Olivocochlear neurons sending axon collaterals into the ventral cochlear nucleus of the rat
Abstract
The olivocochlear projection constitutes the last stage of the descending auditory system in the mammalian brain. Its neurons reside in the superior olivary complex (SOC) and project to the inner and outer hair cell receptors in the cochlea. Olivocochlear neurons were also reported to send axon collaterals into the cochlear nucleus, but controversies about their number and about species differences persist. By injecting the fluorescent retrograde axonal tracers diamidino yellow and fast blue into the cochlea and the ventral cochlear nucleus (VCN), we studied the distribution and number of olivocochlear neurons with and without axon collaterals into the VCN of the rat. We found that olivocochlear neurons residing in the lateral superior olive (LSO), the intrinsic lateral olivocochlear cells (intrinsic LOCs), do not send axon collaterals into the VCN. By contrast, a majority, and possibly all, olivocochlear neurons residing in the ventral nucleus of the trapezoid body (VNTB), the medial olivocochlear cells (MOCs), do have such axon collaterals. These cells may thus affect processing in the ascending auditory pathway at the level of the receptors and concurrently at the level of the secondary sensory neurons in the cochlear nucleus. Belonging to the lateral olivocochlear system, shell neurons reside around the LSO and form a third group of olivocochlear cells (shell LOCs). Like intrinsic LOCs, they innervate the inner hair cells, but like MOCs they do, by means of axon collaterals, project into the VCN. These findings have implications for understanding both auditory signal processing and the plasticity responses that occur following loss of cochlear function. J. Comp. Neurol. 422:95–105, 2000. © 2000 Wiley-Liss, Inc.
Conduction and integration of sensory signals in the auditory system is affected by extensive countercurrent, or descending, projections on all levels (Spangler and Warr, 1991). Compared to other sensory systems of the mammalian brain, this is a unique architecture serving sensory processing. On the lowest level of the descending auditory system, a massive projection originates in the superior olivary complex (SOC) of the brainstem to innervate outer (OHC) and inner hair cells (IHC), or their immediate afferent synapses, in the cochlea. This olivocochlear system shares its developmental origin with facial branchial motor neurons (Bruce et al., 1997) and may be considered to be of the special visceral efferent type.
The olivocochlear system was considered to consist of two main populations of cells in the SOC (White and Warr, 1983; Aschoff and Ostwald, 1987). The medial olivocochlear cells (MOCs) reside in the ventral nucleus of the trapezoid body (VNTB) of both sides of the brain and innervate OHCs in the cochlea (Warr et al., 1986). Lateral olivocochlear cells (LOCs) are associated to the lateral superior olive (LSO) and innervate predominantly the ipsilateral cochlea and terminate beneath the IHCs (Warr et al., 1986, 1997). According to recent reports by Vetter et al. (1991) and Warr et al. (1997), the second group of efferent auditory neurons, the LOCs of the rat, should be further divided into two subgroups. Small and fusiform cells are located within the ipsilateral LSO and are termed intrinsic LOCs; larger olivocochlear neurons are found in the periolivary regions around the LSO of both sides extending dendrites into the LSO (Vetter and Mugnaini, 1992) and may be called shell neurons or shell LOCs.
The collateral projections of olivocochlear cells were extensively studied in the gerbil, mouse, and guinea pig, but several controversies still exist about their pattern. Ryan et al. (1990) reported that the unmyelinated intrinsic LOCs innervate mainly the central part of the ventral cochlear nucleus (VCN) and the myelinated MOCs the peripheral VCN in the gerbil. By contrast, Brown et al. (1988), Brown and Benson (1992), and Brown (1993) found no cochlear nucleus collaterals of LOCs in gerbil, mouse, and cat. However, a majority of MOC axons (60–100%, depending on species) was found to give off collaterals to granule cell-containing regions of the VCN after incomplete filling of the VCN with the retrograde tracer substance. This gave rise to the conjecture that possibly up to 100% of these cells provide axon collaterals into VCN. Winter et al. (1989) described a similar projection pattern in guinea pig. However, they found that only 3.5–9.9% of MOCs give off collaterals into the VCN. White and Warr (1983) showed, by injecting horseradish peroxidase (HRP) into the cochlea, that axons of olivocochlear cells leave the olivocochlear bundle (OCB) at several points and enter the dorsal cochlear nucleus (DCN) and VCN in rat. The position and number of cell bodies that belong to these collaterals have not been evaluated in detail.
Unilateral removal of the cochlea in adult rats causes a substantial reemergence of the growth-associated protein-43 (GAP-43) in the neuropil of the VCN on the side of the lesion (Illing and Horváth, 1995). This protein is tightly related to axonal growth and synaptic plasticity (Benowitz et al., 1990; Benowitz and Routtenberg, 1997). Thus, the increased expression of GAP-43 in the VCN may serve as an indicator for axonal sprouting and remodeling of synapses in the cochlear nucleus following the loss of input from the spiral ganglion. However, following cochlear removal, neuronal cell bodies of the cochlear nucleus do not reexpress GAP-43; the few cells in the cochlear nucleus that express GAP-43 mRNA at low levels do not change this expression (Illing et al., 1997, 1999). It therefore appears that the GAP-43-immunoreactive fibers in the VCN belong to neurons that do not reside in the cochlear nucleus itself, but in other regions of the brainstem. These neurons may synthesize GAP-43 in response to the cochlear lesion and export the protein to the cochlear nucleus. In addition to afferents from the cochlea by the axons of spiral ganglion cells (which degenerate upon cochlear ablation), the VCN also receives major projections from noncochlear sources. These include the DCN (Adams and Warr, 1976; Wickesberg et al., 1991), the SOC (Adams, 1983; Covey et al., 1984; Spangler et al., 1987; Shore et al., 1991; Schofield, 1991, 1994; Warr and Beck, 1996; Ostapoff et al., 1997), the contralateral cochlear nucleus (Cant and Gaston, 1982; Wenthold, 1987; Shore et al., 1992; Alibardi, 1998), the inferior colliculus (Shore et al., 1991; Caicedo and Herbert, 1993), the auditory cortex (Weedman and Ryugo, 1996a,b), as well as some nonauditory projections from the locus coeruleus (Klepper and Herbert, 1991), the dorsal raphe nucleus (Klepper and Herbert, 1991), the dorsal column nuclei, and the spinal trigeminal nuclei (Itoh et al., 1987; Wright and Ryugo 1996). Among these regions, the only population of neurons showing a reemergence of GAP-43 in their cell body following cochleotomy was found in the ipsilateral LSO (Illing et al., 1997, 1999). These cells appear to be intrinsic LOC neurons and are possible candidates for the emergence of GAP-43 and the concomitant plastic reaction in the neuropil of the VCN. However, the distribution of olivocochlear cells having collaterals to the VCN has not yet been described in the rat.
The present study was done to determine the distribution and number of olivocochlear cells that give off collaterals into the VCN en route to the cochlea in the rat. The retrograde axonal tracers Diamidino Yellow (DY) and Fast Blue (FB; Conde, 1987) were used to label cell bodies of individual olivocochlear neurons and to visualize cells that project to the cochlear nucleus. These tracers have been successfully employed to label olivocochlear neurons in previous studies (Robertson et al., 1987a,b; Aschoff and Ostwald, 1988; Robertson and Winter, 1988; Winter et al., 1989).
Abbreviations
-
- AChE
-
acetylcholinesterase
-
- AVCN
-
anteroventral cochlear nucleus
-
- ChAT
-
choline acetyltransferase
-
- DCN
-
dorsal cochlear nucleus
-
- DY
-
diamidino yellow
-
- FB
-
fast blue
-
- GAP-43
-
growth-associated protein-43
-
- HRP
-
horseradish peroxidase
-
- IHC
-
inner hair cell
-
- lfp
-
longitudinal fasciculus of pons
-
- LOCs
-
lateral olivocochlear cells
-
- LSO
-
lateral superior olive
-
- MNTB
-
medial nucleus of the trapezoid body
-
- MOCs
-
medial olivocochlear cells
-
- MSO
-
medial superior olive
-
- n5sp
-
spinal trigeminal tract
-
- n5sr
-
sensory root of trigeminal nerve
-
- n7
-
facial nerve
-
- N7
-
facial nucleus
-
- n8
-
vestibulocochlear nerve
-
- OCB
-
olivocochlear bundle
-
- OHC
-
outer hair cell
-
- Pn
-
pontine nuclei
-
- pt
-
pyramidal tract
-
- PVCN
-
posteroventral cochlear nucleus
-
- SOC
-
superior olivary complex
-
- SPO
-
superior periolivary nucleus
-
- tb
-
trapezoid body
-
- VCN
-
ventral cochlear nucleus
-
- VNTB
-
ventral nucleus of the trapezoid body
MATERIALS AND METHODS
Sixteen adult Wistar rats of both sexes were used in this study. The fluorescent neuronal tracers DY (Sigma-Aldrich, Germany) or FB (Sigma-Aldrich) were introduced into the inner ear or injected into the VCN in order to identify those olivocochlear cells that give off collaterals into the cochlear nucleus. Animals were anesthetized by intraperitoneal (i.p.) injection of a mixture of Ketanest S (50 mg/kg, Parke-Davis, Ann Arbor, MI) and Rompun (5 mg/kg, Bayer Leverkusen, Germany).
Two kinds of operations were done. In the first group (n = 6), the left cochlea was approached by a lateral opening of the tympanic bulla. To expose the cochlea, the facial nerve (n7) was cut at its exit from the skull. A small hole was drilled into the cochlear wall, centered over the middle of its long axis (cp. Voelker et al., 1980), and part of the perilymph was soaked off using small tissue wicks. FB (n = 4) or DY (n = 2) was tamped into the cochlea and the opening closed with surgical bone wax. The bulla was subsequently filled with Gelfoam (Gelita, B. Braun, Germany) and the wound surgically closed.
In the second group (n = 10), FB was injected into the left VCN, immediately followed by application of DY into the cochlea of the same side. To approach the VCN, a small craniotomy was made in the left occipital bone and the overlying cerebellar flocculus and paraflocculus were aspirated to expose the cochlear nucleus. FB was injected through a glass micropipette with a tip diameter of about 50 μm. Using pressure, 0.15 μl saturated solution of FB were slowly injected; the pipette was allowed to remain in place for 10 minutes before being withdrawn. The application of DY into the cochlea was done as described for the first group of animals. The bulla and the cavity of the flocculus were filled with Gelfoam and the wound surgically closed. At completion of the operations, the rats were given the analgesic Novalgin (10 mg/kg intramuscularly [i.m.], Hoechst AG, Frankfurt am Main, Germany). The animals were allowed to recover for a period of 5 or 6 days. They were then deeply anesthetized with an overdose of Nembutal (300 mg/kg i.p., Sanofi, Paris) and fixed by transcardial perfusion with 4% paraformaldehyde in phosphate buffer (0.1 M, pH 7.4, 4°C). Thirty micrometer-thick frozen sections of the brainstem were cut in the frontal plane and mounted on gelatin-subbed slides. Sections were viewed with a Zeiss microscope using epi-illumination. The exciting filter used had a peak at 405 nm and the barrier filter cutoff was 455 nm. To localize the injection site and the labeled cells within the various brainstem nuclei, maps were made based on camera lucida drawings. Individual olivocochlear neurons were examined using a 100× oil-immersion objective in order to distinguish single from double-labeled cells. Care and use of the animals as reported here were approved by Regierungspräsidium Freiburg, permission number 37/9185.81/1/267.2.
The number of labeled cells was determined using profile counts. For neurons labeled by FB, cell bodies recognizable as such were counted; for neurons labeled with DY, cell nuclei were counted. In each case, raw counts were corrected for numerical overestimation resulting from double counts due to sectioning using the equation provided by Abercrombie (1946; cp. Guillery and Herrup, 1997). This was done based on the following measurements: cell body diameter as apparent in FB staining of neurons in LSO: 9.9 ± 0.4 (n = 13), in VNTB: 15.5 ± 0.4 (n = 39), of shell neurons: 13.9 ± 0.5 (n = 14); nucleus diameter as apparent in DY staining in neurons of LSO: 8.4 ± 0.2 (n = 26), of VNTB: 10.9±0.3 (n = 30), in shell neurons: 10.0 ± 0.4 (n = 12). All counting data in this study went through this kind of correction.
RESULTS
Olivocochlear neurons
Following FB or DY application into the cochlea, retrogradely labeled cells were encountered bilaterally in the SOC as described earlier (White and Warr, 1983; Aschoff and Ostwald, 1988). Labeled cells were found in the facial, vestibular, and reticular nuclei, but these will not be dealt with any further in this study. Small (diameter approximately 10 μm) and fusiform neurons inside the LSO (intrinsic LOCs) were labeled virtually exclusively on the ipsilateral side. The majority of larger (approximately 15 μm) multipolar cells of the VNTB (MOCs) resided on the contralateral side. Additionally, larger (about 15 μm) neurons in the periolivary region around the LSO were labeled with an ipsilateral dominance. These neurons were located mainly dorsal, caudal, and rostral to the LSO, and their dendrites were seen to extend almost always into the LSO. This group of olivocochlear cells may be called shell neurons (Vetter and Mugnaini, 1992), or shell LOCs. Figure 1 shows the distribution of neurons in the SOC that were retrogradely labeled following FB application into the left cochlea of one representative animal. There was no difference in the distribution of labeled cells after the intracochlear application of DY or FB. The total number of labeled olivocochlear cells was 901.0 ± 17.7 SEM after tracing with FB and 1,129.0 ± 13.0 SEM after DY application. Averaging over six cases, 509.2 ± 19.8 SEM cells were labeled in the ipsilateral LSO, 54.2 ± 5.0 SEM cells in the ipsilateral shell, and 170.0 ± 13.7 SEM cells in the ipsilateral VNTB. Contralaterally, no labeled neurons were found in the LSO in three cases, but up to two labeled cells were seen in others. There were 14.3 ± 1.3 SEM labeled contralateral shell neurons and 228.3 ± 17.7 SEM labeled cells in the contralateral VNTB. DY-labeled cells could be classified only according to the location of cells and the size of the nucleus as the dendritic morphology of the labeled cells was not revealed by the tracer. Table 1 summarizes the counts of retrogradely labeled olivocochlear neurons over all six experiments.
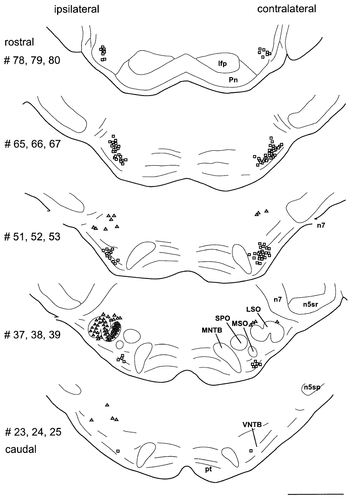
Drawings of serial frontal sections through the brainstem of a representative animal showing the distribution of retrogradely labeled cells following FB application into the left cochlea. Triangles indicate the predominantly ipsilateral distribution of LOCs in the SOC. Squares show the bilateral distribution of MOCs in the VNTB. Each drawing integrates counts from three neighboring sections. One symbol represents one labeled neuron outside the LSO. In the LSO, one triangle represents five labeled neurons. Section numbers were counted from caudal to rostral, starting at the caudal end of the DCN. For abbreviations, see list. Scale bar = 1 mm.
| Experiment no. | LSO | Shell | VNTB | Total | |||
|---|---|---|---|---|---|---|---|
| Ipsilateral | Contralateral | Ipsilateral | Contralateral | Ipsilateral | Contralateral | ||
| M441 (FB) | 519 | 2 | 40 | 11 | 154 | 200 | 926 |
| M314 (FB) | 497 | 2 | 56 | 14 | 166 | 201 | 936 |
| M341 (FB) | 491 | 2 | 55 | 20 | 121 | 189 | 878 |
| M398 (FB) | 430 | 0 | 41 | 14 | 163 | 216 | 864 |
| M417 (DY) | 559 | 0 | 61 | 15 | 211 | 296 | 1,142 |
| M421 (DY) | 559 | 0 | 72 | 12 | 205 | 268 | 1,116 |
| Mean ± SEM | 509.2 ± 19.8 | 1.0 ± 0.4 | 54.2 ± 5.0 | 14.3 ± 1.3 | 170.0 ± 13.7 | 228.3 ± 17.7 | 977.0 ± 49.5 |
Double-labeling experiments
In the second series of experiments, we combined the retrograde labeling of olivocochlear cells with the visualization of cells that project to the VCN. DY was applied to the left cochlea to trace olivocochlear cells and FB was injected into the VCN of the same side. The average number of labeled olivocochlear cells (929.0 ± 42.6 SEM) was indistinguishable to the counts obtained after a single application of FB or DY into the cochlea as detailed above (977.0 ± 49.5 SEM). In five experiments, the injection site of FB, including their diffusion range, was restricted to the VCN and included both the posteroventral (PVCN) and anteroventral cochlear nucleus (AVCN). Figures 2 and 4 document the injection sites of FB in the VCN of two representative cases. The most medial and rostral parts of the VCN were not filled with the tracer.
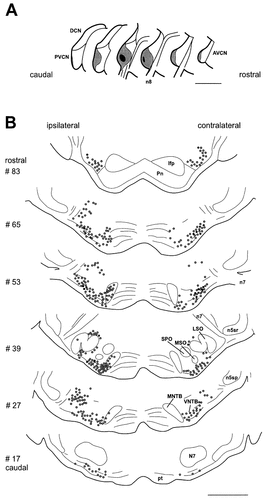
Distribution of neurons on the level of SOC projecting to the VCN. A: Injection site into the cochlear nucleus, representative for this experimental series in both position and size. Dark gray area indicates peak concentration of FB and the gray surround shows diffusion range of the tracer. B: Representative distribution of retrogradely labeled neurons following FB injection into the left VCN together with DY application into the left cochlea. Neurons labeled from cochlea only are not shown. Open circles indicate FB-labeled cells that project to the VCN. Filled circles represent double-labeled cells, i.e., olivocochlear cells sending an axon collateral into the VCN. Each drawing shows counts from one section only. Note that there are many more cells in the SOC projecting to the VCN as compared to those projecting to the cochlea (see Fig. 1). Section numbering as for Figure 1. For abbreviations, see list. Scale bars = 1 mm in A,B.
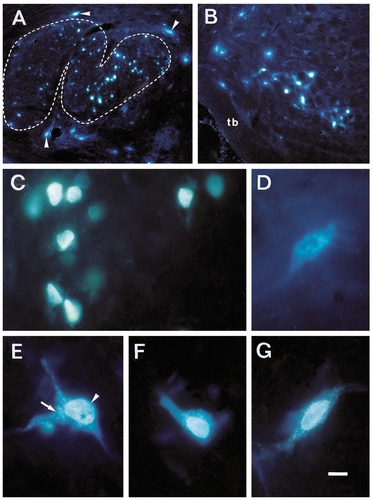
Retrogradely labeled cells in the SOC following DY application into the left cochlea and FB injection into the left VCN. A: Labeled cells in and around the ipsilateral LSO. Outline of LSO is indicated by dashed line. Arrowheads point to shell neurons. B: Labeled neurons in the VNTB on the side of the injection; MOC neuron may be recognized by their yellow nucleus. Double-labeled cells cannot easily be identified at this magnification. tb, trapezoid body. C: Single-labeled cells in the ipsilateral LSO showing only yellow nuclear staining through axonal transport of the tracer DY. D: Single-labeled neuron in the ipsilateral VNTB, showing only blue staining of the cytoplasm through axonal transport of FB. E–G: Double-labeled neurons in the ipsilateral (E) and contralateral (F, G) VNTB showing yellow fluorescence of the nucleus and blue staining of the cytoplasm. These cells prove, by virtue of their double labeling, that they send axon collaterals to both the cochlea and the cochlear nucleus. Arrow points to FB labeling of the cytoplasm of a neuron; arrowhead points to DY labeling of its nucleus. Scale bar = 100 μm for A,B; 10 μm for C–G.
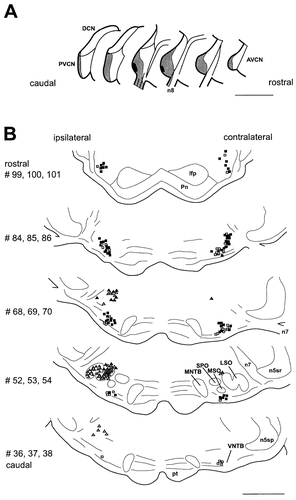
Olivocochlear neurons in the SOC labeled after DY application into the left cochlea and FB injection into the left VCN. A: Injection site into the cochlear nucleus, representative for this experimental series in both position and size. Dark gray area indicates peak concentration of FB and the gray surround shows diffusion range of the tracer. B: Drawings of frontal sections with single and double-labeled neurons through the SOC. Each drawing represents data from three neighboring sections. Neurons single labeled from the cochlear nucleus are not shown (see Fig. 2). Triangles indicate LOCs, squares indicate MOCs. In the LSO, each triangle represents five labeled neurons; outside the LSO each symbol marks one cell. Open symbols represent neurons single labeled by DY, filled symbols represent double-labeled neurons. Filled symbols indicate olivocochlear cells that also project to VCN. Section numbering as in Figure 2. For abbreviations, see list. Scale bars = 1 mm in A,B.
Following the FB injection into the VCN, a large number of periolivary cells were labeled, showing an ipsilateral dominance (ipsilateral vs. contralateral 1.5:1). Principal neurons of the ipsilateral medial nucleus of the trapezoid body (MNTB) were also labeled. The ipsilateral LSO had a few (8.8 ± 2.3 SEM), the contralateral had no labeled neurons. Additionally, multipolar neurons of the contralateral VCN, some cells in the deeper layers of the contralateral DCN, and neurons of all three subnuclei of the inferior colliculus were also labeled. Figure 2 provides data of one representative experiment, showing the distribution of neurons in the SOC that were retrogradely labeled after injection of FB into the VCN. In five other experimental cases, the injection site extended beyond the VCN and covered part of the white matter immediately medial of it. As axons of the olivocochlear neurons run in this bundle, tracer uptake by its fibers resulted in retrograde labeling of cells in several regions of the brainstem, including the ipsilateral LSO. These experiments were not considered for our quantitative evaluations.
Olivocochlear neurons with collaterals to the VCN
The combination of DY and FB allowed us to identify olivocochlear cells giving off collaterals into the VCN. The combined application of these tracers resulted in partly intermingled populations of blue and/or yellow-labeled neurons (Figs. 3A,B). Single-labeled neurons showed only yellow fluorescence of the nucleus (Fig. 3C) or blue staining of the cytoplasm (Fig. 3D). Double-labeled neurons were clearly distinguishable by the intense yellow fluorescence of their nucleus and their blue cytoplasm (Figs. 3E–G). We found double-labeled cells in the periolivary region around the LSO (shell LOCs) and in the VNTB (MOCs) of both sides of the brain. Across five experiments, only six double-labeled neurons were found in the ipsilateral LSO. Figure 4 shows the distribution of single and double-labeled olivocochlear cells in the SOC. Fifty-six percent of the MOCs in the VNTB and 39% of shell neurons were seen by virtue of their double labeling to send collaterals into the VCN. These percentages resulted from retrograde transport originating in a restricted area of the VCN. They are expected to grow, possibly up to 100%, with a larger or even complete coverage of the VCN with tracer, which is difficult to achieve for practical reasons. Table 2 summarizes counts and SEMs of single and double-labeled olivocochlear neurons in the LSO, shell region, and the VNTB.
| Experiment no. | LSO | Shell | VNTB | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ipsilateral all | dl | Contralateral all | Ipsilateral all | dl | Contralateral all | dl | Ipsilateral all | dl | Contralateral all | dl | All | dl | |
| M422 | 497 | 0 | 0 | 51 | 24 | 15 | 9 | 176 | 106 | 197 | 129 | 936 | 268 |
| M424 | 410 | 0 | 0 | 45 | 9 | 12 | 7 | 191 | 113 | 305 | 170 | 963 | 299 |
| M436 | 377 | 1 | 0 | 30 | 10 | 7 | 6 | 158 | 70 | 205 | 94 | 777 | 180 |
| M437 | 486 | 4 | 0 | 57 | 22 | 12 | 9 | 160 | 106 | 216 | 128 | 931 | 270 |
| M439 | 545 | 1 | 0 | 66 | 18 | 9 | 4 | 213 | 126 | 205 | 89 | 1038 | 237 |
| Mean ± SEM | 463.0 ± 30.5 | 1.2 ± 0.7 | 0 | 49.8 ± 6.0 | 16.6 ± 3.1 | 11.0 ± 1.4 | 7.0 ± 0.9 | 179.6 ± 10.3 | 104.2 ± 9.3 | 225.6 ± 20.1 | 122.0 ± 14.6 | 929.0 ± 42.6 | 250.8 ± 20.2 |
- 1 Double-labeled (dl) cells sending collaterals into the VCN.
DISCUSSION
The major results of the present study are that (1) the intrinsic lateral olivocochlear neurons in the LSO of the rat do not have axon collaterals reaching into VCN; (2) a significant number, and possibly a majority, of shell neurons around the LSO of both sides give off collaterals into VCN and, by virtue of their specific connectivity, constitute a distinct population of olivocochlear cells; and (3) a majority of MOCs, but possibly even all of them, residing in the VNTB of both sides send axon collaterals into the VCN. They are therefore in a position to massively influence the ascending auditory pathway, not only at the level of the hair cells, but also on the level of the cochlear nucleus.
Single labeling of olivocochlear cells
In the present study, we could identify and quantify two groups of LOCs — one in the ipsilateral LSO (intrinsic LOCs) and another in the periolivary region around the LSO of both sides (shell LOCs). MOCs were found in the VNTB of both sides. The distribution and number of olivocochlear cells are in general agreement with previous studies. White and Warr (1983) described the dual (i.e., lateral and medial) origin of the OCB in the rat by using axonal transport of HRP. Injection of free HRP into the cochlea resulted in the retrograde labeling of 350 cells in the LSO and 300 cells in the VNTB. The numbers of labeled olivocochlear cells in the present study are significantly larger than these counts (509.2 ± 19.8 cells in the LSO and 398.3 ± 30.8 cells in the VNTB), but somewhat smaller than found previously with the same fluorescent tracers (540 cells in the LSO and 556 in the VNTB, Aschoff and Ostwald, 1988; cp. Robertson et al., 1989). In addition, we also recognized labeled shell neurons first described by Vetter et al. (1991). Shell neurons were also traced by intracochlear injection of cholera toxin tracers. It was shown that their dendrites penetrate into the LSO and surrounding areas (Vetter and Mugnaini, 1992). In six cases, we counted 68.5 ± 5.3 SEM shell neurons, 54.2 ± 5.0 SEM cells around the ipsilateral, and 14.3 ± 1.3 SEM cells around the contralateral LSO. Injection of cholera toxins resulted in 111 labeled shell neurons, 105 ipsilateral, and 6 contralateral (Vetter and Mugnaini, 1992). These differences may be explained by a difference in uptake efficiency of the different tracers.
Following tracer application into the cochlea, marginal to low levels of FB remained in the middle ear cavity. This may have resulted in labeling of a comparatively sparse population of motor neurons of middle ear muscles (Spangler et al., 1982; Keller et al., 1983; Rouiller et al., 1989) and salivatory glands (Hiura, 1977). However, areas of overlap with olivocochlear cells only exist for stapedial motor neurons and are marginal even there (Rouiller et al., 1989). Should this labeling have occurred, our counting error would not exceed 5%.
Double-labeling experiments
In our second experimental group, we have combined the retrograde tracing of the olivocochlear cells with DY and the injection of FB into the VCN. This tracer combination has been proven useful in numerous neuroanatomical studies dealing with virtually all projectional systems in the mammalian brain. Double-labeled cells can be easily identified by a yellow nucleus and blue cytoplasm (Figs. 3E–G). This combination of tracers has also been used to study the collateral projections of olivocochlear cells in the guinea pig (Robertson and Winter 1988; Winter et al., 1989).
Quantitative evaluation was based only on those experiments in which the injection and local diffusion of FB were confined to the VCN and included both the PVCN and the AVCN (Figs. 2, 4) but not adjacent white matter. Regions that were found to project to the VCN included the ipsilateral DCN, the ipsilateral and contralateral SOC, the contralateral DCN and VCN, and the inferior colliculus of both sides. These projections are known and were analyzed in previous studies in the cat (Adams and Warr, 1976; Cant and Gaston, 1982; Adams, 1983; Spangler et al., 1987), guinea pig (Wenthold, 1987; Schofield, 1991, 1994; Shore et al., 1991, 1992; Ostapoff et al., 1997), and rat (Weedman and Ryugo, 1996a,b; Warr and Beck, 1996; Alibardi, 1998). Our study specifically focuses on olivocochlear cells projecting to the VCN.
MOCs with collaterals to the VCN
Our results show that the majority of MOCs, located in the VNTB of both sides, send collaterals into the VCN (Fig. 4, Table 2). Double-labeled cells were found along the whole rostrocaudal extent of the VNTB and the percentage of double-labeled cells was almost the same on the two sides (ipsilateral 58%, contralateral 54%). Because the tracer injection into the VCN could not cover the whole nucleus, the counts we obtained must necessarily be minimal estimates. We suggest that in fact 80–100% of MOCs send collaterals into VCN. This estimate would be consistent with previous observations in some other species. Using HRP injection into the cochlea, 67–100% (depending on the species) of thick MOC axons were found to give off collaterals into the VCN in the cat (Brown at al., 1988), mouse (Brown et al., 1988; Brown and Benson, 1992), and gerbil (Brown et al., 1988; Ryan et al. 1990). In the rat, HRP injection into the cochlea showed that collaterals leave the OCB and enter the VCN at two points: at the junction between the DCN and PVCN and from the main OCB as it descends on the medial side of the vestibular nerve, entering the AVCN (White and Warr, 1983). These collaterals are also apparent with stains for acetylcholinesterase (AChE; White and Warr, 1983). Injection of 3H-leucine and biotinylated dextran amine into the VNTB of the rat resulted in strong anterograde labeling in the cochlear nuclei and identification of the OCB (Warr and Beck, 1996). However, indirect evidence from other studies suggested that collateral projections from the OCB to the VCN are very sparse in the rat. Cutting the entire OCB causes only slight reductions in levels of AChE in the cochlear nucleus (Osen et al., 1984). In microassays for choline acetyltransferase (ChAT), a reduction of only about 15% was observed in the cochlear nucleus granule cell region of the rat (Godfrey et al., 1987); a reduction of 70% was found in the cat (Godfrey et al., 1990).
Injecting FB into the cochlear nucleus and DY into the cochlea of the guinea pig, Winter et al. (1989) saw only 3.5–9.9% of MOCs projecting to the cochlear nucleus. This difference to the data obtained in the present study could either be an interspecies difference between guinea pig and rat or the result of methodological differences. In three experimental cases, Winter et al. (1989) injected a 2% aqueous solution of FB into the cochlear nucleus (including the DCN). However, the 9.9% double labeling of MOCs was reported in only one experiment, whereas the others showed even fewer double-labeled cells.
The local targets of the collateral projections from MOCs could not be determined in the present study as our injection site occupied both the peripheral and central VCN (Figs. 2, 4). Previous ultrastructural investigations showed that cochlear nucleus collaterals of MOCs terminate on varicose dendrites of small cells and on large dendrites of multipolar cells in mice (Benson and Brown 1990; Benson et al., 1996). The massive collateral projection of MOCs found in the present study indicates that MOCs must play a crucial role in feedback control of auditory processing. These cells can directly influence the motility of OHCs (Dallos et al., 1990; Liberman et al., 1990) as well as the secondary sensory neurons in the cochlear nucleus. They are themselves under the influence of an excitatory input from the contralateral cochlea by way of the cochlear nucleus, thus serving to link both cochlea and both cochlear nuclei (Robertson and Winter, 1988; Thompson and Thompson, 1991). This regional feedback loop, together with descending pathways from the inferior colliculus (Faye-Lund, 1986; Caicedo and Herbert, 1993; Vetter et al., 1993) and auditory cortex (Feliciano et al., 1995; Weedman and Ryugo, 1996a,b), constitutes the anatomical basis for the efferent control of auditory processing in the lower brainstem.
LOCs with collaterals to the VCN
The second main finding of the present study is that the two types of lateral olivocochlear neurons differ in their collateral projection pattern. Although intrinsic LOCs in the LSO, apart from extremely rare single cells, do not send collaterals into the VCN, a majority of shell neurons around the LSO may send axon branches into the VCN. Previous studies have shown that the projections from the LSO to the cochlear nucleus are very sparse in the guinea pig (Winter et al., 1989; Schofield, 1994; Ostapoff et al., 1997) and cat (Spangler et al., 1987). Weedman and Ryugo (1996a) did not observe any labeled cells in the LSO following FB injection into the rat cochlear nucleus. On average, we found only 8.8 ± 2.3 SEM LSO cells that project to the VCN. Across all five experiments, only six of these neurons (1.2 ± 0.7 SEM) proved, by virtue of their double labeling, to be olivocochlear cells. When our injection extended medially to the VCN and touched the OCB, nearly all LOCs in the LSO were double labeled. This is evidence that the thin unmyelinated axons are capable of transporting either tracer. The failure of labeling these cells by tracer injections strictly confined to the VCN does imply the absence of axon terminals there.
There is virtually no projection from intrinsic LOCs to the VCN in the normal adult rat. Therefore, we must conclude that the cochleotomy-induced reemergence of GAP-43 in the neuropil of the VCN and in cell bodies of intrinsic LOCs reported earlier (Illing et al., 1997, 1999) are independent consequences of the cochlear lesion — unless there occurs a cochleotomy-dependent sprouting of the axons of these olivocochlear neurons into the cochlear nucleus, a possibility currently investigated in this laboratory.
Our results support the evidence that LOCs are divided into two subclasses in the rat: the small (diameter approximately 10 μm) and fusiform intrinsic neurons in the LSO and the larger (diameter approximately 15 μm) and multipolar shell neurons around the LSO (Vetter and Mugnaini, 1992). It has already been reported that these groups of cells have different terminal arborizations in the cochlea (Warr et al., 1997). Here we show that the axonal projection pattern of these neurons is also different: many shell LOCs send collaterals into the VCN, whereas the intrinsic LOCs in the LSO project only to the cochlea. Apparently, shell neurons share size, dendritic morphology, and the collateral projection pattern including the possibility to project to both cochleae, with MOCs (Aschoff and Ostwald, 1988; Robertson et al., 1989), but their localization close to the LSO, and their innervation of IHCs in the cochlea with the LOCs.
Acknowledgements
We thank Mrs. B. Hobmaier, Mr. W. Kuhn, and Mrs. G. Wittmann for technical assistance. M.H. was supported by OTKA, Hungary (grant T 025231), and received a fellowship from the Deutsche Akademische Austauschdienst (DAAD).




