SNAP-25 regulation during adrenal gland development: Comparison with differentiation markers and other SNAREs
Abstract
Synaptosomal-associated protein of 25 kDa (SNAP-25) is one of a limited number of soluble N-ethylmaleimide-sensitive fusion attachment protein receptors (SNAREs) that play a major role in membrane docking of synaptic vesicles and secretory granules during regulated exocytosis. We have previously shown that SNAP-25 levels differ between noradrenergic and adrenergic chromaffin cell populations of the adult adrenal gland. We examine SNAP-25 expression by immunofluoresence in cells of the sympathoadrenal lineage in the rat during late embryonic and postnatal development. In parallel, tyrosine hydroxylase was used to identify sympathoadrenal cells, phenylethanolamine N-methyltransferase to distinguish adrenergic from noradrenergic chromaffin cells, and chromogranin A to define the presence of secretory granules. In addition, SNAP-25 protein and mRNA levels were followed in adrenal gland extracts by immunoblotting and reverse transcription-polymerase chain reaction (RT-PCR). Protein levels were compared with those of other molecules also implicated in organelle trafficking, including syntaxin 1 and vesicle-associated membrane protein (VAMP-2) and the nonneuronal analogues SNAP-23 and cellubrevin. This study provides evidence that SNAP-25 is expressed early during development in sympathoadrenal neurons and migrating cells. It is detected in intra-adrenal chromoblasts as soon as they enter the adrenal primordium. Its differential expression between catecholamine chromaffin cell phenotypes is already evident from the 17th embryonic day, future noradrenergic cells appearing to express higher levels than adrenergic cells. The granule maturation marker chromogranin A is expressed in chromaffin cells later than SNAP-25. Both SNAP-25 protein and mRNA increased rapidly in the adrenal gland in the perinatal period to peak during the first postnatal week, after which levels dropped dramatically to adult values. In contrast, levels of both syntaxin and SNAP-23 appeared to remain fairly constant throughout adrenal gland development. VAMP-2 expression increased gradually around birth to reach maximal levels during the first two postnatal weeks, and then decreased slightly. Cellubrevin levels also appeared to increase gradually until adult values were attained by the end of the second postnatal week. The threefold increase of SNAP-25 mRNA shortly after birth compared to the low adult levels suggests that during this period SNAP-25 is implicated in additional functions than regulated secretion, possibly associated with cellular growth or maturation. J. Comp. Neurol. 421:533–542, 2000. © 2000 Wiley-Liss, Inc.
Adrenal medullary chromaffin cells are derived from precursor cells that migrate from the thoracolumbar region of the neural crest into the primary sympathetic chain (Le Douarin, 1980). They pass the mesenchyme neural tube-notochord complex, where they are exposed to factors that induce sympathoadrenal (SA) characteristics, including the capacity to synthesize catecholamines (Landis and Patterson, 1981). On their arrival at the mesoderm-derived adrenal cortical primordium, these chromoblasts aggregate to form a blastema before entering it. At the earliest stages of adrenal medulla ontogenesis, at least two phenotypes of intra-adrenal chromoblasts can be distinguished (Henion and Landis, 1990; Leon et al., 1992). Larger cell groups destined to become intra-adrenal neurons and noradrenergic (NA) chromaffin cells contrast with small dispersed groups of future adrenergic cells (A). At later stages, the medulla becomes more compact to form homotypic groups of either A or NA cells with rare groups of large ganglionic neurons. During chromoblast differentiation and maturation, levels of tyrosine hydroxylase (TH) are regulated in both phenotypes, phenylethanolamine N-methyltransferase (PNMT) is induced in a major population of chromaffin cells, and granule synthesis and maturation occurs as a forerunner of functional maturation by the end of the first postnatal week. This stage can be monitored by the appearance of granule constituents such as chromogranin A (CGA; Kent and Coupland, 1989).
It is currently accepted that the secretion of catecholamines from chromaffin cells is governed in part by the interaction of secretory granule membrane-associated proteins with receptor proteins on the plasma membrane called soluble N-ethylmaleimide-sensitive fusion attachment protein receptors (SNAREs), which leads to their docking. Synaptosomal-associated protein of 25 kDa (SNAP-25), which occurs as two isoforms “a” and “b” (Bark and Wilson, 1994b), is one of several proteins that constitute the docking complex (Sollner et al., 1993; Sudhof et al., 1993; Martin, 1994; Sollner and Rothman, 1994; Burgoyne and Morgan, 1998). A role for SNAP-25 in neurotransmitter release in chromaffin cells has been suggested (Roth and Burgoyne, 1994; Gutierrez et al., 1997; Lawrence et al., 1997). Apart from regulated exocytosis, SNAP-25 is also likely to play a role in constitutive exocytosis involved in the renewal of plasma membrane constituents. In this regard, it has been implicated in morphological plasticity of central neurons, in particular membrane expansion (Rothman, 1994) and axon or neurite outgrowth (Oyler et al., 1989; Osen-Sand et al., 1993; Soriano et al., 1994; Boschert et al., 1996; Patanow et al., 1997; Grosse et al., 1999).
In the adult rat, our previous studies on the adrenal gland have shown that the expression of SNAP-25 is not identical in both chromaffin cell phenotypes. NA cells express higher levels and it appears to be distributed in the cytoplasm, whereas it is restricted to the plasma membrane in A cells (Kannan et al., 1996). As SNAP-25 is a presumed key element in regulated exocytosis, the present studies were performed to address the question of whether this expression of SNAP-25 in both phenotypes is modified during functional maturation of the chromaffin cell (i.e., during the period leading up to the initiation of regulated secretory activity). In order to better understand the likely roles of this protein during development, possible correlations were examined between SNAP-25 expression and the expression of other proteins known to be involved in exocytosis. In particular, the developmental profiles of the neuronal SNAREs (syntaxin 1), the vesicle-associated membrane protein (VAMP-2), and the nonneuronal SNARE analogues (SNAP-23 and cellubrevin) were determined. In addition, possible local factors that might influence regulation of SNAP-25 expression during adrenal gland development are discussed.
Abbreviations
-
- A
-
adrenergic
-
- CGA
-
chromogranin A
-
- NA
-
noradrenergic
-
- PNMT
-
phenylethanolamine N-methyltransferase
-
- SA
-
sympathoadrenal
-
- SNAP
-
synaptosomal-associated protein
-
- SNARE
-
soluble N-ethylmaleimide-sensitive fusion attachment protein receptor
-
- TH
-
tyrosine hydroxylase
-
- VAMP
-
vesicle-associated membrane protein
MATERIALS AND METHODS
Animals
Wistar rats were maintained in a constant light-dark cycle (12 hour: 12 hour) with free access to water and food. The first day of gestation (E0) was defined as the day following an overnight mating. Embryos at different embryonic ages (from E14 to E19) were removed from the uterine horns of females anesthetized in a chamber saturated with isoflurane (Abbot Laboratories, Queenborough, UK). Adrenal glands were also isolated from rat pups and young animals at various ages (from newborn P0 to P60). All animal experimentation was carried out according to European Community Council guidelines.
Immunofluorescence
Cryostat sections (10 μm thick) of 4% formaldehyde-fixed rat adrenal glands were prepared and processed for immunofluorescence as previously described (Kannan et al., 1996). SNAP-25 was detected using the monoclonal SMI-81 antibody (1/500, Sternberger Monoclonals, Baltimore, MD). TH was detected with a rabbit antibody (1/500 Institut Boy, Reims, France). A rabbit polyclonal antibody was used to detect the adrenalin-synthesizing enzyme PNMT (1/500, a generous gift from Dr. L. Denoroy, Université Claude Bernard, Lyon, France). A rabbit antibody against the bovine CGA peptide 316-329 sequence, produced in our laboratory, was employed to label the granule protein CGA (1/500). Secondary antibodies against mouse and rabbit were coupled to rhodamine and Cy2, respectively (1/150, Chemicon, Temecula, CA; 1/1,000, Amersham, Arlingron Heights, IL). Various antibody combinations were used for double labeling (SNAP-25/TH; SNAP-25/PNMT; SNAP-25/CGA).
Immunoblots
For proteins, three series of duplicate samples of different ages were analyzed. For each extract, glands were pooled: 10 glands for E16, 8 glands for E19, 4 glands for P2, P7, and P14, and 2 glands for P30 and P60. Triton X-100 soluble protein tissue extracts (15 μg/ lane, except for VAMP-2, 20 μg, and cellubrevin, 30 μg) were electrophoresed on 12% (15% for cellubrevin and VAMP-2) sodium dodecyl sulfate (SDS) polyacrylamide gels and transferred to nitrocellulose sheets, as previously described (Kannan et al., 1996). TH was detected on Western blots with a mouse monoclonal antibody against rat TH (1/5,000 Chemicon), PNMT with the rabbit anti-PNMT (1/2,000), SNAP-25 with the mouse monoclonal SMI-81 antibody used for immunofluorescence (1/10,000), SNAP-23 with a rabbit polyclonal antibody, TG7 (1/1,000; Galli et al., 1998), syntaxin 1 with the HPC-1 mouse monoclonal (1/2,000; Sigma, France), VAMP-2 with a mouse monoclonal (1/5,000 clone 69-1; Synaptic Systems, Göttingen, Germany), cellubrevin with MC16 a rabbit polyclonal (1/1,000; Galli et al., 1994), and CGA with a rabbit anti-bovine CGA raised in our laboratory (a mixture of two poyclonal antibodies raised against either peptide sequence 4-16 or the conserved amino acid sequence 65-76 of bovine CGA [each at 1/1,000; Majdoubi et al., 1996]). Actin, employed as internal standard, was detected with a monoclonal anti β-actin antibody (1/5,000; Sigma, St. Quentin). After incubation with appropriate secondary antibodies coupled to biotin (1/10,000 Tropix, Bedford, MS) and alkaline phosphatase-conjugated avidin (1/10,000; Tropix), or directly with alkaline phosphatase-conjugated secondary antibodies (1/2500; Biorad, Paris, France), antigens were detected by chemiluminescence using CSPD, according to the manufacturer's instructions (Tropix).
Reverse transcription-polymerase chain reaction (RT-PCR)
For each age, RNA extractions were performed in triplicate: either four (E19, P2, P14) or two glands (P30) were pooled for each extract. Total RNA was prepared from frozen adrenal glands by directly lysing tissues in extraction buffer (guanidinium thiocyanate/phenol/chloroform). mRNAs were transcribed into cDNA using oligo-dT and Superscript RNAase H- M-MLV reverse transcriptase (GIBCO-BRL, France), as previously described (Grant et al., 1999). The following PCR cycle profile was used: denaturation at 94°C for 45 seconds, annealing at 60°C for 60 seconds, and polymerization at 72°C for 60 seconds, followed by an additional polymerization after the last cycle at 72°C for 120 seconds. The number of cycles was adjusted according to the abundance of each mRNA so that amplifications were in the linear range of the PCR reaction. cDNA corresponding to 50 ng of RNA in RT-PCRs was used to amplify PCR products for SNAP-25 (559 bp), SNAP-25a and SNAP-25b (202 bp), SNAP-23 (392 bp), as previously described (Grant et al., 1999). As an internal standard, a β-actin PCR product (645 bp) was amplified using 5′-gatggtgggtatgggtcagaaggactccta-3′ and 5′-gcatcggaaccgctcattgccgatagtgat (J00691) as forward and reverse primers. A TH PCR product (469 bp) was amplified with the primers 5′-aatgcacccagtatatccgccatgcctcct-3′ and 5′-gccagtgtgtacgggtcaaacttcacagag-3′ (M10244). A PNMT product (440 bp) was amplified with the primers 5′-ttcgctgcatggcacaagtctttgccaccg-3′ and 5′-cagcgcggtgatatgatacaaagcctgccg-3′ (X14211).
Southern blot analysis
After electrophoresis of PCR products for SNAP-25 (5 μl) for each age (n = 3), DNA was transferred by capillarity onto a nylon membrane. After fixation, DNA was hybridized with a biotinylated probe on exon 4 (Grant et al., 1999) for 3 hours at 50°C. After washing, the membrane was incubated with alkaline phosphatase-coupled avidin (1/20,000) and then bands were revealed by chemiluminescence with CSP-Star (Tropix). Band intensities were analyzed by scanning the films obtained and determining their optical density using the PC-BAS program of a Bio-Imaging Analyzer (FUJIX BAS 1000; Fuji, Tokyo, Japan).
RESULTS
Immunofluorescence
Cellular labeling for SNAP-25 was examined in transverse sections of embryos from E14 or neonatal rats or in adrenal gland sections of older animals and correlated with that of maturation and phenotypic markers, by using TH labeling as a criterion to identify SA cells. At E14, SNAP-25 was found to be expressed strongly at surface membranes of sympathetic ganglionic neurons, migrating SA cells, SA cells in para-aortic cellular accumulations and in the adrenal blastema, and also in nerve fibers (Fig. 1). The extra-adrenal chromoblasts were strongly TH positive from the earliest ages examined. A few TH- or SNAP-25-positive intra-adrenal cells were observed at this stage, but no intra- or extra-adrenal SA cells were found to be stained for CGA or PNMT. At E17 and E19, inside the developing adrenal primordium, relatively large groups of chromoblasts, representing future NA chromaffin cells and neurons (Leon et al., 1992), were observed: these were positive for SNAP-25 and weakly TH positive (Fig. 2a), but were negative for PNMT (Figs. 2c,e). In contrast, many small groups of PNMT-positive chromoblasts, the future A chromaffin cells, were seen dispersed throughout the embryonic gland. These expressed SNAP-25 less strongly, but TH more strongly (Fig. 2a). At these stages, SNAP-25 labeling of unmyelinated nerve fibers traversing both the cortex and medullary region was intense (Fig. 2d). CGA was found at E17 to be expressed by a few intra-adrenal cells, but these cells were not necessarily those that were most intensely stained for SNAP-25 (data not shown). At E19, CGA was detectable in more, but not all, chromoblasts; intensity varied between cells and no strict correlation existed between relative labeling intensity of CGA-positive and SNAP-25-positive cells, although labeling intensity was often greater in chromoblasts that were less well stained for SNAP-25 (Figs. 2g,h). At birth and during the first postnatal week (Fig. 3), immunofluorescence for SNAP-25 appeared to increase in both PNMT-positive and PNMT-negative cells. At the end of the first week, SNAP-25 labeling, as at earlier stages, remained less intense in A compared with NA cells (Figs. 3e,f), a situation characteristic of the adult gland (Kannan et al., 1996). During this period, both the number and the immunofluorescence intensity of CGA-positive cells increased, although not all cells appeared to be well stained (Fig. 3g). CGA was found in both A and NA cells, but in general at lower levels in NA cells. In young animals (P30 and P60), the differential intensity of SNAP-25 labeling found between catecholamine phenotypes at earlier stages was maintained (Figs. 4c,d). No consistent differences in TH labeling staining between cell groups were noted at these ages. CGA labeling extended to all chromaffin cells, although variations in labeling intensity between cell groups were noted.
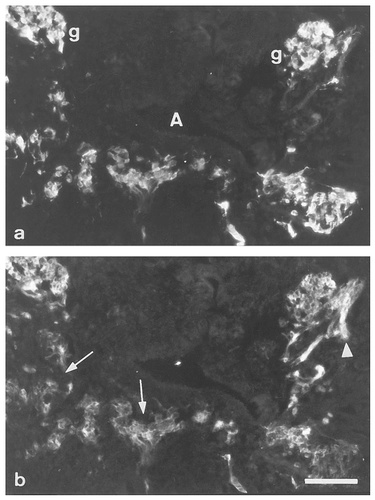
Double-immunolabeled transverse sections of E14 rat embryos. Immunoreactivity for TH (a) and SNAP-25 (b) of sympathetic ganglia (g) and migrating SA cells (arrows) in para-aortic regions. Nerve fibers (arrowhead) are also intensely labeled for SNAP-25. A, aorta. Scale bar = 100 μm.
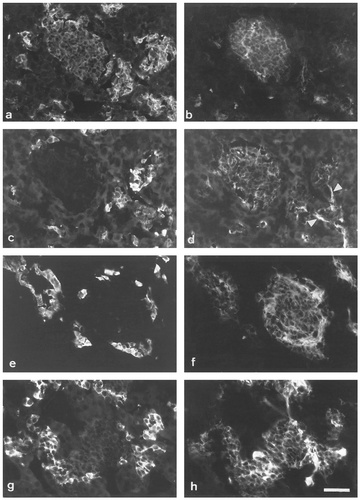
Double immunofluorescent labeling of adrenal glands of E17 (a–d) and E19 (e–h) rat embryos: TH (a), PNMT (c,e), CGA (g), and SNAP-25 (b,d,f,h). Micrographs (a,b; c,d) are from adjacent sections. Both large and small cell aggregates are present: cells in small groups are strongly TH and PNMT-positive; cells in the larger aggregates are PNMT-negative and more weakly labeled for TH. At both ages, the larger aggregates are clearly labeled for SNAP-25, whereas labeling of smaller groups is barely detectable. Note that not all cells strongly labeled for SNAP-25 are detectably CGA-positive (g,h). Arrowheads, nerve fibers (d). Scale bar = 50 μm.
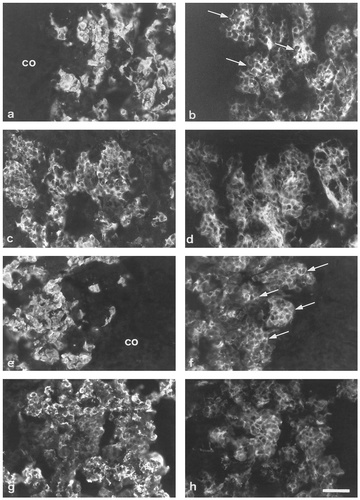
Double immunofluorescent labeling of adrenal glands at birth (a–d) and P7 (e–h): PNMT (a,e), CGA (c,g), and SNAP-25 (b,d,f,h). Note that cell groups (arrows) more strongly labeled for SNAP-25 are PNMT-negative (a,b; e,f). No clear-cut correlation exists between intensity of SNAP-25 and CGA labeling. co, cortex. Scale bar = 50 μm.
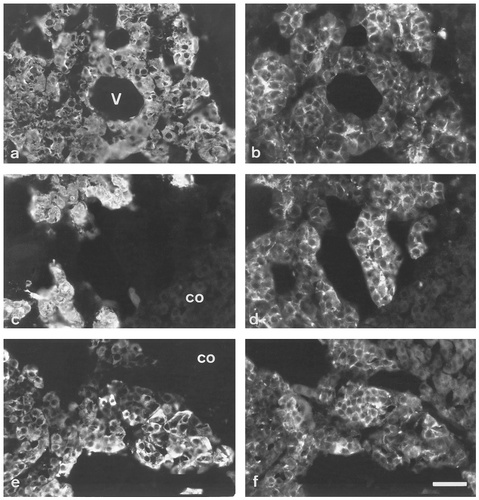
Rat adrenal gland at P30 double immunolabeled for TH (a), PNMT (c), CGA (e), and SNAP-25 (b,d,f). Although variations in intensity of TH labeling are apparent, no correlation exists with the intensity of SNAP-25 labeling (a,b). SNAP-25 labeling of PNMT-positive cells (c,d) appears more intense than at earlier ages and differences in intensity of CGA labeling are still evident at this age. V, blood vessel; co, cortex. Scale bar = 50 μm
Western blotting
Immunoblots were performed on adrenal gland extracts from animals between E16 and P60. Actin was used as internal standard, the level of which did not appear to vary significantly during development from E16 to P60, as judged from immunoblots for which identical amounts of protein extracts at different ages were analyzed (Fig. 5). TH levels were also fairly constant over the same period except during the first postnatal week, when they appeared to be slightly higher (Fig. 5). In contrast, PNMT protein levels were not detected under the conditions used here at E16, but they increased significantly between E19 and P2, after which they appeared to remain constant. The secretory granule protein CGA was below detectable levels until P2, but it then increased sharply and subsequently remained fairly constant.
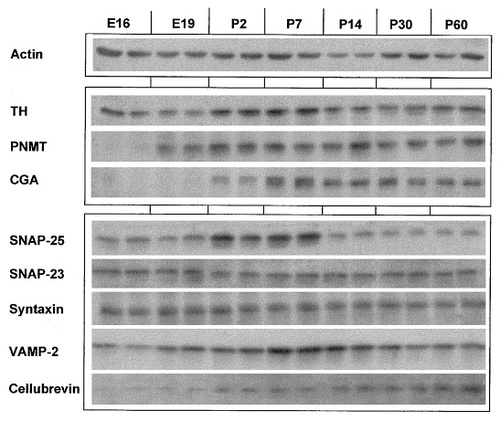
Western blot of rat adrenal gland protein extracts from E16 to P30. Two different samples were run side by side for each age. Actin levels remain constant between E16 and P60. TH levels are apparently higher during the first postnatal week and then remain constant. PNMT is barely detectable at E16, but then increases to adult levels by P2. SNAP-25 increases slightly between E16 and E19 and then considerably to reach a maximum at P2–P7, after which it decreases rapidly to adult levels. SNAP-23 and syntaxin remain relatively constant throughout development. CGA and cellubrevin are expressed at low or undetectable levels until P2, after which they remain fairly constant.
SNAP-25 expression showed three phases: the protein was detectable at low levels between E16 and E19. Expression then increased rapidly (2.7-fold) to reach a maximum by P2–P7, before declining dramatically by P14, when levels were similar to those typical of the adult gland. Levels in the adult were approximately one-half those in prenatal glands. In contrast, expression of the nonneuronal analogue protein SNAP-23 showed quite a different developmental pattern: its levels did not vary significantly throughout development and embryonic levels were comparable to those found in the adult gland. The neuronal membrane-associated t-SNARE, syntaxin 1, although it associates with both SNAP-25 and synaptobrevin/VAMP to form a complex involved in secretion (Oho et al., 1995), surprisingly did not follow the same developmental pattern as SNAP-25. Levels of this protein, like those of SNAP-23, remained fairly constant throughout adrenal gland development and postnatal life. The synaptic vesicle-associated v-SNARE, VAMP-2, increased gradually from E16 to reach maximal levels during the first two postnatal weeks; subsequently a slight decrease was noted. The nonneuronal vesicle-associated SNARE analogue, cellubrevin, increased from barely detectable levels between E16 and E19 until the end of the second postnatal week, after which levels appeared to stabilize at adult values. No notable changes between P14 and P60 were observed for any of the proteins investigated.
RT-PCR
As found for the protein, levels of actin mRNA were also relatively constant throughout the period studied, as judged from ethidium bromide-stained agarose gels of PCR products amplified from identical amounts of adrenal RNA extracts (Fig. 6). The PCR-amplified product for the general SA cell marker, TH, however, showed a peak at around P2. Levels had decreased by P14, as did the TH protein. The PNMT PCR product, reflecting the expression of the A marker, increased slightly between E19 and P2, but then did not vary significantly. In contrast, the SNAP-25 message appeared to increase considerably between E19 and P2 before declining to close to adult levels by P14, which were similar to those found in the embryonic gland at E19. Southern blot semiquantitative analysis of SNAP-25 products (Fig. 7) permitted an estimation of relative amounts of the SNAP-25 mRNA at different ages. This showed an approximately threefold increase in the SNAP-25 message between E19 and P2 and that expression was similar in late embryonic and adult glands. In addition, using primers capable of distinguishing between the two isoforms (Grant et al., 1999), no change in the ratio of SNAP-25a to SNAP-25b was noted between E19 and P60 (data not shown). SNAP-25a was always the predominant form. The profile for SNAP-23 mRNA, in contrast to the SNAP-25 analogue, remained constant from E19 to adulthood (Fig. 6). Thus, both SNAP-25 and SNAP-23 mRNA levels appear to follow a similar profile as their corresponding proteins.
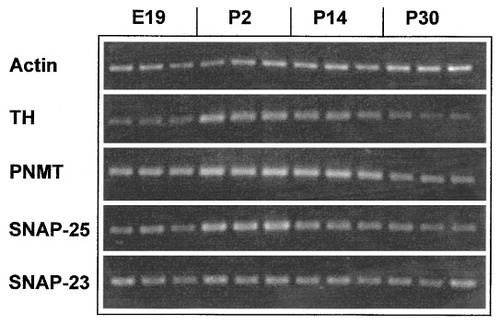
PCR amplification products from rat adrenal extracts at different ages for actin, TH, PNMT, SNAP-25, SNAP-23. Both the actin and SNAP-23 signals remain constant throughout development. Both TH and SNAP-25 peak at P2. PNMT increases between E19 and P2 and then stays constant.
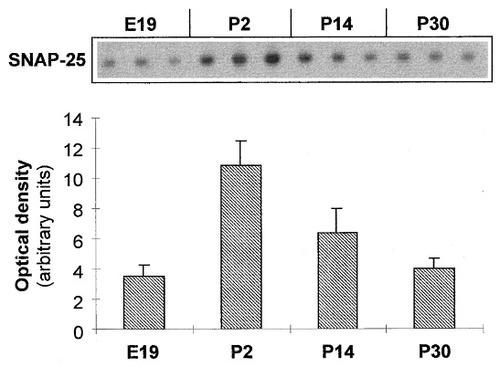
Southern blot (upper) and quantitative estimation (lower) of SNAP-25 PCR product amplified from rat adrenal glands at different ages. Densities are expressed as means ± standard deviations for three independant extractions.
DISCUSSION
This study shows that expression of SNAP-25 in the adrenal gland is precocious and that it is regulated during development. Unlike other SNAREs, an unexpected transient increase in SNAP-25 is observed during the first postnatal week. The low overall level at the outset of adrenal medullary histogenesis correlates with the small intra-adrenal population of chromaffin cells between E14 and E16. However, although the number of intra-adrenal chromoblasts triples between E13.5 and E18.5 (Deimling et al., 1998), and the majority of adult chromaffin cells is already present by birth (Coupland and Tomlinson, 1989), the level of SNAP-25 protein in the adrenal medulla does not increase commensurately, even though cellular labeling intensity does increase slightly over the same period. The high SNAP-25 expression between P2 and P7 thus correlates with a period of relatively low cell proliferation that, as shown by 5-bromodeoxyuridine incorporation, is still relatively high in the medulla at E18, but is low after birth (Coupland and Tomlinson, 1989; Mitani et al., 1999). The low overall embryonic adrenal SNAP-25 levels therefore reflect the relatively low expression in the majority of the immature intra-adrenal chromoblast population during the proliferative phase. The subsequent very significant increase in this molecule immediately after birth accompanies the early stages of functional maturation, when cells are increasing in size (Coupland and Tomlinson, 1989).
Two functional roles, both of which involve vesicle fusion with the plasma membrane, have been proposed for SNAP-25: one is implicated in the regulated exocytosis of neurotransmitters from synaptic vesicles or secretory granules (Roth and Burgoyne, 1994; Bark and Wilson, 1994a; Sudhof, 1995; Calakos and Scheller, 1996), the second is associated with increases in membrane surface area produced by plasmalemmal precursor vesicle fusion with the plasma membrane (Osen-Sand et al., 1993, 1996; Catsicas et al., 1994; Rothman, 1994; Igarashi et al., 1997; West Greenlee et al., 1998). This second constitutive role could be of particular importance during tissue development. A marked upregulation of SNAP-25 protein expression in the developing brain is well documented, with a shift in localization from cell bodies to cell extensions and presynaptic terminals (Oyler et al., 1991, 1992; Duc and Catsicas, 1995). Also, strong evidence points to a function in cell elongation, neurite extension, synaptogenesis, and growth cone expansion (Oyler et al., 1989; Osen-Sand et al., 1993, 1996; Soriano et al., 1994; Boschert et al., 1996; Goutan et al., 1999; Morihara et al., 1999). Thus, some similarities exist between the adrenal gland and brain. However, although SNAP-25 expression increases in both tissues during a phase of cell maturation, adrenal levels decrease significantly within 2 weeks after birth.
In contrast, although brain levels are always much higher than in the adrenal (Grant et al., 1999), they only decrease gradually with aging (Bark et al., 1995; Shimohama et al., 1998). In addition, brain development is accompanied by a switch from SNAP-25a to SNAP-25b (Boschert et al., 1996; Aguado et al., 1999), whereas SNAP-25a predominates throughout rat adrenal development. Such data suggest isoform-specific functions for SNAP-25, supporting a role for SNAP-25a in neural plasticity in the brain (Bark et al., 1995; Boschert et al., 1996; Jacobsson et al., 1996) and a neurotransmission-related role for SNAP-25b specific to classical synaptic vesicles (Bark and Wilson, 1994a; Langley and Grant, 1997). The timing of the high SNAP-25 expression in the adrenal gland during the cellular growth and maturation phase just after birth fits in with the view that the SNAP-25a isoform is implicated in morphological plasticity at this time, i.e., it is involved in the constitutive membrane expansion of chromoblasts rather than in regulated secretory events. The first postnatal week represents a critical period for chromaffin cells. Not only is this the time during which they become capable of coordinated secretory activity (see below), this is also the period during which they are considered to possess greater phenotypic plasticity (Coupland and Tomlinson, 1989); they subsequently become less responsive to trophic factors such as nerve growth factor (NGF). However, it is not clear why the SNAP-25a isoform persists in the rat adrenal. In other species, higher levels of SNAP-25b are found in the adult (Grant et al., 1999). Its persistence could reflect either a difference in exocytotic mechanisms in endocrine tissues (Langley and Grant, 1995) or a greater potential of rat chromaffin cells for phenotypic plasticity.
Transient expression of SNAP-25 has also been reported during retinal development. It is relatively strongly expressed in cholinergic amacrine cells only during a distinct period prior to eye-opening in both the Brazilian opossum and the rat, after which cell body labeling diminishes (West Greenlee et al., 1998). It has been proposed that SNAP-25 plays a role in the establisment of appropriate retinal circuitry during a critical period of visual system development. This molecule may thus perform specific roles at defined stages in the ontogenesis of various tissues.
The fact that other SNAREs studied here, i.e. syntaxin1, VAMP-2, cellubrevin, and SNAP-23, do not follow the same developmental profile in the adrenal gland accords with what has been found for other tissues. For example, the developmental expression profile of syntaxin 1 in the hippocampus differs from that of SNAP-25 (Shimohama et al., 1998). This suggests that they are not involved in identical processes or they play other roles at the same time, a conclusion that has also been drawn for SNAP-25 and syntaxin involvement in growth cone extension (Morihara et al., 1999). In addition, other data indicate that whereas SNAP-25 is involved in axonal growth, synaptobrevin/VAMP is not (Grosse et al., 1999). In agreement with this conclusion, we have found that although cellubrevin is confined to medullary cells (unpublished data), its expression is delayed compared to that of SNAP-25. SNAP-23 differs from these SNAREs by its expression both in cortical and chromaffin cells (Grant et al., 1999). This molecule is therefore likely to be involved in different processes than SNAP-25.
Because the dramatic upregulation of SNAP-25 occurs immediately after birth, a birth-related event, possibly involving a massive release of humoral factors (Lagercrantz and Bistoletti, 1973), cannot be excluded as a potential trigger. This maximal expression of SNAP-25 also occurs a few days before the onset of coordinated secretory activity of the adrenal gland (Seidler and Slotkin, 1985; Holgert et al., 1994). However, the time courses for SNAP-25, syntaxin 1, and SNAP-23 expression differ from those of total catecholamines (Verhofstad et al., 1989) and other granule constituents such as CGA (Kent and Coupland, 1989). In contrast, the timing of cellubrevin and VAMP-2 expression is more closely correlated with granule maturation, as reflected by expression of the secretory granule protein CGA. It thus appears that the molecules involved in the secretory process are in place well before granule maturation is finally completed and before splanchnic innervation becomes functional (Seidler and Slotkin, 1985). The relatively high level of expression of SNAP-25 up until the end of the first postnatal week and its subsequent abrupt decrease to adult levels when secretory activity commences lend further support to the idea that it is involved in processes other than regulated secretion during this period.
The present study also shows that SNAP-25 appears to be expressed more strongly by ganglionic neurons, migrating cells, and immature extra-adrenal chromaffin cells of the blastema, than by intra-adrenal chromaffin cells at early stages. The reason for this is uncertain. It is more likely to reflect the influence of environment, particularly when cells migrate into the adrenal primordium, rather than the functional maturity of the cells. In addition, the more intense SNAP-25 labeling in NA cells, already evident at E17, could also be due to local factors that appear to both downregulate cellular levels and regulate the molecule differently in the two phenotypes. Several factors have been shown to up or downregulate adrenal gland proteins including SNAP-25 (see Langley and Grant, 1999, for review): growth factors (Takei et al., 1997; Feng et al., 1999), depolarization (Sepulveda et al., 1998), and steroids (Jacobsson et al., 1998). The embryonic expression of SNAP-25 in cells migrating into the cortical anlage might be affected by the very high levels of glucocorticoids secreted after E17 by cortical cells (Seidl and Unsicker, 1989). Moreover, previous reports have demonstrated that glucocorticoids exert a regulatory action on the chromaffin cell expression of certain neuronal markers (Vogel and Weston, 1990) including L1, GAP-43 (Federoff et al., 1988; Grant et al., 1996), two proteins that are also differentially expressed between the chromaffin cell phenotypes (Leon et al., 1992; Grant et al., 1994), and SCG10 (Federoff et al., 1988; Stein et al., 1988). The current data suggest that glucocorticoids might participate in the early regulation of SNAP-25 in chromaffin cells of the adrenal gland. Further studies are necessary to confirm this.
Acknowledgements
We thank Dr. L. Denoroy and T.Galli for their generous gifts of antibodies, and Ms. S.Wallerich for valuable technical assistance.




