left side) to a BDA-labeled axon terminal (BDA-t). The immunogold particles (arrowheads) in the MOR-labeled terminal are associated exclusively with the plasma membrane and are found on all of the surfaces viewed in this field. There are several immunogold-labeled axons (MOR-a) in this field, one of which (MOR-a3) is directly apposed to the MOR-t. MOR-a3 contains a dense core vesicle (dcv) that has an immunogold particle associated with it. There are astrocytic glial processes (asterisks) between the MOR-labeled axons at the top of the photomicrograph (MOR-a's) and the unlabeled dendrite (Ud). The BDA-labeled terminal (BDA-t, left side) also is apposed to an immunogold labeled profile (arrowheads) and contains dense core vesicles (dcv) along with scvs. Scale bar = 0.5 μm.
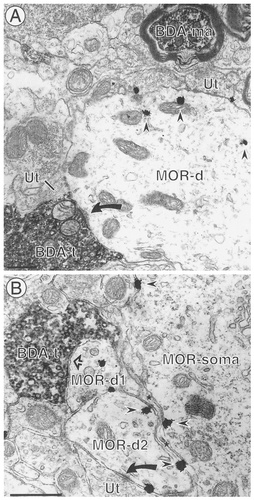
BDA-labeled terminals contact dendrites that contain MOR. A: An axon terminal containing BDA (BDA-t) forms an asymmetric synapse (arrow) with a dendrite containing gold-silver deposits for MOR (MOR-d). The dendrite also receives contacts from several unlabeled terminals (Ut). The immunogold particles (arrowheads) are associated primarily with intracellular membranous organelles. A BDA-labeled myelinated axon (BDA-ma) is seen at the top right. B: A BDA-labeled axon terminal (BDA-t) is apposed (open straight arrow) to an immunogold-labeled dendrite (MOR-d1), which is directly apposed to another immunogold-labeled dendrite (MOR-d2). MOR-d2 receives an asymmetric synapse (solid curved arrow) from an unlabeled terminal (Ut). The MOR-labeled dendrites are separated from an immunogold-labeled cell body (MOR-soma) by astrocytic glial processes (asterisks). Scale bars = 0.5 μm.
Localization of MOR in axons, including vagal afferents
Immunogold labeling for MOR often was seen in axons and axon terminals in the medial NTS (Table 1). In these axons, with or without BDA, MOR-immunogold labeling was seen at both cytoplasmic sites and membrane sites (Figs. 2, 3). Approximately half of the MOR-labeled axons and axon terminals had immunogold labeling that was associated exclusively with intracellular membranes, including vesicles (Fig. 2A), whereas the remainder had immunogold particles that also were associated with the plasma membrane (Figs. 2B,C, 3, 4). Most of these immunogold particles were associated with nonsynaptic portions of the plasma membrane, with apposing profiles that usually were either glia (Figs. 2C, 3) or dendrites (Fig. 4).
| Neuronal profiles | Glia (%) | No. | |||
|---|---|---|---|---|---|
| Somata (%) | Dendrites (%) | Axons (%)2 | Terminals (%)2 | ||
| 2 (1) | 98 (32) | 56 (18) | 133 (44) | 14 (5) | 303 |
- 1 Data were collected from six rats (two Vibratome sections per rat) from the intermediate nucleus tractus solitarius.
- 2 Includes 28 biotinylated dextran amine (BDA)-labeled axons and 55 BDA-labeled axon terminals.
In addition to colocalization in the same axon, we also found 19 examples of appositions between MOR-labeled axons or axon terminals and BDA-labeled axonal profiles (Fig. 4). The majority of the vagal afferents identified with BDA (n = 285 afferents), however, were apposed to unlabeled axons or axon terminals (n = 112 terminals; Figs. 2C, 5A).
MOR localization in dendrites, including targets of vagal afferents
MOR immunoreactivity also was present in many dendrites (Table 1), some of which were contacted by BDA-labeled vagal afferents; specifically, of the BDA-labeled terminals that contacted dendrites (n = 136), 26 targeted dendrites that contained immunogold labeling for MOR (19%; Fig. 5), whereas the remainder were unlabeled (Fig. 2C). Only 5 of the BDA-labeled terminals that contacted MOR-labeled dendrites also contained MOR-immunogold labeling within the axon terminal.
MOR immunogold particles were associated with the plasma membrane of many dendrites (62%), whereas the remaining dendrites contained gold particles that were associated exclusively with cytoplasmic membranous organelles (Fig. 5A). Plasmalemmal gold particles usually were found at nonsynaptic portions of the membrane in dendrites that were targets of BDA-labeled terminals as well as those that were contacted by unlabeled axon terminals (Fig. 5B). Specifically, only 3% of the MOR-labeled dendrites containing plasmalemmal gold particles had gold particles that were associated directly with a synaptic density, 27% of the dendrites had gold particles near a synaptic contact but not directly associated with the density, and 42% had gold particles exclusively at nonsynaptic portions of the dendritic membrane.
DISCUSSION
In the current study, we demonstrated that MOR receptors are present at potentially functional sites along plasma membranes of selective vagal afferent terminals and in dendritic targets of other vagal afferent terminals in cardiorespiratory portions of the rat medial NTS. These results are discussed in relation to methodological considerations and functional implications for the differential involvement of MORs in the presynaptic modulation versus the postsynaptic modulation of subsets of second-order sensory neurons in the medial NTS.
Methodological considerations
Antiserum.
Like all immunocytochemical studies, the current methods were limited by the specificity of the antibody to its designated antigen and by the sensitivity of the detection method. Although the antibody used in the current studies has been well characterized, it is possible that a portion of the labeling obtained with this antibody may reflect detection of similar or identical peptides in other proteins. Thus, for all data and discussions, the terms label or immunoreactivity infer antigen-like immunoreactivity. The MOR antibody used in the current study has been characterized fully, however, and is likely to represent MOR protein (Cheng et al., 1996b). This conclusion is supported by the consistency between immunocytochemical localization of MOR (Arvidsson et al., 1995; Mansour et al., 1995) and receptor autoradiography (LaMotte et al., 1976; Besse et al., 1990, 1992).
Anterograde transport.
The detection of vagal afferents was based on anterograde transport of BDA after unilateral injections into the nodose ganglion. BDA has been shown to provide excellent anterograde labeling of pathways throughout the nervous system (Veenman et al., 1992; Alisky and Tolbert, 1994; Aicher et al., 1995), and we showed previously that it also can be used to label afferents arising from peripheral ganglia (Aicher et al., 1999). The current results confirm and extend this observation. This method will label only the afferents of cells that are filled adequately with the tracer; therefore, the lack of labeling in a terminal cannot be used to conclude that the terminal is not a vagal afferent. Unlabeled terminals may arise from the contralateral nodose ganglion (Kalia and Mesulam, 1980a,b), from other primary afferents to the NTS (Contreras et al., 1982), or from central projections (Lowey, 1990; Silva-Carvalho et al., 1995; Pickel et al., 1996). BDA appears to identify a more diverse population of vagal afferent terminals than other anterograde tracers (Sumal et al., 1983; Velley et al., 1991) based on frequency of detection and the diversity in size of labeled terminals. However, the light microscopic distribution of vagal afferents was comparable to that described in prior studies (Kalia and Mesulam, 1980a,b; Kalia and Sullivan, 1982). In addition, the proportions of unmyelinated and myelinated axons was comparable to previous studies of the sensory component of the cat vagus nerve identified by electron microscopy and electrophysiology (Mei et al., 1980) and our own previous description (Aicher et al., 1999).
MOR in vagal afferents and apposed axons
The current identification of MOR in vagal afferents in the medial NTS suggests the involvement of MOR ligands in modulating the presynaptic release of glutamate, which is the primary transmitter in these afferents (Talman et al., 1980; Saha et al., 1995; Sykes et al., 1997). This finding supports other data indicating that MOR ligands act at presynaptic sites to block calcium channels in the NTS (Rhim and Miller, 1994) as well as on nodose ganglion cells (Rusin and Moises, 1998). We recently described the localization of the N-methyl-D-aspartate (NMDA)-type glutamate receptor relative to vagal afferents (Aicher et al., 1999) and found NMDA receptors located presynaptically on 40% of these afferents to the caudal NTS. The 33% localization of MOR in vagal afferents in the same region in the current study suggests that MOR and NMDA receptors often are colocalized in vagal afferents. Indeed, MOR and NMDA receptors often are colocalized in presynaptic profiles in this region of the NTS (Huang et al., 2000), as reported in other brain regions (Gracy et al., 1997; Commons et al., 1999).
In addition to containing MOR, BDA-labeled vagal afferents were apposed to a few MOR-immunoreactive and many unlabeled axons or axon terminals in the NTS. The frequent appositions between BDA-labeled vagal afferents and other axonal profiles confirms similar earlier observations (Sumal et al., 1983; Velley et al., 1991; Aicher et al., 1999). These axonal appositions largely may reflect transit in common axon bundles, but they also may play a role in the functional integration of mulitmodal sensory information in NTS neurons (Mifflin, 1993; Hines et al., 1994; Silva-Carvalho et al., 1998).
MOR distribution in vagal target dendrites
We observed MOR at extrasynaptic sites in many dendritic targets of BDA-labeled vagal afferents. Our findings are consistent with electrophysiologic studies in the NTS slice preparation, suggesting that MOR ligands can act at postsynaptic sites at which they have been shown to inhibit neurons through potassium channels (Rhim et al., 1993). The extrasynaptic distribution of MOR in dendrites is consistent with that shown previously in the NTS (Cheng et al., 1996a) and in other brain regions (Wang et al., 1997; Wang and Pickel, 1998) and supports a modulatory role for MOR in the postsynaptic responses to excitatory vagal afferents. Extrasynaptic receptors for peptides, such as the opioids, are consistent with the release of the peptide from dense core vesicles in axon terminals, and these vesicles are not associated preferentially with synaptic contacts (Pierce et al., 1999). Therefore, we do not necessarily expect the localization of MOR to synaptic sites that has been reported for glutamatergic and GABAergic receptors (Craig et al., 1994).
Functional implications
The current results suggest that MOR ligands are involved in both presynaptic and postsynaptic modulation of vagal input within the NTS. However, presynaptic and postsynaptic modulation of vagal afferents and their targets in the NTS by MOR ligands largely are separate mechanisms that involve distinct populations of vagal afferents, because MORs rarely were present at both the presynaptic site and the postsynaptic site of a single synaptic contact.
Although the type of sensory stimuli encoded by the afferents labeled in the current study was not determined (Paintal, 1973), the intermediate region of the NTS receives input primarily from cardiopulmonary afferents (Loewy, 1990). Our data suggest that MOR agonists modulate the release of excitatory transmitters from vagal afferents as well as the responses in vagal afferent targets. Both actions are consistent with the cardiovascular effects of microinjections of MOR ligands in the NTS (Hassen et al., 1982; Lawrence and Jarrott, 1996). The increase in arterial pressure and heart rate produced by MOR ligands is consistent with a blockade of baroreceptor input, because baroreceptor activation would excite NTS cells and lead to a fall in blood pressure and heart rate. It has been suggested that MOR ligands in the NTS are capable of modulating these baroreceptive neurons but are not active tonically, because NTS injections of the opioid antagonist naloxone did not alter baroreflex function (Gordon, 1990). However, others have reported changes in baroreflex gain after naloxone administration (Szilagyi, 1988; Feldman et al., 1996). Elevated levels of endogenous opioids also have been reported in a two-kidney model of hypertension (Szilagyi, 1988), and opioid receptors within the brain may be responsible for the maintenance of this form of hypertension and the accompanying alterations in baroreflex function (Szilagyi, 1988).
Acknowledgements
This work was supported by an Established Investigator Award from the American Heart Association (S.A.A.) and by the following grants from the National Institutes of Health: HL56301 (S.A.A.), DE12640 (S.A.A.), MH48776 (V.M.P.), MH00078 (V.M.P.), DA04600 (V.M.P.), and HL18974. The authors are grateful to Dr. Peter Y. Cheng for assistance with the tracer injections and initial data collection and to Dr. Lee-Yuan Liu-Chen of Temple University School of Medicine for her generous gift of the MOR antibody.




