Morphologic fate of diencephalic prosomeres and their subdivisions revealed by mapping cadherin expression
Abstract
The expression of four cadherins (cadherin-6B, cadherin-7, R-cadherin, and N-cadherin) was mapped in the diencephalon of chicken embryos at 11 days and 15 days of incubation and was compared with Nissl stains and radial glial topology. Results showed that each cadherin is expressed in a restricted manner by a different set of embryonic divisions, brain nuclei, and their subregions. An analysis of the segmental organization based on the prosomeric model indicated that, in the mature diencephalon, each prosomere persists and forms a coherent domain of gray matter extending across the entire transverse dimension of the neural tube, from the ventricular surface to the pial surface. Moreover, the results suggest the presence of a novel set of secondary subdivisions for the dorsal thalamus (dorsal, intermediate, and ventral tiers and anteroventral subregion). They also confirm the presence of secondary subdivisions in the pretectum (commissural, juxtacommissural, and precommissural). At most of the borders between the prosomeres and their secondary subdivisions, changes in radial glial fiber density were observed. The diencephalic brain nuclei that derive from each of the subdivisions were determined. In addition, a number of previously less well-characterized gray matter regions of the diencephalon were defined in more detail based on the mapping of cadherin expression. The results demonstrate in detail how the divisions of the early embryonic diencephalon persist and transform into mature gray matter architecture during brain morphogenesis, and they support the hypothesis that cadherins play a role in this process by providing a framework of potentially adhesive specificities. J. Comp. Neurol. 421:481–514, 2000. © 2000 Wiley-Liss, Inc.
During early embryonic development, the vertebrate brain is divided into a number of histogenetic fields in which distinct sets of neurons and glial cells are generated and differentiate. These embryonic fields are arranged adjacent to one another and divide the neural tube into longitudinal regions and transverse regions. The spatial arrangement of the embryonic divisions, in general, is similar among all vertebrate species and, thus, represents a general scheme for vertebrate brain development (Puelles, 1995).
Each embryonic division can be characterized early on by the expression of specific gene regulatory proteins (for reviews, see Simeone et al., 1992; Krumlauf et al., 1993; Parr et al., 1993; Puelles and Rubenstein, 1993; Stoykova and Gruss, 1994), morphogenetic molecules (Gänzler and Redies, 1995; for reviews, see Redies and Takeichi, 1996; Redies, 2000), or other molecular and biochemical markers (e.g., acetylcholine esterase, calcium-binding proteins; Puelles et al., 1987; De Castro et al., 1998). The borders between individual divisions often show morphologic features, such as indentations, ridges, or changes in histologic appearance (for reviews, see Lumsden and Keynes, 1989; Puelles and Rubenstein, 1993). Moreover, the molecular characteristics and density of the radial glial processes have been shown to change at some of the borders (Geisert and Bidanset, 1993; Silver et al., 1993; Steindler, 1993; Gänzler and Redies, 1995; Heyman et al., 1995; Stoykova et al., 1997; Götz et al., 1998).
In each embryonic division, neurons are born and migrate to the mantle layer, where they aggregate into brain nuclei or layers (Bergquist, 1932; Sauer, 1935; Bergquist and Källén, 1954). In this way, the gray matter of the mature brain is formed gradually. The origin of most gray matter structures of the mature brain can be followed during brain morphogenesis and can be assigned to specific embryonic divisions. The large majority of neurons settle and differentiate in the embryonic division where they are born (Rendahl, 1924; Puelles et al., 1987; Fraser et al., 1990; Figdor and Stern, 1993; Marin and Puelles, 1995; Wingate and Lumsden, 1996), although there are a few examples for migration of early neurons across divisional boundaries (Harkmark, 1954; Tan and Le Douarin, 1991; Birgbauer and Fraser, 1994; Anderson et al., 1997).
It has been shown previously that, from early stages of brain development, members of the cadherin family of adhesion molecules are expressed differentially by some of the early embryonic divisions of the diencephalic alar plate of the chicken (Gänzler and Redies, 1995; Fushimi et al., 1997; Yoon et al., 2000). Similar expression patterns also have been found in the embryonic mouse brain. A particularly striking feature is the abrupt change in cadherin expression by neuroepithelial cells and radial glia at the divisional boundaries. These potentially adhesive changes were proposed to help maintain the segmental structure of the neural tube (for reviews, see Redies, 1995; Redies and Takeichi, 1996). A restricted expression of cadherins also is seen in the early anlagen of gray matter structures developing within the embryonic divisions (Gänzler and Redies, 1995; Inoue et al., 1997; Korematsu and Redies, 1997; Yoon et al., 2000). In the current study, the expression of four cadherins [cadherin-6B (cad6B), cadherin-7 (cad7), R-cadherin (Rcad), and N-cadherin (Ncad)] is mapped in detail at intermediate stages of diencephalic development. By comparing immunostaining results to histologic features and radial glial density, we followed the development of individual embryonic divisions (for earlier stages, see the accompanying paper: Yoon et al., 2000) and determined which gray matter structures derive from each of them.
Our analysis is based on the prosomeric model of the vertebrate forebrain developed by Puelles, Rubenstein, and coworkers (Bulfone et al., 1993; Puelles and Rubenstein, 1993; Puelles, 1995; Rubenstein et al., 1998). In addition, reference also is made to earlier work on the development of the chicken diencephalon (Rendahl, 1924; Kuhlenbeck, 1937, Kuhlenbeck, 1939; Martínez-de-la-Torre et al., 1990; Martínez et al., 1991; Puelles et al., 1991). In the prosomeric model, the vertebrate forebrain is divided into six neuromeres, termed prosomeres 1–6 (p1–p6). A schematic diagram of the prosomeric model in the chicken brain is shown in Figure 1. The conventional diencephalon comprises parts of all six prosomeres, whereas the telencephalon comprises only extreme dorsal regions of p4–p6 (Fig. 1). The prosomeres, their abbreviations, and alternative names that have been used in the literature are listed in Table 1.
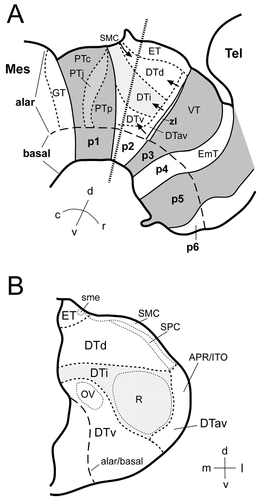
A: Schematic diagram of the prosomeric model applied to the embryonic chicken brain. The diencephalon is composed of prosomeres 1–3 (p1–p3) and the basal domains of p4–p6. The pretectum (p1), the ventral thalamus (p3), and p5 are shaded in gray. Additional (secondary) subdivisions are proposed for the pretectum and the dorsal thalamus (for discussion, see text). Light gray areas represent specific subdivisions within the pretectum (PTj) and the dorsal thalamus (DTi). The thick dotted line through p2 indicates the sectioning level for the schematic diagram shown in B. B: Schematic diagram of secondary subdivisions in the dorsal thalamus (p2) in the transverse plane (indicated by thick dotted line in A). The light gray area represents the intermediate tier (DTi). Some nuclear boundaries are indicated by thin dotted lines. For abbreviations, see Table 1 and list.
|
Name |
Abbreviation1 |
Prosomere2 |
Terminology of Rendahl (1924) |
|---|---|---|---|
|
Pretectum |
PT |
p1 (alar) |
Synencephalon (alar) |
|
Commissural |
PTc |
— |
— |
|
Juxtacommissural |
PTj |
— |
— |
|
Precommissural |
PTp |
— |
— |
|
Dorsal thamalus |
DT |
p2 (alar) |
Posterior prosencephalon (alar) |
|
Dorsal tier |
DTd |
— |
— |
|
Intermediate tier |
DTi |
— |
— |
|
Ventral tier |
DTv |
— |
— |
|
Anteroventral subdivision |
DTav |
— |
— |
|
Ventral thalamus |
VT |
p3 (alar) |
Anterior prosencephalon (alar) |
|
Hypothalamus |
— |
p4–p6 (basal) |
— |
- 1 Abbreviation used in the current study.
- 2 After the model by Puelles and Rubenstein (1993). p, Prosomere.
The prosomeres form complete transverse segments that are divided further into longitudinal subdivisions (e.g., alar and basal plates); these are not necessarily homogeneous internally and usually show further subdivisions, particularly in the alar plate. Indeed, our results support the existence of additional (secondary) subdivisions within the pretectum (alar p1; Table 1), as proposed before for avian and other species (Rendahl, 1924; Martínez-de-la-Torre, 1985; Caballero-Bleda et al., 1992; Figdor and Stern, 1993; Redies et al., 1997; De Castro et al., 1998; Pombal and Puelles, 1999). We also found a set of secondary subdivisions in the dorsal thalamus (alar p2; Table 1). These subdivisions are defined in detail. The structures of gray matter deriving from all (primary and secondary) diencephalic (sub)divisions are determined. In addition, a number of regions of gray matter in the chicken diencephalon that previously were defined insufficiently are characterized in greater detail according to their cadherin expression profile and histologic appearance. The analysis presented here is restricted to the diencephalic gray matter. A description of the fiber tracts expressing the four cadherins will be published elsewhere (L. Medina, L. Puelles, and C. Redies, unpublished observations).
Abbreviations
-
- AB
-
anterobasal nucleus
-
- AChE
-
acetylcholine esterase
-
- ADSV
-
anterior nucleus of the ventral supraoptic decussation
-
- al
-
ansa lenticularis
-
- ALA
-
anterior nucleus of the ansa lenticularis
-
- alar/basal
-
boundary between alar plate and basal plate
-
- AM
-
anteromedial preoptic nucleus
-
- AP
-
area pretectalis
-
- APd
-
area pretectalis diffusa
-
- APR
-
perirotundic area
-
- AT
-
area triangularis
-
- bor
-
basal optic root
-
- BOR
-
nucleus of the basal optic root
-
- BSTa
-
bed nucleus of the stria terminalis, anterior part
-
- BSTp
-
bed nucleus of the stria terminalis, posterior part
-
- c
-
caudal
-
- ca
-
anterior commissure
-
- cad6B
-
cadherin-6B
-
- cad7
-
cadherin-7
-
- cgt
-
commissure of the griseum centrale
-
- CP
-
nucleus of the posterior commissure
-
- cp
-
posterior commissure
-
- cpa
-
pallial commissure
-
- CT
-
nucleus of the tectal commissure
-
- ct
-
tectal commissure
-
- d
-
dorsal
-
- DA
-
dorsal anterior nucleus
-
- DC
-
dorsal complex
-
- DF
-
nucleus dorsofrontalis
-
- DIP
-
dorsointermediate posterior nucleus
-
- DIVA
-
dorsal intermediate ventral anterior nucleus
-
- DLA
-
dorsolateral anterior nucleus
-
- DLL
-
dorsolateral lateral nucleus
-
- DLM
-
dorsolateral medial nucleus
-
- DLP
-
dorsolateral posterior nucleus
-
- DM
-
dorsomedial hypothalamic nucleus
-
- DMA
-
dorsomedial anterior nucleus
-
- DMP
-
dorsomedial posterior nucleus
-
- DPo
-
dorsal posterior nucleus
-
- Dr
-
nucleus of Darkschewitsch, rostral portion
-
- dsd
-
dorsal supraoptic decussation
-
- dss
-
dorsal suprapallial sulcus
-
- dsv
-
ventral supraoptic decussation
-
- DT
-
dorsal thalamus
-
- DTav
-
dorsal thalamus, anteroventral subdivision
-
- DTd
-
dorsal thalamus, dorsal subdivision
-
- DTi
-
dorsal thalamus, intermediate subdivision
-
- DTv
-
dorsal thalamus, ventral subdivision
-
- EmT
-
eminentia thalami
-
- ET
-
epithalamus
-
- fr
-
retroflex fascicle
-
- GT
-
griseum tectale
-
- GV
-
ventral geniculate nucleus
-
- HL
-
lateral habenula
-
- HM
-
medial habenula
-
- HMd
-
medial habenula, dorsal portion
-
- HMv
-
medial habenula, ventral portion
-
- IC
-
interstitial nucleus of Cajal
-
- ICr
-
interstitial nucleus of Cajal, rostral portion
-
- ict
-
isthmo-commissural tract
-
- ICT
-
nucleus intercalatus
-
- IH
-
inferior hypothalamic nucleus
-
- IM
-
intermediomedial nucleus
-
- IN
-
infundibular nucleus of hypothalamus
-
- io
-
isthmooptic tract
-
- IP
-
intermedioposterior nucleus
-
- IPl
-
intermedioposterior nucleus, lateral part
-
- IPm
-
intermedioposterior nucleus, medial part
-
- IPS
-
interstitial nucleus of the pretectosubpretectal tract
-
- ITO
-
prospective interstitial nucleus of the optic tract
-
- JCP
-
juxtacommissural plate
-
- l
-
lateral
-
- LA
-
lateroanterior nucleus
-
- lfb
-
lateral forebrain bundle
-
- LH
-
lateral hypothalamic region
-
- lmd
-
lamina medullaris dorsalis
-
- LPA
-
lateral preoptic area
-
- LSO
-
lateral septal organ
-
- lv
-
lateral ventricle
-
- m
-
medial
-
- MA
-
mamillary area
-
- ME
-
median eminence
-
- mes
-
mesencephalon
-
- ML
-
lateral mamillary nucleus
-
- Ncad
-
N-cadherin
-
- NH stalk
-
stalk of neurohypophysis
-
- NH
-
neurohypophysis
-
- oh
-
olfactohabenular tract
-
- OM
-
nucleus of the occipitomesencephalic tract
-
- om
-
occipitomesencephalic tract
-
- ot
-
optic tract
-
- OV
-
nucleus ovoidalis
-
- p1–p6
-
prosomeres 1–6
-
- Pa
-
paraphysis
-
- pch
-
choroid plexus
-
- PDSV
-
posterior nucleus of the ventral supraoptic decussation
-
- PE
-
external pretectal nucleus
-
- PH
-
periventricular hypothalamic nucleus
-
- Pi
-
pineal gland (epiphysis)
-
- PI
-
posterointermediate nucleus
-
- PM
-
nucleus paramedianus internus
-
- Po
-
posterior nucleus
-
- POM
-
medial (main) portion of preoptic nucleus
-
- POMl
-
lateral portion of preoptic nucleus
-
- POV
-
nucleus periovoidalis
-
- PPC
-
principal precommissural nucleus
-
- PPT
-
principal pretectal nucleus
-
- PT
-
pretectum
-
- PTc
-
pretectum, commissural subdivision
-
- PTj
-
pretectum, juxtacommissural subdivision
-
- PTM
-
medial pretectal nucleus
-
- PTp
-
pretectum, precommissural subdivision
-
- PV
-
posteroventral nucleus
-
- PVN
-
paraventricular nucleus
-
- PVO
-
periventricular hypothalamic organ
-
- R
-
nucleus rotundus and subdivisions
-
- r
-
rostral
-
- R5
-
immunostain for radial glia
-
- Ral
-
nucleus rotundus, anterolateral portion
-
- Ram
-
nucleus rotundus, anteromedial portion
-
- Rcad
-
R-cadherin
-
- Ri
-
nucleus rotundus, intermediate portion
-
- RM
-
retromamillary region of hypothalamus
-
- ROV
-
retroovoidal area
-
- Rp
-
nucleus rotundus, posterior portion
-
- Rpf
-
nucleus rotundus, parafascicular portion
-
- Rpl
-
nucleus rotundus, posterolateral portion
-
- Rpm
-
nucleus rotundus, posteromedial portion
-
- RR
-
retrorotundic area
-
- RS
-
superior reticular nucleus
-
- RSd
-
superior reticular nucleus, dorsal portion
-
- RSv
-
superior reticular nucleus, ventral portion
-
- Rvl
-
nucleus rotundus, ventrolateral portion
-
- SCE
-
stratum cellulare externum
-
- SCI
-
stratum cellulare internum
-
- SCNl
-
lateral suprachiasmatic nucleus
-
- SCNm
-
medial suprachiasmatic nucleus
-
- SH
-
subhabenular region
-
- SHl
-
subhabenular region, lateral portion
-
- SHm
-
subhabenular region, medial portion
-
- SL
-
lateral septum
-
- SM
-
medial septum
-
- SMC
-
microcellular superficial nucleus
-
- sme
-
stria medullaris
-
- SP
-
subpretectal nucleus
-
- SPC
-
parvocellular superficial nucleus
-
- SpL
-
lateral spiriform nucleus
-
- SpM
-
medial spiriform nucleus
-
- SpMcl
-
medial spiriform nucleus, caudolateral subdivision
-
- SpMrm
-
medial spiriform nucleus, rostromedial subdivision
-
- SPO
-
nucleus semilunaris periovoidalis
-
- SRt
-
subrotundic nucleus
-
- SS
-
superficial synencephalic nucleus
-
- STm
-
striatum, medial portion
-
- T
-
triangular nucleus
-
- Tect
-
optic tectum
-
- Tel
-
telencephalon
-
- thio
-
thionine stain
-
- tov
-
tectoovoidal tract
-
- tsm
-
septomesencephalic tract
-
- tt
-
tectothalamic tract
-
- Uva
-
nucleus uvaeformis
-
- v
-
ventral
-
- v3
-
third ventricle
-
- VIA
-
ventrointermediate area
-
- VL
-
ventrolateral nucleus
-
- VM
-
ventromedial hypothalamic nucleus
-
- vss
-
ventral suprapallial sulcus
-
- VT
-
ventral thalamus
-
- VTD
-
dorsal area of ventral thalamus
-
- Z
-
nucleus zeta
-
- ZI
-
zona incerta
-
- zl
-
zona limitans intrathalamica
MATERIAL AND METHODS
The materialsand methods employed are described in the accompanying paper by Yoon et al. (2000), except where noted below.
Animals
Embryos from the following stages (according to Hamburger and Hamilton, 1951) were studied: embryonic day 11 (E11; stage 37), E15 (stage 41), and E19 (stage 45).
Antibodies
In addition to the antibodies against cadherins described in the accompanying paper (Yoon et al., 2000), mouse monoclonal antibody R5 was used to visualize radial glial cells and their processes. This antibody specifically reacts with a vimentin-associated protein (Dräger et al., 1984; Herman et al., 1993; kind gift of U. Dräger).
Histochemistry
Immunostaining procedures are as described in the accompanying paper by Yoon et al. (2000). Brains from chicken embryos at E11, E15, and E19 were fixed for 2–6 hours at 4°C. Several complete series of sections were cut through entire brains (E11: three transverse series, one horizontal series, and one sagittal series; E15: one transverse series, one horizontal series, and one sagittal series; E19: one transverse series and one sagittal series) and were stained with antibodies. For each antibody, sections within the set of immunostains were spaced 100–140 μm apart.
For embryos at E15 and E19, one of the parallel section series was reacted for acetylcholine esterase (AChE) activity, which was visualized with a modified Karnovsky-Roots technique, as described in detail previously (Gänzler and Redies, 1995). This material provided additional evidence for the identification of gray matter structures.
All stains were visualized with Axiophot or Ultraphot microscopes (Zeiss, Oberkochen, Germany). Photographic images of selected sections were processed as described elsewhere (Yoon et al., 2000). To display the staining results for two or three cadherins simultaneously (Fig. 11), photographic images were enhanced in contrast, color coded, and superimposed by using the Photoshop software program (Adobe Systems, Mountain View, CA).
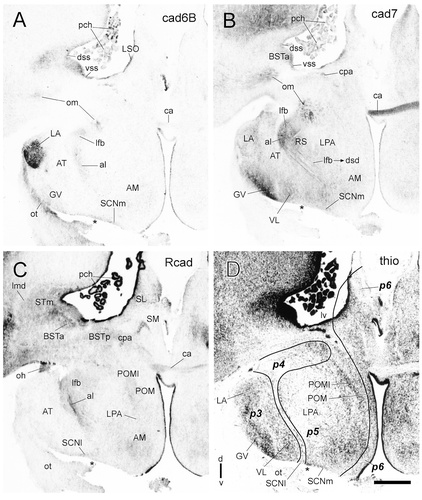
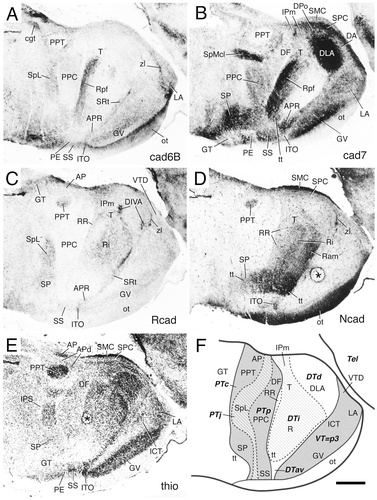
Cadherin expression in adjacent transverse sections through the diencephalon of the embryonic day 11 chicken at the level of the lateroanterior nucleus. Sections were immunostained with antibodies against cadherin-6B (cad6B; A), cadherin-7 (cad7; B), and R-cadherin (Rcad; C). Thionine (thio) staining of an adjacent section is shown in D, in which the divisional borders are represented by solid lines. Asterisks indicate cutting artifacts. For abbreviations, see list. Scale bar = 500 μm.
Cadherin expression in adjacent transverse sections through the diencephalon of the embryonic day 11 chicken at the level of the nucleus dorsolateralis anterior. Sections were immunostained with antibodies against cad6B (A), cad7 (B), and Rcad (C). Thionine staining of an adjacent section is shown in D. The asterisk indicates a staining artifact. The inset in C shows the R-cadherin-positive zona limitans cut lengthwise at a slightly more caudal level. A schematic representation of the diencephalic subdivisions for this section is shown in Figure 7A. For abbreviations, see list. Scale bars = 500 μm in D (also applies to A–C); 200 μm in inset.
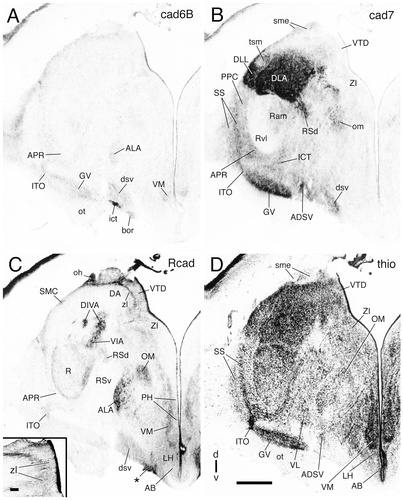
Cadherin expression in adjacent transverse sections through the diencephalon of the embryonic day 11 chicken at the level of the nucleus ovoidalis. Sections were immunostained with antibodies against cad6B (A), cad7 (B), and Rcad (C). Thionine staining of an adjacent section is shown in D. The single asterisk indicates a staining artifact. Double asterisks indicate artifactual staining (false contrast due to high cell density; compare with Fig. 17, which shows enlargements of the areas boxed in D). A schematic representation of the diencephalic subdivisions for this section is shown in Figure 7B. For abbreviations, see list. Scale bar = 500 μm in D (also applies to A–C).
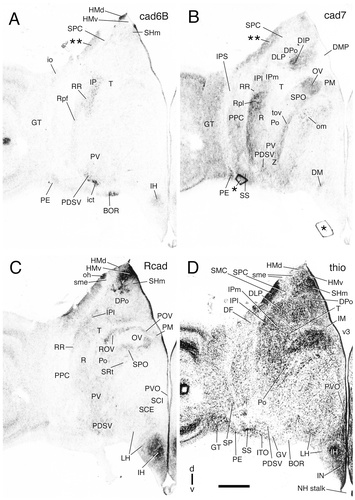
Cadherin expression in a rostral-to-caudal series of transverse sections through the diencephalon of the embryonic day 11 chicken at the level of the posterior nucleus of the ventral supraoptic decussation. Sections were immunostained with antibodies against cad6B (A), cad7 (B), and Rcad (C). Thionine staining of an adjacent section is shown in D. The single asterisks indicate artifacts. Double asterisks indicate artifactual staining (false contrast due to high cell density). A schematic representation of the diencephalic subdivisions for this section is shown in Figure 7C. For abbreviations, see list. Scale bar = 500 μm.
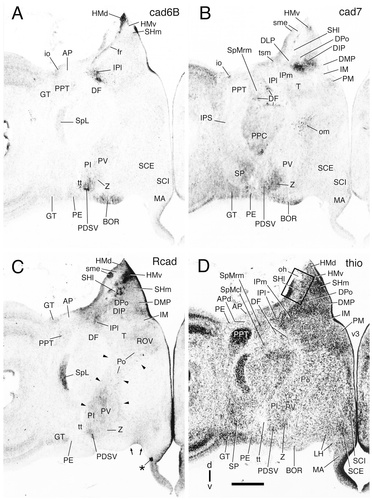
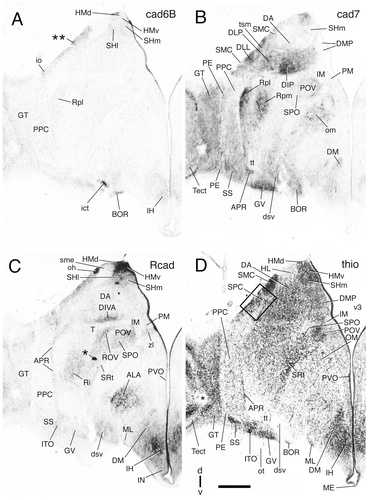
Cadherin expression in adjacent transverse sections through the diencephalon of the embryonic day 11 chicken at the level of the lateral spiriform nucleus. Sections were immunostained with antibodies against cad6B (A), cad7 (B), and Rcad (C). Thionine staining of an adjacent section is shown in D. The single asterisk indicates a staining artifact. An enlargement of the area boxed in D is shown in Fig. 17. In C, arrows indicate R-cadherin-positive fibers reaching the pial surface; the arrowheads point at the caudal and rostral borders of the ventral tier of the dorsal thalamus (DTv). A schematic representation of the diencephalic subdivisions for this section is shown in Figure 7D. For abbreviations, see list. Scale bar = 500 μm.
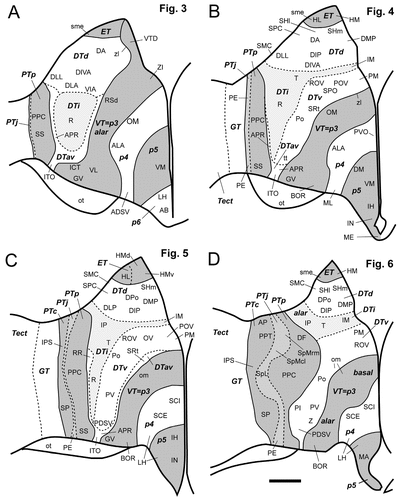
Schematic diagrams of the postulated diencephalic divisions for the sections displayed in Figure 3 (A), Figure 4 (B), Figure 5 (C), and Figure 6 (D). The pretectum, the ventral thalamus, and p5 are shaded in gray, whereas the dorsal thalamus, p4, and p6 have no shading. The solid lines indicate borders between prosomeres, and the dashed lines indicate borders between the secondary subdivision within the pretectum and the dorsal thalamus. The areas shaded in light gray represent the juxtacommissural pretectum (PTj) and the intermediate tier of the dorsal thalamus (DTi), respectively. For other abbreviations, see list. Scale bar = 500 μm.
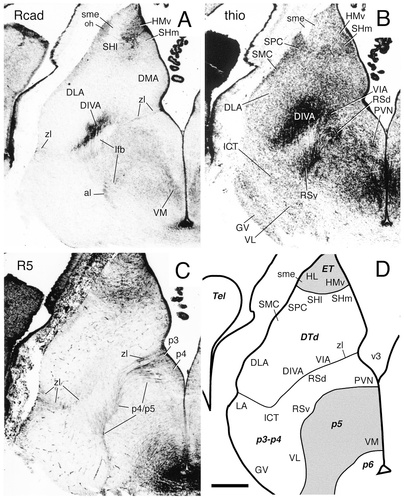
A–D: Cadherin expression and radial glial topology in transverse sections through the diencephalon of the embryonic day 11 chicken at the level of the nucleus dorsolateralis anterior. Adjacent sections were immunostained with antibodies against Rcad (A) and the radial glial marker R5 (C). A thionine stain of a corresponding section is shown in B, and a schematic diagram displaying the postulated diencephalic divisions is shown in D. A color-coded superposition of the immunostaining results for Rcad (A) with results for cad7 is shown in Figure 11A. The different shadings in D represent different postulated divisions, as indicated by the bold abbreviations. For abbreviations, see list. Scale bar = 500 μm.
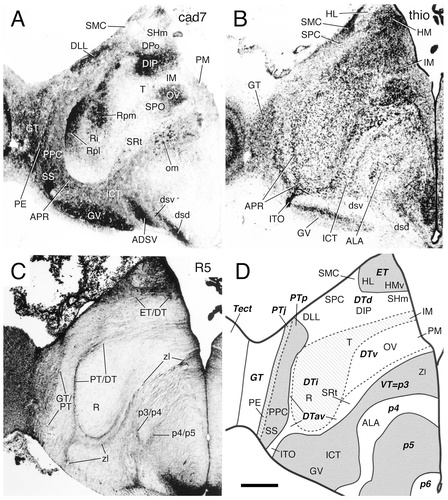
A–D: Cadherin expression and radial glial topology in transverse sections through the diencephalon of the embryonic day 11 chicken at the level of the nucleus rotundus. Adjacent sections were immunostained with antibodies against cad7 (A) and the radial glial marker R5 (C). A thionine stain of a corresponding section is shown in B, and a schematic diagram displaying the postulated diencephalic divisions is shown in D. A color-coded superposition of the immunostaining results for cad7 (A) with results for Rcad and Ncad is shown in Figure 11B. The different shadings in D represent different divisions, as indicated by the bold abbreviations. For abbreviations, see list. Scale bar = 500 μm.
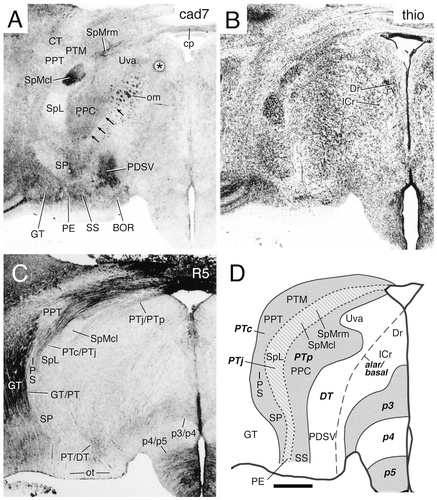
A–D: Cadherin expression and radial glial topology in transverse sections through the diencephalon of the embryonic day 11 chicken at the level of the spiriform nuclei. Adjacent sections were immunostained with antibodies against cad7 (A) and the radial glial marker R5 (C). A thionine stain of a corresponding section is shown in B, and a schematic diagram displaying the postulated diencephalic divisions is shown in D. A color-coded superposition of the immunostaining results for cad7 (A) with results for Ncad is shown in Figure 11C. The different shadings in D represent different divisions, as indicated by the bold abbreviations. The arrows in A indicate the border between the pretectal and dorsal thalamic divisions. The asterisk in A indicates an artifact. For abbreviations, see list. Scale bar = 500 μm.
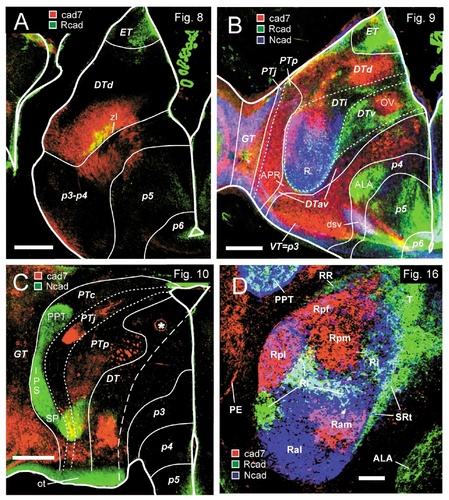
Color-coded overlays of immunostains for various combinations of cadherins. Results displayed in each photomicrograph are the result of the superposition of adjacent sections. A: Results from adjacent transverse sections through the embryonic day 11 diencephalon at the level of the nucleus dorsolateralis anterior. The Rcad data are the same as those shown in Figure 8. B: Results from adjacent transverse sections through the embryonic day 11 diencephalon at the level of the nucleus rotundus. The cad7 data are the same as those shown in Figure 9. C: Results from adjacent transverse sections through the embryonic day 11 diencephalon at the level of the spiriform nuclei. The cadherin-7 data are the same as those shown in Figure 10. D: Results from adjacent transverse sections through the nucleus rotundus region of the embryonic day 15 diencephalon. The cadherin data are the same as those shown in Figure 16B–D. The different colors represent the cadherin immunostaining results, as indicated by the boxes in A–D. Note the partial overlap of cadherin expression indicated by the mixed colors (in A, B, and D, yellow for cad7/Rcad, pink for cad7/Ncad, and turquoise for Rcad/Ncad; in C, yellow for cad7/Ncad). The lines in A–C represent the borders of postulated diencephalic divisions, as indicated by the bold abbreviations (compare with Figs. 8D, 9D, and 10D, respectively). The asterisk in C indicates an artifact. For abbreviations, see list. Scale bars = 500 μm in A–C; 200 μm in D.
Cadherin expression in parasagittal sections through the diencephalon of the embryonic day 11 chicken. Sections were immunostained with antibodies against cad6B (A), cad7 (B), Rcad (C), and Ncad (D). A thionine stain of a corresponding section is shown in E, and a schematic diagram displaying the postulated diencephalic divisions is shown in F. The sections in A, C, D, and E are adjacent, whereas the section in B is from the other side of the same brain at a corresponding parasagittal level. The areas with different shadings in F represent different divisions, as indicated by the bold abbreviations. The asterisks in D and E indicate artifacts. For abbreviations, see list. Scale bar = 500 μm.
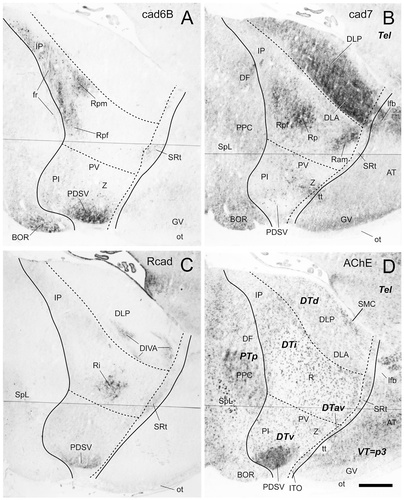
Cadherin expression in parasagittal sections through the diencephalon of the embryonic day 15 chicken. Adjacent sections were immunostained with antibodies against cad6B (A), cad7 (B), and Rcad (C). A histochemical stain for acetylcholine esterase (AChE) of an adjacent section is shown in D. Note that these sections are cut at a plane more medial than those displayed in Figure 12. The lines indicate postulated divisional boundaries, as indicated by bold letters in D. For abbreviations, see list. Scale bar = 500 μm.
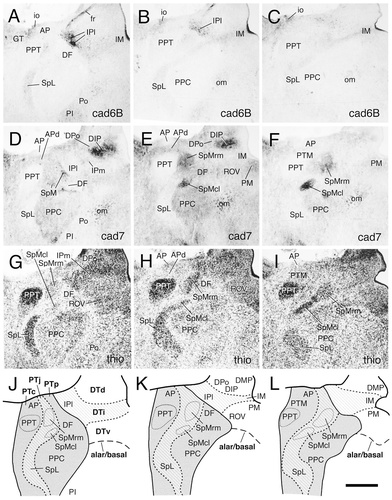
Immunostaining results for cad6B (A–C) and cad7 (D–F) in a series of transverse sections through the dorsal pretectal area of an embryonic day 11 chicken. Thionine stains are shown in G–I. The sections shown in A,D,G, B,E,H and C,F,I represent adjacent sections, respectively. The sequence of the section levels is from rostral to caudal (from left to right). A schematic diagram for each section level is shown in the bottom row (J–L). In these diagrams, the solid lines indicate borders between postulated divisions. The thicker dashed lines indicate borders between the pretectal and dorsal thalamic subdivisions. The thinner dashed lines indicate the borders of individual pretectal brain nuclei, as indicated. The alar/basal plate boundary is indicated by a long dashed line. The different shadings represent diencephalic divisions, as indicated in the legend to Figure 1. For abbreviations, see list. Scale bar = 500 μm.
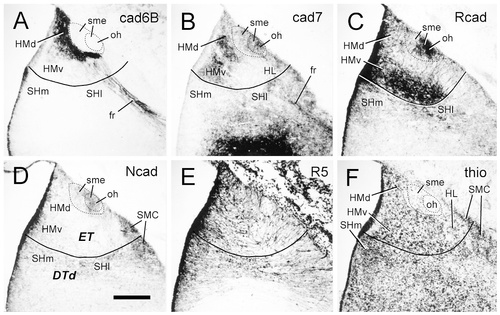
Cadherin expression in transverse sections through the epithalamus of the embryonic day 11 chicken. Sections were immunostained with antibodies against cad6B (A), cad7 (B), Rcad (C), Ncad (D), and the radial glial marker R5 (E). A thionine stain of an adjacent section is shown in F. The solid lines indicate the boundary between the epithalamus and the dorsal thalamus. The dashed lines surround the entire stria medullaris (outer circle) and its densely fasciculated part (inner circle). For abbreviations, see list. Scale bar = 200 μm.
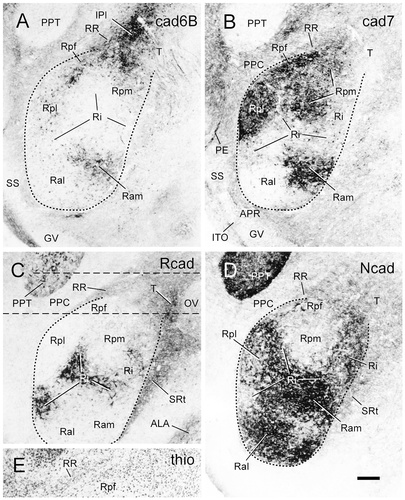
Cadherin expression in transverse sections through the area of the nucleus rotundus of embryonic day 15 chicken. Sections were immunostained with antibodies against cad6B (A), cad7 (B), Rcad (C), and Ncad (D). Note the differential cadherin expression by the subdivisions of the nucleus rotundus. The dashed lines outline the nucleus rotundus. The straight dashed lines in C indicate the region for which a thionine stain of an adjacent section is shown in E. A color-coded superposition of the immunostaining results for cad7 (B), Rcad (C), and Ncad (D) is shown in Figure 11D. For abbreviations, see list. Scale bar = 200 μm.
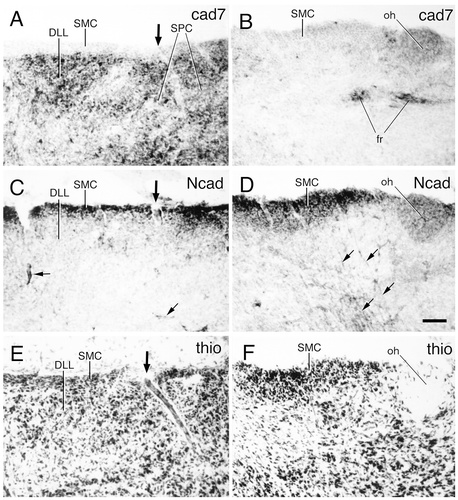
Cadherin expression in transverse sections through the area of the nucleus superficialis microcellularis (SMC) of the dorsal thalamus of an embryonic day 11 chicken. Two series of adjacent sections were immunostained with antibodies against cad7 (A,B) and Ncad (C,D). A thionine stain of adjacent sections is shown in E,F. A, C, and E are enlargements of the boxed area in Figure 4D, and B, D, and F are enlargements of the boxed area in Figure 6. Note the differences in cadherin expression and histologic appearance between the nucleus microcellularis (SMC) and the nucleus parvocellularis (SPC). The large arrows in A, C, and E indicate the position of a blood vessel entering the brain. The small arrows in C indicate Ncad-positive blood vessels within the brain parenchyma. The small arrows in D indicate Ncad-positive radial glial fibers. For abbreviations, see list. Scale bar = 50 μm.
Data analysis and terminology
Data analysis was based on the prosomeric model of the vertebrate brain by Puelles, Rubenstein, and coworkers (Bulfone et al., 1993; Puelles and Rubenstein, 1993; Puelles, 1995). In addition, the seminal studies by Rendahl (1924) and Vaage (1969) as well as more recent publications on the development of the chicken diencephalon (Martínez-de-la-Torre, 1985; Puelles et al., 1987; De Castro et al., 1998) were consulted. For identification of gray matter structures, we generally followed the terminology used in the atlases of the chicken brain by Kuenzel and Masson (1988) and of the pigeon brain by Karten and Hodos (1967). For some gray matter areas, additional studies served as a frame of reference for subdivision schemes and terminology, e.g., the studies by Martínez-de-la-Torre et al. (1990) and Puelles et al. (1991).
RESULTS
General observations
The four cadherins studied in the current report (cad6B, cad7, Rcad, and Ncad) each are expressed in a spatially restricted manner consistent with patterns of radial glial heterogeneity in the chicken diencephalon at E11 and E15 (E11: Figs. 2-12; E15: Fig. 13). In Part I below, evidence is described that supports the general usefulness of mapping cadherin expression and studying radial glial topology for the localization of prosomere boundaries and subdivisions in the chicken diencephalon at intermediate stages of development. In Part II, the pattern of cadherin-expressing gray matter structures in each embryonic division is described in more detail. Some novel aspects for specific diencephalic regions that have been studied in less detail previously also are described (Figs. 14-17).
Part I: Observations on prosomeric boundaries and subdivisions
Prosomere-derived nuclear domains in the diencephalon (Fig. 1) were identified in the past largely by analysis of cytoarchitectonic development. They were referred to as prosomeres and named the synencephalon (our p1), the posterior parencephalon (p2), and the anterior parencephalon (see Table 1; Rendahl, 1924; Puelles et al., 1991). Note that Rendahl's anterior parencephalon was subdivided later into p3–p6 by Puelles and Rubenstein (1993) and Puelles (1995). Alar derivatives of the prosomeres are the pretectal nuclei in p1, the dorsal thalamic and epithalamic nuclei in p2, and the ventral thalamic formations in p3 (Fig. 1A). The classical hypothalamus (mamillary, tuberal, and retrochiasmatic nuclei) largely falls across basal p4–p6, as postulated more recently on morphologic and molecular evidence (Puelles and Rubenstein, 1993; Puelles, 1995; Shimamura et al., 1995). In contrast, anterior, suprachiasmatic, and preoptic parts of the conventional hypothalamus fall within the alar plate of the p4–p6 domains together with the eminentia thalami (Fig. 1). Observed patterns of cadherin expression relative to cytoarchitectonic features (Figs. 2-6) can be interpreted readily in terms of this neuromeric morphologic model, as summarized in Figure 7.
Relation of radial glial topology to prosomeres.
Figures 8-12 show evidence suggesting that, in addition to cytoarchitectonic and chemoarchitectonic limits, local changes in radial glial density serve as landmarks to confirm the postulated locations of permanent prosomeric boundaries in the mature diencephalon. Radial glia are assumed to reflect how the ventricular territory of a histogenetic unit (prosomere or secondary subdivision) and its boundaries project onto the pial surface of the brain, irrespective of morphogenetic deformations caused by differential growth across the wall of the neural tube.
Here, radial glial topology was studied with the R5 monoclonal antibody. Although several diencephalic regions exhibit only a few radial glial fibers [e.g., the nucleus rotundus (R) in Fig. 9C], fiber density is relatively high in other regions (e.g., in the basal p5 region of the hypothalamus; Figs. 8-10C). Moreover, in several diencephalic regions, prominent bands or raphes of increased radial glial fiber density are seen (Figs. 8C, 9C). A closer look at adjacent Nissl-stained sections reveals that these raphes frequently are located at the cytoarchitectonic boundaries between prosomeres. One example is the border of the principal precommissural nucleus (PPC), which lies in a rostral, precommissural part of p1, with the nucleus rotundus (R) of the dorsal thalamus (DT) in p2 [Fig. 1A, precommisural subdivision of the pretectum (PTp) and DT; Fig. 9, PPC and R]. The border between these two prosomeres is marked by dense radial glial fibers (Fig. 9C, PT/DT border). Another example is the border between the dorsal thalamus in p2 and the ventral thalamus (VT) in p3 (Fig. 1A, DT and VT). This border region is known as the zona limitans (zl) intrathalamica (Figs. 8, 9C, zl), and it contains dense radial glial fibers that express Rcad uniformly near the ventricle (Fig. 8A); this staining appears more extensive and uniform at earlier stages (Gänzler and Redies, 1995; Yoon et al., 2000). The PT/DT and the DT/VT boundaries approach one another near the pial surface, midways along the dorsoventral dimension of the dorsal thalamus, where they are separated only by the thin prospective interstitial nucleus (ITO) of the optic tract (Fig. 9B, ITO), a superficially migrated cell population (see also Martínez et al., 1991; Puelles et al., 1991). A third example of such a glial arrangement is the border between p4 and p5 within the preoptopeduncular region of the hypothalamus (Figs. 8C, 9C, 10C, p4/p5). Some other prosomere boundaries are marked by changes in radial glial density without showing an increased density at the border itself. For example, the griseum centrale (GT) at the rostral end of the alar midbrain (Figs. 9, 10, GT) contains many radial glial fibers, and there is an abrupt change across the GT/PT border (mesencephalon/p1 border) to the much lower radial glial density of the pretectum.
Additional subdivisions within the pretectum and dorsal thalamus.
By mapping the cadherin-expressing diencephalic neuronal populations (see below), we found that it was convenient to divide the pretectum (alar p1) into three approximately transverse subregions and the dorsal thalamus (alar p2) into four subregions (Fig. 1A,B). Although there is abundant evidence for additional internal differentiation in each of them, these subdivisions nevertheless each have a unitary developmental and cytoarchitectonic aspect, including distinct patterns of radial glia. These findings suggest that the subdivisions represent intermediate histogenetic primordia and warrant assigning them specific names. The three pretectal parts are called commissural (PTc), juxtacommissural (PTj), and precommissural regions (PTp), respectively (Fig. 1A). These terms refer to their positions relative to the posterior commissure and follow Rendahl (1924), who distinguished PTp from PTc, and Martínez-de-la-Torre (1985), who introduced PTj (see also Puelles et al., 1996; Redies et al., 1997; De Castro et al., 1998; Pombal and Puelles, 1999). Figures 10 and 11C display in transverse sections the distinction between these pretectal regions, as determined on the basis of Nissl stains, cadherin staining, and differential radial glial distribution. The cad7 and Ncad immunostaining (Fig. 11C) shows an example of how nuclei of the same subdivision can become chemoarchitectonically diverse, acquiring a differential profile of cadherin expression [in Fig. 10A, compare the medial and lateral spiriform nuclei (SpMcl and SpL, respectively) of PTj]. The three subdivisions of the pretectum also are shown in parasagittal sections in Figure 12.
The four novel secondary subdivisions of the dorsal thalamus are defined as the dorsal, intermediate, and ventral tiers, and there also is an anteroventral subregion (Fig. 1A,B). These subdivisions can be seen particularly clearly in parasagittal sections (Figs. 12, 13).
Rostrally, the boomerang-shaped anteroventral subregion (DTav; Fig. 1A) separates the three superposed tiers from the transverse zona limitans (p2/p3 limit), and it also separates the ventral tier from the longitudinal alar-basal plate border of p2 (Rendahl, 1924; Puelles et al., 1991, Puelles et al., 1999b). The unity of this subdivision is supported by the common dates of birth and migration pattern of its cellular constituents, similar cell typology of its derivatives (Martínez et al., 1991; Puelles et al., 1991), and molecular data (see Discussion). The DTav cells invade the superficial stratum, covering the three dorsoventral tiers, and give rise to cells of the dorsal geniculate formation and to the perirotundic nuclear complex (Martínez et al., 1991; Puelles et al., 1991). The latter extends back to the PT/DT boundary in birds by tangential, caudalward migration of its cells superficial to the ventral and intermediate tiers (Fig. 1B, DTav; see also Puelles et al., 1991).
The dorsal tier of the dorsal thalamus lies under the epithalamus or habenular nuclear complex, which, in fact, is the dorsalmost portion of the p2 alar plate, although columnar (i.e., nonneuromeric) tradition gives it a separate name and category. Note that neither the dorsal tier nor the epithalamus extends back to the PT/DT boundary from which they clearly are separated by the caudodorsally expanded intermediate tier, which is the site of origin of the nucleus rotundus and some other associated cell groups, including the intermedioposterior nucleus (Figs. 1A,B, 12, 13). The boundary between the dorsal and intermediate tiers usually is visible as a discontinuity in Nissl-stained material (Figs. 3-7). Medially, the ventral tier generates the nucleus ovoidalis and associated grisea (Fig. 1B), and, laterally, it contains some poorly known cell groups (represented in Figs. 5, 6, 7C,D, 13A–D). Evidence for an at least partial delineation of these tiers by bundles of radial glia is presented in Figure 9C,D. The dorsal tier encompasses dorsal thalamic nuclei projecting to dorsal pallial telencephalic targets (the Wulst, among others), whereas the intermediate and ventral tier nuclei project to ventral pallial telencephalic targets in the dorsal ventricular ridge (Discussion).
Overview of cadherin distribution in the diencephalon.
Although the expression patterns of the four cadherins studied in this report (cad6B, cad7, Rcad, and Ncad) are distinct from one another, partial overlaps between any two cadherins were observed. On the whole, the overlap of expression is greater between cad6B and cad7 than between the other cadherins. In this section, we present an overview of the expression data. Figures 2-6 display immunostaining examples in a rostral-to-caudal series of selected transverse sections and are supplemented by the transverse sections in Figures 8-10, which relate the expression data to the radial glial topology, and by the sagittal sections in Figures 12 and 13. Together, these data illustrate the overall correlation of various cadherin immunostaining patterns to Nissl-stained prosomeric divisions, secondary subdivisions, individual diencephalic nuclei, and even some internal divisions within single nuclei.
Changes of cadherin expression at interprosomeric boundaries.
The cadherin immunostaining patterns frequently show abrupt changes in cadherin expression. Some of these changes coincide with previously suggested cytoarchitectonic boundaries of mature prosomere-derived domains (Rendahl, 1924; Martínez-de-la-Torre, 1985; Puelles et al., 1991; De Castro et al., 1998) as well as coinciding with the R5-immunoreactive radial glial raphes at the boundaries and the sudden changes in glial density described above. The following four examples illustrate this point:
1) In the dorsal thalamus (alar p2), the expression of the four cadherins within the nucleus rotundus abruptly falls off caudally at the p1/p2 (PT/DT) border, most markedly for cad7 and cad6B (Figs. 9A, 11B, 12A–B). Fainter expression of Rcad and Ncad in the retrorotundic area (RR), the nucleus triangularis (T), and the medial part of intermedioposterior nucleus (IPm), all of which belong to the dorsal thalamus, also falls off along the curved dorsal continuation of the PT/DT limit (Fig. 12C,D). More ventrally, the PT/DT boundary separates the perirotundic area (APR) and the prospective interstitial nucleus of the optic tract (ITO), which are derivatives of the anteroventral region of the dorsal thalamus, from the principal precommissural (PPC) and superficial synencephalic (SS) nuclei of the pretectum. More superficially, the boundary also is delineated by a change in cadherin expression (compare APR, ITO, PPC, and SS in Fig. 12A–D). Caudally, the PT/DT border is partially demarcated by a change in cad7 expression (Fig. 10A, arrowheads).
2) The zona limitans intrathalamica (zl), i.e. the DT/VT (p2/p3) boundary, aligns with the rostral border of the Rcad-positive (also weakly cad6B- and cad7-positive) perirotundic area (APR) and subrotundic nucleus (SRt), components of the anteroventral subregion of the dorsal thalamus (Figs. 4C, 9C, 12C). This boundary can be followed dorsalward as the anteroventral subregion thins out in front of the dorsal tier [Fig 8A,B, dorsal intermediate ventral anterior nucleus (DIVA); Fig. 12B, nucleus dorsolateralis anterior (DLA)]. Superficially within the anteroventral subregion, the ITO nucleus appears intercalated between the cad7-positive superficial synencephalic nucleus (SS) and the ventral geniculate nucleus (GV). The ITO nucleus is strongly positive for Ncad but negative for cad7 (Fig. 12A–E). Periventricularly, the zona limitans demarcates a change in cadherin expression from Rcad in DT to cad7 in VT (Fig. 11B). At E11, the radial glial raphe associated with the zona limitans has lost most of its earlier Rcad immunoreactivity (Gänzler and Redies, 1995; Yoon et al., 2000). It remains Rcad-positive only periventricularly, although it is more extensively positive at its probably less mature dorsal portion (zl in Figs. 8A, 9C, 12C).
3) The anterior nucleus of the ansa lenticularis (ALA in Figs. 3C, 4C, 9B, 11B) is part of p4 (p4 basal plate, migrated out of the mamillary area). The radial glial pattern emphasizes that its lateral (topologically caudal) margin coincides with the p3/p4 border and shows an abrupt change from Rcad expression in ALA (p4) to cad7 expression in VT (p3; Fig. 11B). Moreover, the medial (topologically rostral) margin of ALA coincides with the p4/p5 border, where Rcad expression also falls off (Fig. 11B).
4) Part of the border between the pretectum and the rostralmost alar midbrain formation, the griseum tectale (GT/PT or mesencephalon/p1 border), is formed by the caudal margins of the principal pretectal nucleus (PPT), the interstitial nucleus of the pretectosubpretectal tract (IPS), and the subpretectal nucleus (SP in Fig. 13E,F). This border colocalizes at an intermediate radial level with the margin of strong Ncad expression in these nuclei, abutting differential cad7 expression in the midbrain griseum tectale (GT in Fig. 11C). Note the prominent change in density of radial glial fibers at the GT/PT border (Fig. 10C).
There are also examples of cadherin-immunoreactive structures that extend across prosomeric boundaries without any change in expression level. However, most if not all of these structures represent fiber tracts running in a topologically longitudinal direction. For example, parts of the tectothalamic tract (tt in Fig. 12B,D) express different cadherins (Redies et al., 1993; Wöhrn et al., 1999) and can be followed across all the prosomeric boundaries from the midbrain into the ventral supraoptic decussation (dsv in Figs. 9A,B, 11B) in p6. Another example is the cadherin-positive optic tract (ot in Figs. 10, 11C, 12), which originates in the retina (p6; Puelles and Rubenstein, 1993) and crosses all prosomeres to terminate in the optic tectum of the midbrain. Likewise, the cad7-positive fibers of the dorsal peduncle of the lateral forebrain bundle originate in the dorsal thalamus and run longitudinally to traverse the zona limitans intrathalamica (zl in Figs. 8, 11A; lfb in Figs. 8A, 13B) as well as p3 and p4. They enter the main lateral forebrain bundle at the hemispheric stalk in p5 (lfb in Fig. 8) and finally project dorsalward into the telencephalon (Tel in Figs. 1A, 2).
Changes of cadherin expression at borders of intraprosomeric (secondary) subdivisions.
Changes in radial glial density within some of the prosomeres, especially within the pretectum (Fig. 10) and the dorsal thalamus (Fig. 9), as discussed above, seem to coincide with the borders of additional subdivisions within the alar plate of these prosomeres. For instance, Figures 8C,D and 9C,D show differential density of radial glial fibers in the epithalamus as well as in the dorsal, intermediate, and ventral tiers of the dorsal thalamus; moreover, the common border between these three tiers and the anteroventral subregion also is delineated partially by a glial raphe found at the ventrolateral aspect of nucleus rotundus (Fig. 9C). Comparison with cad7 immunoreactivity indicates that expression in the anteroventral subregion respects the boundary with the intermediate tier (Figs. 9A, 11B). In addition, periventricular cad7-negative or cad7-positive nuclei alternate from dorsal to ventral, corresponding to the epithalamus, the tiers of the dorsal thalamus (dorsal, intermediate, and ventral tiers), and the ventral thalamus, respectively, as identified on the basis of changes in radial glial density and cytoarchitecture (Fig. 9). Similar correspondences can be found for cadherin expression and the radial glial patterns of the pretectal alar subdivisions (see Figs. 10, 11C, 12A–C,F).
Changes of cadherin expression within alar subdivisions or single nuclei.
In a number of cases, cadherin expression selectively characterizes a nucleus within a subdivision [e.g., cad6B for the lateroanterior nucleus (LA) in Fig. 2A; Rcad for SpL in Fig. 6C; cad7 for the nucleus ovoidalis (OV) in Fig. 9A; or Ncad for PPT in Fig. 11C]. Moreover, discrete portions of some nuclei also express cadherins differentially. In these cases, there usually is no particular delineation by radial glial raphes or by changes in glial density, at least in the material studied by us. Differential cadherin expression is a common property of cell groups in the dorsal tier of the dorsal thalamus. Some of these cell groups may be identified tentatively as corresponding to hodologically characterized loci within dorsal thalamic nuclei. The nucleus rotundus represents a marked example of internal subdivisions that are distinguished by different combinations of cadherin immunoreactivities in a histoarchitectural rather homogeneous nucleus (Figs. 9A, 11B,D, 12A–D, 13A–C, 16). The subdivisions identified in the current work (described in more detail below) can be related to those demonstrated previously with AChE staining (Martínez-de-la-Torre et al., 1990). Subdivisions of the nucleus rotundus are known to differ in their functional properties (Wang et al., 1993) and connectivities (Benowitz and Karten, 1976; Deng and Rogers, 1998; Hellmann and Gunturkun, 1999). Note that there also are gradient-like changes in cadherin expression within the nucleus rotundus and some other diencephalic nuclei [e.g., cad7 expression in the dorsointermediate posterior nucleus (DIP); Fig. 4B].
Part II: Detailed description of cadherin expression in the diencephalon
The transverse sections shown in Figures 2-6 illustrate five levels of sectioning through the E11 diencephalon that were immunoreacted with either cad6B, cad7, or Rcad antibodies plus an adjacent section that was stained with thionine for Nissl substance. The levels are arranged in a rostral-to-caudal sequence. Figure 7 schematically identifies the prosomeric domains and main alar subdivisions for the section levels shown in Figures 3-6; for the rostralmost level (Fig. 2), the same information is drawn in Figure 2D.
Table 2 lists all gray matter structures identified in this study, grouped according to the subdivisions in which each structure is located, together with the profile of cadherin expression at E11. Table 2 also indicates the figure(s) in which each structure is shown. In Figure 7 and in the other schematic diagrams (Figs. 1, 2C, 8D, 9D, 10D, 12F, 14J–L, 18), the pretectum (PT or p1), the ventral thalamus (VT or p3), and p5 are shaded in gray, whereas the dorsal thalamus (DT or p2) and p4 and p6 are not shaded. Intermediate shading and striped areas represent alar subdivisions within the pretectum (PTj) and the dorsal thalamus (DTi).
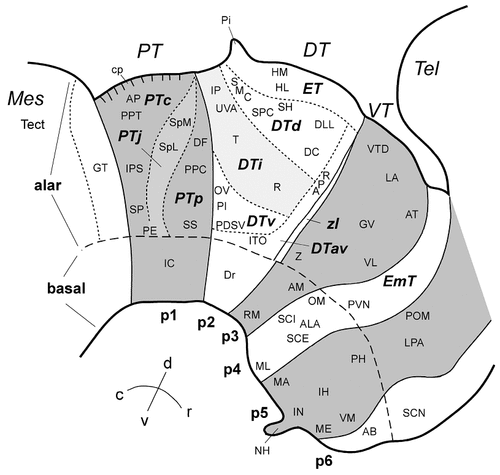
Schematic diagram of the origin and location of selected diencephalic nuclei in the prosomeric model of the embryonic chicken brain. The pretectum (p1), the ventral thalamus (p3), and p5 are shaded in gray. Striped areas represent secondary subdivisions within the pretectum (PTj) and the dorsal thalamus (DTi). Abbreviations in large bold letters indicate primary and secondary diencephalic divisions (see Table 1). Small letters indicate diencephalic nuclei. For abbreviations, see list and Table 2.
| Structure | Immunoreactivity | Abbreviation2 | Shown in Figure(s) | |||
|---|---|---|---|---|---|---|
| Cad6B | Cad7 | Rcad | Ncad | |||
| Pretectum (alar plate of prosomere 1) | ||||||
| Commissural pretectum (PTc) | ||||||
| Area pretectalis | − | − | p | p | AP | 6, 7, 12, 14, 18 |
| Area pretectalis diffusa | − | + | − | − | APd | 6, 12, 14 |
| Nucleus of posterior commissure | − | + | − | − | CP | — |
| Interstitial nucleus of the pretectosubpretectal tract | − | − | s | + | IPS | 5–7, 10–12, 18 |
| Principal pretectal nucleus | − | − | s | + | PPT | 6, 7, 10–12, 14, 16, 18 |
| Medial pretectal nucleus | − | (+) | − | − | PTM | 10, 14 |
| Subpretectal nucleus | − | p | − | p | SP | 5–7, 10–12, 18 |
| Juxtacommissural pretectum (PTj) | ||||||
| External pretectal nucleus | + | + | − | − | PE | 4–7, 9–12, 16, 18 |
| Lateral spiriform nucleus | (s) | − | + | − | SpL | 6, 7, 10, 12–14, 18 |
| Medial spiriform nucleus | SpM | 14, 18 | ||||
| Caudolateral part | − | + | − | − | SpMcl | 6, 7, 10, 12, 14 |
| Rostromedial part | − | + | − | − | SpMrm | 6, 7, 10, 14 |
| Precommissural pretectum (PTp) | ||||||
| Nucleus of the basal optic root3 | p | p | − | − | BOR | 13 |
| Nucleus dorsofrontalis | − | p | − | − | DF | 5–7, 12–14, 18 |
| Principal precommissural nucleus | − | + | − | − | PPC | 3–6, 7, 9, 10, 12–14, 16, 18 |
| Superficial synencephalic nucleus | − | + | − | − | SS | 3–5, 7, 9, 10, 12, 16, 18 |
| Dorsal thalamus (alar plate of prosomere 2) | ||||||
| Epithalamus (ET) | ||||||
| Lateral habenula | − | p | − | − | HL | 4, 7–9, 15, 18 |
| Medial habenula | HM | 4–6, 9 | ||||
| Dorsal portion | + | p | p | − | HMd | 4–7, 15 |
| Ventral portion | − | + | + | (+) | HMv | 4–9, 15 |
| Pineal gland (epiphysis) | s | p | p | + | Pi | 18 |
| Dorsal tier (DTd) | ||||||
| Dorsal anterior nucleus | − | − | − | − | DA | 3, 4, 7, 12 |
| Dorsal posterior nucleus | − | (+) | p | (+) | DPo | 5–7, 9, 12, 14 |
| Dorsointermediate posterior nucleus | − | + | p | − | DIP | 4–7, 9, 14 |
| Dorsal intermediate ventral anterior nucleus | − | + | + | − | DIVA | 3, 4, 7, 8, 12, 13 |
| Dorsolateral anterior nucleus | − | + | p | − | DLA | 3, 7, 8, 12, 13 |
| Dorsolateral lateral nucleus4 | − | + | − | − | DLL | 3, 4, 7, 9, 13, 17, 18 |
| Dorsolateral medial nucleus | − | + | − | − | DLM | — |
| Dorsolateral posterior nucleus | − | p | (+) | − | DLP | 4–7, 15 |
| Dorsomedial anterior nucleus | − | − | − | − | DMA | 8 |
| Dorsomedial posterior nucleus | − | − | − | − | DMP | 4–7, 14 |
| Microcellular superficial nucleus | − | − | (+) | + | SMC | 1, 3–5, 7–9, 12, 13, 15, 17, 18 |
| Parvocellular superficial nucleus4 | − | p | − | − | SPC | 1, 4, 5, 7–9, 12, 13, 17, 18 |
| Subhabenular region, lateral | − | p | p | p | SHl | 4, 6–8, 15 |
| Subhabenular region, medial | − | − | − | − | SHm | 4–8, 15 |
| Ventrointermediate area | − | + | + | + | VIA | 3, 7, 8 |
| Intermediate tier (DTi) | ||||||
| Intermediomedial nucleus | − | − | − | − | IM | 4–7, 9, 14 |
| Intermedioposterior nucleus | IP | 7 | ||||
| Lateral portion | + | p | p | − | IPl | 5, 6, 14, 16 |
| Medial portion | (p) | + | (+) | (+) | IPm | 5, 6, 12, 14 |
| Rotundus nucleus | p | p | p | p | R | 1, 3, 5, 7, 9, 11–13, 18 |
| Triangular nucleus | − | − | p | (+) | T | 4–7, 9, 11, 12, 16, 18 |
| Nucleus uvaeformis | − | − | − | − | Uva | 10, 18 |
| Ventral tier (DTv) | ||||||
| Nucleus ovoidalis | − | + | − | − | OV | 1, 5, 7, 9, 11, 16, 18 |
| Posterior nucleus of the ventral supraoptic decussation | s | + | + | (+) | PDSV | 5–7, 10, 13, 18 |
| Posterointermediate nucleus | (+) | s | + | (+) | PI | 6, 7, 13, 14, 18 |
| Nucleus paramedianus internus | − | (+) | s | − | PM | 4–7, 9, 14 |
| Posterior nucleus | − | (+) | (+) | − | Po | 5–7, 14 |
| Nucleus periovoidalis | − | (+) | + | (+) | POV | 4, 5, 7 |
| Posteroventral nucleus | s | (+) | + | (+) | PV | 5–7, 13 |
| Retroovoidal area | − | (p) | + | (+) | ROV | 4–7, 14 |
| Nucleus semilunaris periovoidalis | − | p | + | + | SPO | 4, 5, 7, 9 |
| Nucleus zeta | − | + | (+) | + | Z | 5–7, 13, 18 |
| Anteroventral subdivision (DTav) | ||||||
| Perirotundic area | (+) | + | + | − | APR | 1, 3, 4, 7, 9, 11, 12, 16, 18 |
| Retrorotundic area | s | (+) | + | (+) | RR | 5, 7, 11, 12, 16 |
| Prospective interstitial nucleus of the optic tract | (+) | − | + | + | ITO | 1, 3–5, 7, 9, 12, 13, 16, 18 |
| Subrotundic nucleus | − | − | s | − | SRt | 4, 5, 7, 9, 11–13, 16 |
| DT/VT boundary region | ||||||
| Zona limitans intrathalamica | p | − | p | p | zl | 3, 4, 7–9, 12 |
| Ventral thalamus (alar plate of prosomere 3) | ||||||
| Area triangularis | − | − | − | − | AT | 2, 18, 13 |
| Ventral geniculate nucleus | p | + | − | − | GV | 2–5, 7–9, 12, 13, 16, 18 |
| Nucleus intercalatus | − | s | − | − | ICT | 3, 7–9, 12 |
| Lateroanterior nucleus | + | n, s | − | − | LA | 2, 8, 12, 18 |
| Superior reticular nucleus | RS | 2 | ||||
| Dorsal portion | (s) | p | (s) | − | RSd | 3, 7, 8 |
| Ventral portion | − | (+) | − | − | RSv | 3, 8 |
| Ventrolateral nucleus | − | (p) | − | − | VL | 2, 3, 7, 8, 18 |
| Dorsal area of ventral thalamus | − | − | − | − | VTD | 3, 7, 12, 18 |
| Zona incerta | − | p | (s) | − | ZI | 3, 7, 9 |
| Basal plate of prosomeres 1–3 | ||||||
| Retromamillary region | + | − | + | − | RM | 18 |
| Interstitial nucleus of Cajal, rostral portion | + | + | − | − | ICr | 10 |
| Nucleus of Darkschewitsch, rostral portion | − | + | − | (+) | Dr | 10, 18 |
| Anteromedial preoptic nucleus | − | s | p | − | AM | 2, 18 |
| Alar and roof plates of prosomeres 4–6 | ||||||
| Paraphysis | − | p | p | + | Pa | |
| Lateral preoptic area | − | + | − | − | LPA | 2, 18 |
| Medial (main) portion of preoptic nucleus | − | − | − | − | POM | 2, 18 |
| Lateral portion of preoptic nucleus | − | − | + | − | POMl | 2 |
| Medial suprachiasmatic nucleus | + | p | (+) | − | SCNm | 2 |
| Lateral suprachiasmatic nucleus | − | − | + | + | SCNl | 2 |
| Basal and floor plate of prosomere 4 | ||||||
| Paraventricular nucleus | − | − | + | − | PVN | 8, 18 |
| Anterior nucleus of the ventral supraoptic decussation | + | + | + | − | ADSV | 3, 7, 9 |
| Anterior nucleus of the ansa lenticularis | (s) | (s) | + | − | ALA | 3, 4, 7, 9, 11, 16, 18 |
| Lateral mamillary nucleus | − | − | (+) | + | ML | 4, 7, 18 |
| Nucleus of the occipitomesencephalic tract | (s) | s | + | − | OM | 3, 4, 7, 18 |
| Periventricular hypothalamic organ | − | − | − | − | PVO | 4, 5, 7 |
| Stratum cellulare externum | − | (s) | s | − | SCE | 5–7, 18 |
| Stratum cellulare internum | − | (s) | − | − | SCI | 5–7, 18 |
| Lateral hypothalamic region | − | − | p | p | LH | 3, 5–7 |
| Basal and floor plate of prosomere 5 | ||||||
| Dorsomedial hypothalamic nucleus | − | + | + | + | DM | 4, 5, 7 |
| Inferior hypothalamic nucleus | + | − | + | − | IH | 4, 5, 7, 18 |
| Infundibular nucleus | − | − | + | + | IN | 4, 5, 7, 18 |
| Mamillary area | − | (+) | − | − | MA | 6, 7, 18 |
| Periventricular hypothalamic nucleus | s | − | − | − | PH | 3, 18 |
| Ventromedial hypothalamic nucleus | p | − | + | − | VM | 3, 7, 8, 18 |
| Median eminence | + | − | − | + | ME | 4, 7, 18 |
| Stalk of neurohypophysis | + | − | − | − | NH stalk | 5 |
| Basal and floor plate of prosomere 6 | ||||||
| Anterobasal nucleus | − | − | (+) | + | AB | 3, 7, 18 |
| Juxtacommissural plate | + | − | + | p | JCP | — |
- 1 −, Structure is not immunoreactive; +, structure is immunoreactive; n, only neuropil is immunoreactive; p, only parts of the structure are immunoreactive; s, only scattered cells are immunoreactive. Parenthesis denote weak immunoreactivity.
- 2 Abbreviation used in the current work.
- 3 This nucleus extends rostrally into p2 (Fig. 10) and p3 (Figs. 4-7).
- 4 Likely to originate in the anteroventral subregion (DTav; Puelles et al., 1999b).
Pretectum (alar p1).
The sets of nuclei derived from the three subdivisions of the pretectum display prominent differences in cadherin expression (Figs. 3-6, 10, 11C, 12, 14). Ncad is expressed by the migrated nuclei of the commissural pretectum (PPT, IPS, and SP; Figs. 11C, 12D; Redies et al., 1993) but not by any of the nuclei of the other two subdivisions. In the area pretectalis, which lies at the dorsal pial surface of the commissural pretectum, a diffuse (deep) region (APd) can be distinguished from a dense (superficial) part (AP) on the basis of differential cad7 expression (AP, APd; Figs. 14D–F; compare with Medina et al., 1994; Puelles and Medina, 1994).
In the juxtacommissural pretectum, Rcad expression is prominent in SpL (Figs. 6C, 12C), and there also are some scattered, Rcad-positive cells in PPT and IPS (Arndt and Redies, 1996). Cad7 expression is restricted to the medial spiriform nucleus, which appears to be divided into rostromedial and caudolateral parts (SpMrm and SpMcl in Figs. 10A, 12B, 14E,F); the rostralmost parts of SpM show less Cad7 expression (SpMrm, SpM in Figs. 6B, 14D). Cad7 immunoreactivity is conspicuously absent from pretectal nuclei that express Ncad and Rcad, except for SP, which shows cad7 immunoreactivity medially. Cad6B is expressed weakly in SpL (Figs. 6A, 12A, 14A–C) and in the external pretectal nucleus (PE in Figs. 5A, 6A, 12A).
The precommissural pretectum is dominated by cad7 immunoreactivity that is especially prominent in its principal precommissural and superficial synencephalic nuclei (PPC and SS in Figs. 3B, 4B, 5B, 6B, 10A, 12B, 14D–F). The location of the dorsofrontal nucleus (DF), which is a dorsal prolongation of PPC and weakly expresses cad7, is rostromedial to SpM (DF in Figs. 6, 7D, 12B,E,F, 14D,E,G,H). The caudalmost part of the nucleus of the basal optic root originates in p1 and shows partially overlapping expression of cad6B and cad7 (BOR in Fig. 13A,B; Wöhrn et al., 1998).
Epithalamus and dorsal thalamus (alar p2)
Epithalamus.
The epithalamus, which usually is divided into medial and lateral habenular nuclei (HM and HL, respectively), is quite heterogeneous in our cadherin-stained material (Figs. 4-6; enlargement in Fig. 15). A dorsal part of the medial habenular nucleus (HMd) placed medial and under the stria medullaris is strongly cad6B positive (Figs. 4-6, 15A); a ventral subarea of HMd is cad7 positive (Fig. 15B). The larger ventral part of the medial habenular nucleus (HMv) is labeled strongly for Rcad and shows weaker expression of cad7 periventricularly (Figs. 4-6, 15B,C). The ventricular stratum associated medially with HM strongly expresses Rcad and Ncad (Fig. 15C,D) and does not express cad6B, which is in contrast to the remaining p2 alar plate (Fig. 15A). The expression of cad6B and cad7 also marks projection fibers of HMd and HMv entering the retroflex fascicle (fr in Figs. 6A, 15A,B). No Rcad-immunoreactive fibers could be followed from HMv into fr, but Rcad is expressed in the radial glial processes of the habenular region (Fig. 15C,E). A compact bundle of Rcad-positive nerve fibers appears within the stria medullaris tract (sme in Fig. 2-6, 15). Here, this bundle is identified tentatively as an olfactohabenular tract, because it can be traced all the way from the telencephalon (oh in Figs. 2C–6C, 8, 15). This tract also stands out in glia-immunostained and Nissl preparations (Fig. 15E,F). Its fibers weakly express cad7 and Ncad (oh in Fig. 15B,D). The lateral habenular nucleus (HL), placed interstitially to passing fibers largely ventral to the stria medullaris, shows only limited expression of cad7. Some Ncad-positive cells of the dorsal thalamic subpial cell lamina, which we call the superficial microcellular nucleus (SMC), seem to invade slightly the surface of the epithalamus (SMC in Fig. 15D,F).
Dorsal tier.
The dorsal tier of the dorsal thalamus predominantly expresses cad7, particularly by cell masses adjoining the boundary with the intermediate tier (see sagittal sections in Figs. 12B, 13B). This pattern is displayed in more detail in Figures 3B–6B. The entire DLA, including the contained DIVA and ventrointermediate area (VIA) cell groups (which selectively coexpress Rcad), strongly expresses cad7 and shows a straight boundary with the nucleus rotundus of the underlying intermediate tier (Figs. 3B,C, 12B, 13B). Medially, the cad7 expression gradually diminishes toward the periventricular dorsomedial anterior nucleus, which is largely cad7 negative (DMA in Figs. 8A, 11A). Caudally, the cad7-positive complex of the dorsal tier becomes reduced to the dorsal intermedioposterior nucleus (DIP), which also shows a gradual loss of signal toward the periventricular dorsomedial posterior nucleus (DMP; Figs. 4B, 5B, 6B, 9A, 11B, 14D–L). The dorsal tier neurons that dorsally and caudolaterally fill the space between the cad7-positive DLA/DIP complex and the epithalamus hardly express cad7. This cell group can be divided into a rostrodorsally placed dorsal anterior nucleus (DA in Figs. 3C, 4B,C, 12B) and a caudodorsally located dorsal posterior nucleus (DPo in Figs. 5B,C, 6B,D, 9A, 12B, 14D–L). Laterally and medially, both of these nuclei are covered by the subhabenular nuclei. In addition, lateral to DIP, the dorsolateral posterior nucleus is found that shows only weak cad7 and Rcad immunoreactivity (DLP in Figs. 4B–D, 5B–D). The DLP, DIP, and DMP nuclei are aligned with the dorsalmost, cell-poor stripe of the intermediate tier (Figs. 4D, 5D). The subhabenular region of the dorsal tier coincides topographically with a separate ventricular bulge and the dorsalmost ventricular expression of cad6B (Figs. 4A–6A); this region shows a rather heterogeneous cadherin immunoreactivity pattern that is distinct laterally but less prominent medially (SHm and SHl in Figs. 4-6, 15).
Some superficially placed cell populations in the dorsal tier of the dorsal thalamus strongly express cad7 and more weakly express cad6B. Apart from the nucleus dorsolateralis lateralis (DLL), which originates in the anteroventral compartment (Puelles et al., 1999), these populations are formed by the superficial parvicellular nucleus (SPC) and a previously scarcely known thin subpial layer of diminutive cells termed here the superficial microcellular nucleus (SMC; Guillén, 1992). Ncad is expressed by SMC but not by SPC, and vice versa for cad7 (Figs. 12B,D, 17A–F).
Intermediate tier.
The intermediate tier of the dorsal thalamus has a narrow periventricular portion occupied by the intermediomedial nucleus that does not express any of the four cadherins studied here (IM in Figs. 4D–6D, 9D). The ventricular surface of this tier is marked by particularly strong cad6B expression (Fig. 14A–C).
The largest nucleus of the intermediate tier, the nucleus rotundus, is located ventrolaterally and extends practically along the entire rostrocaudal dimension of the dorsal thalamus. The nucleus rotundus is separated from the zona limitans by the subrotundic nucleus and from the pretectum by perirotundic and retrorotundic areas. All of these surrounding regions are derivatives of the anteroventral region (see below). The nucleus rotundus itself (R) contains posterior, parafascicular, posterolateral, posteromedial, intermediate, anterolateral, and anteromedial subregions (Rp, Rpf, Rpl, Rpm, Ri, Ral, and Ram, respectively, in Figs. 3-5, 9A, 11B,D, 12, 13, 16; see Martínez-de-la-Torre et al., 1990) that differentially express all four cadherins. At its ventrolateral border, the nucleus rotundus is demarcated (and separated from the brain surface) by migrated anteroventral region derivatives (perirotundic area and ITO; see Fig. 1B). Medially, the nucleus rotundus contacts the nucleus triangularis (T in Figs. 4-6, 7B–D, 9A, 12A–F, 13, 16C,D), a deep domain of the intermediate tier that forms a connection to the periventricular IM nucleus. Both nuclei share a similar profile of cadherin expression.
The intermediate tier approaches the pial surface of the brain only dorsocaudally, behind the dorsal tier, where the SPC and SMC cell populations covering the intermediate tier become thinner. For consistency of terminology and to avoid confusion, we have renamed the intermediate tier area at this position the intermedioposterior nucleus (IP; see Discussion). This nucleus contains distinct medial and lateral subregions (IPm and IPl in Figs. 5, 6, 7B–D, 12, 14) that express several of the cadherins. Cad6B immunoreactivity predominates in IPl, whereas weak cad7 expression is found in IPm, and Rcad appears throughout IP. Some Rcad expression appears throughout IPl, whereas Ncad expression is weak.
Ventral tier.
Like the other tiers, the ventral tier has a distinct periventricular stratum that contains a small cell population, the so-called nucleus paramedianus internus of classic literature (Rendahl, 1924; PM in Figs. 4-6, 7B–D, 9A, 14E). This nucleus weakly expresses cad7 and Rcad. Superficial to it, the nucleus ovoidalis (OV) expresses cad7 strongly but is distinctly negative for Rcad and Ncad (OV in Figs. 5B,C, 9A, 11B). It is surrounded by the following Rcad-positive regions: a rostrally located, dense periovoidal nucleus (POV); a caudal retroovoidal area (ROV); and a lateroventrally adjacent nucleus semilunaris periovoidalis (SPO; Figs. 4-6, 9, 14). Extending radially in an apparently oblique plane from the ovoidal complex toward the brain surface, there is a group of ventral tier nuclei that differentially express all four cadherins: In a deeper position, there is a less well known, faintly Rcad-positive region that we call the posterior nucleus (Po in Figs. 5C, 6C, 7C,D, 14A,D). This nucleus appears to be divided into two superposed strata: a deep stratum that contains larger neurons and an intermediate stratum that contains small cells and is traversed by some fiber fascicles of the occipitomesencephalic tract (om in Figs. 5C, 6C,D, 14D–F).
Near the brain surface, the ventral tier derivatives form an enlarged, ovoid complex that may be confused with the nucleus rotundus due to similarities in general shape and position in transverse sections. In sagittal sections (Fig. 13), this nuclear complex is found clearly ventral to the nucleus rotundus. It consists of the posterior nucleus of the ventral supraoptic decussation (PDSV), the posterointermediate nucleus (PI; or posterior nucleus of the ansa lenticularis), the posteroventral nucleus (PV), and the nucleus zeta (Z; Figs. 5, 6, 7, 13). This nuclear complex expresses Rcad, which distinguishes it from adjacent p1 and p3 regions (Fig. 6C, arrowheads). The PDSV nucleus is traversed longitudinally by the tectothalamic tract. This nucleus distinctly expresses both cad6B and Rcad and is marked by intense AChE activity (Figs. 5A,C, 6A,C, 13A,C,D). It has a thin, tail-like, superficial extension that reaches the brain surface under the optic tract after sorting between the SS, the GV, and the BOR nuclei (not shown; best seen in AChE material, see Martínez-de-la-Torre et al., 1990). The PV nucleus weakly expresses cad7 and Rcad and lies just under the nucleus rotundus, whereas PI protrudes somewhat into the pretectum and weakly expresses cad6B, with some interspersed cad7-positive cells (Fig. 13A,B). An anteromedial subregion of this complex, which is interstitial to the cad7-positive tectoovoidal tract, is called here the nucleus zeta (z in Figs. 5B, 6B, 13B). This nucleus lies at a topologic position similar to that of the nucleus zeta (ζ) in reptiles (Beccari, 1923), warranting the use of the same name in the avian brain.
Anteroventral subregion.
In the chicken, cells originating in the boomerang-shaped anteroventral histogenetic zone of the dorsal thalamus (see above) migrate to the surface, partly filling the area of origin in depth and partly dispersing tangentially caudalward at the surface, to finally largely cover the dorsal, intermediate, and ventral tiers (Fig. 1A,B; Rendahl, 1924; Puelles et al., 1991, Puelles et al., 1999; Yoon et al., 2000). The best known derivatives of this region (nucleus subrotundus, perirotundic area, prospective interstitial nucleus of the optic tract; see Table 2) differentiate in close spatial relation to the nucleus rotundus of the intermediate tier. They are characterized mainly by their expression of Rcad but also show differential expression of the other cadherins (SRt, APR, and ITO in Figs. 3, 4, 12). For example, the prospective interstitial nucleus of the optic tract (ITO) is the only nucleus in this region that shows strong Ncad expression (Fig. 13D). This finding supports its distinction from the perirotundic area (APR), as proposed by Puelles et al. (1991) based on other evidence. Caudodorsally, the anteroventral derivatives extend behind the nucleus rotundus as a nucleus that we call the retrorotundic nucleus (RR in Figs. 5, 7C, 12, 16; see also Yoon et al., 2000).
Ventral thalamus (alar p3).
Rostrally, parts of the ventral thalamus protrude into our rostralmost section levels (p3 in Fig. 2D). The ventrolateral nucleus (VL) and the overlying area triangularis (AT) show only weak or no cad7 expression (Figs. 2B, 3B, 13B). In contrast, the lateroanterior nucleus (LA) is the only structure in the ventral thalamus that strongly expresses cad6B (Figs. 2A, 12A; see also Wöhrn et al., 1998); it is only moderately cad7-positive (Figs. 2B, 12B). There also is practically no Rcad or Ncad expression in the ventral thalamus except for some weak, dispersed Rcad signals at the periventricular zona incerta (ZI in Fig. 3C,D). The ventral geniculate nucleus (GV) strongly expresses cad7, particularly in its superficial neuropil (Figs. 2B, 3B, 4B, 12B, 13B; see also Wöhrn et al., 1998). Medial to GV, the p3 (rostral) portion of the nucleus of the basal optic root is found (BOR in Figs. 4-7). Other cad7-positive populations in p3 include the dorsal part of the superior reticular nucleus (RSd in Fig. 3B) and a deeper reticular subpopulation at the limit of the zona incerta (ZI in Fig. 3B). The intercalate nucleus (ICT) contains some isolated, strongly cad7-immunoreactive neurons (Figs. 3B, 9A,B). On the whole, cad7 is the predominant cadherin expressed in the ventral thalamus (Fig. 11B).
In the rostral section levels, there is a periventricular area situated immediately below the diencephalic roof. This area does not express any of the four cadherins and is separated from the dorsal thalamus by the Rcad-positive glial raphe of the zona limitans intrathalamica (p2/p3 border). We conclude that this area represents the topologically dorsalmost part of ventral thalamus. Consequently, we call it the ventral thalamic dorsal area (VTD in Figs. 3, 7A, 12C,F). Caudal to VTD, the dorsalmost parts of the ventricular surface are occupied by the zona limitans (Fig. 3C, inset) and, even more caudally, by the dorsal thalamus (Figs. 4-7), as expected.
Other divisions.
In general, the divisions of the prosomeric basal and floor plates are less well demarcated by cytoarchitectonic features or by the expression of the four cadherins than those of the alar plate. The basal and floor plate nuclei that can be characterized by their cadherin expression profiles are listed in Table 2. Many of these nuclei, especially those of the hypothalamus (basal p4–p6), are shown in Figures 3-5 and Figures 8-10. In the p4–p6 roof plates, the paraphysis shows regionalized expression of cad7, Rcad, and Ncad (data not shown).
DISCUSSION
In the current paper, the gray matter architecture of the chicken diencephalon was studied at intermediate stages of development (E11 and E15) by mapping the expression of four cadherins. For Rcad and Ncad, our results confirm and extend previous studies showing that these cadherins are expressed in a spatially restricted manner by developing diencephalic brain nuclei (Redies et al., 1993; Gänzler and Redies, 1995; Arndt and Redies, 1996). Similar results were obtained for a number of other cadherins in the brains of chicken and mouse (for review, see Redies, 2000). A large majority of the cadherin-positive brain nuclei identified in the current study already exhibit expression as primordia at earlier stages of development (Yoon et al., 2000; for Rcad and Ncad, see also Gänzler and Redies, 1995).
The results of the current study lend further support to the general idea that cadherins provide a system of potentially adhesive cues that specify morphologic and functional brain structures, such as embryonic subdivisions, brain nuclei, and their subregions, during development (Redies et al., 1993; Redies, 1995; see discussion in the accompanying paper: Yoon et al., 2000). It has been shown previously in the visual and cerebellar system of the chicken embryo that the four cadherins studied here also are expressed by specific sublayers and regions of laminated structures of the brain (e.g., retina, tectum, and cerebellar cortex) as well as by specific neural circuits within these systems (Redies et al., 1993; Arndt and Redies, 1996; Arndt and Redies, 1998; Arndt et al., 1998; Miskevich et al., 1998; Wöhrn et al., 1998, Wöhrn et al., 1999). A full account of the fiber tracts and neural circuits expressing the four cadherins will be published separately (L. Medina, L. Puelles, and C. Redies, unpublished observations).
Cadherins as markers for developing gray matter structures
The current study focuses on gray matter architecture at a stage of development when most gray matter structures already have formed and assumed their final topologically invariant position. Taking into account cytoarchitectonic features, radial glial topology, and previously published data, the results from the cadherin-expression mapping allow us to study developing gray matter structures, including the prosomeres and their secondary subdivisions, as well as diencephalic brain nuclei and their regional differentiation with considerable spatial resolution and detail.
In general, the diencephalic regions expressing a given cadherin maintain their expression from E11 to E15. Results from prehatching stages (E19; data not shown) were found to be very similar to those at E15. Thus, cadherins can be used as expression markers for specific gray matter structures. The immunostaining data displayed in the current paper are predominantly from sections through E11 diencephalon, because, at these stages, neurons still are packed more densely, and the borders of the main embryonic subdivisions can be related more easily to radial glial fiber density. At E15 (Figs. 13, 16) and E19 (data not shown), cadherin immunoreactivity has become more restricted to individual diencephalic brain nuclei, as described previously for Rcad (Arndt and Redies, 1996). Consequently, the embryonic subdivisions at E19 are not as clearly visible as they are at E11.
General similarities in spatial extent and overlap of cadherin expression
The expression of many cadherins by neurons in the living brain is combinatorial, both at the regional level (Fushimi et al., 1997; Suzuki et al., 1997) and at the cellular level (Kohmura et al., 1998; Wöhrn et al., 1999). In the current study, many brain regions showed immunoreactivity for more than one of the four cadherins, in some cases also at the cellular level (data not shown). Generally speaking, the number of cad6B-expressing gray matter areas is relatively low, whereas cad7, Rcad, and Ncad are expressed more widely. Many cad6B-positive regions coexpress cad7. There is relatively little overlap between Ncad and Rcad, and the overlap between Ncad and cad7 is only moderate. Similar observations on the extent and overlap of expression were made for the same four cadherins in the visual and cerebellar systems (Arndt and Redies, 1998; Arndt et al., 1998; Wöhrn et al., 1998, Wöhrn, et al., 1999) and in the telencephalon and hindbrain (our unpublished data). It is therefore conceivable that the genetic patterning processes regulating the expression of the four cadherins and the spatial relation of their expression domains are similar in different parts of the brain.
Persistence of neuromeric subdivisions in mature diencephalic gray matter
The importance of early embryonic regionalization and segmentation for understanding vertebrate brain development has been supported by numerous studies during the last decade. What is less well known is how this type of pattern formation relates to the appearance of mature brain architecture. Although seminal work studying this issue was published a long time ago (Rendahl, 1924; Coggeshall, 1964; Vaage, 1969; Keyser, 1972; Vaage, 1973), there have been relatively few modern studies on this topic (Puelles et al., 1991, Puelles et al., 1992; Figdor and Stern, 1993; Puelles, 1995). In the hindbrain, migration of early neurons is predominantly radial after rhombomere formation has taken place (Fraser et al., 1990; Birgbauer and Fraser, 1994). For the hindbrain, it has been shown that, as a consequence of the restricted tangential migration, segmental organization persists and provides the basis for nucleus formation (Vaage, 1973; Puelles and Martínez-de-la-Torre, 1987; Marin and Puelles, 1995; Wingate and Lumsden, 1996).
To our knowledge, the current study is the first comprehensive description of the diencephalic prosomeres and their gray matter derivatives in the chicken. Previous studies in the diencephalon have shown that, like in the hindbrain, some of the major divisional borders restrict tangential migration (Figdor and Stern, 1993; Golden et al., 1997). In addition, morphologic and histochemical studies have revealed that the neuromeric divisions persist as neuromere-derived domains of the mantle layer in the mature diencephalic gray matter of the chicken (Rendahl, 1924; Puelles et al., 1991), like in other vertebrate species (Martínez-de-la-Torre, 1985; Puelles et al., 1992, Puelles et al., 1996; Pombal and Puelles, 1999).
Previous studies have demonstrated that, in chicken, mouse, frog, and zebrafish, the borders of embryonic divisions often coincide with changes in cadherin expression at early stages of brain development. The regional changes in cadherin expression are subserved mostly by neuroepithelial cells and radial glial cells that extend processes all the way to the pial surface (Espeseth et al., 1995; Gänzler and Redies, 1995; Matsunami and Takeichi, 1995; Kimura et al., 1996; Redies and Takeichi, 1996; Inoue et al., 1997; Korematsu and Redies, 1997; Yoon et al., 2000). Thus, at early stages, many cadherins are expressed across the entire thickness of the neural tube wall. As the mantle layer of the diencephalic neural tube increases in thickness and approaches its mature appearance, cadherin-immunoreactive radial glial processes are no longer a prominent feature, although some radial glial fibers still are present in specific regions, as demonstrated in the current study. At the same time, regional differences in cadherin expression appear in the mantle layer (Gänzler and Redies, 1995; Yoon et al., 2000). During subsequent development, the mantle layer increases in thickness and differentiates into the anlagen of brain nuclei. In parallel, differential cadherin expression by brain nuclei begins to be a prominent feature in the mantle layer. Despite these overall changes in the predominant cell types expressing cadherins, the divisional borders remain clearly detectable in the immunostained sections (see, e.g., Figs. 11B,C; 12). However, in contrast to the earlier stages, the divisional borders now coincide with the borders of cadherin-expressing brain nuclei situated adjacent to the borders. Thus, at different levels of mantle layer stratification, several different cadherins may demarcate a given divisional border, thereby increasing the complexity of cadherin expression as morphologic and functional differentiation of diencephalic gray matter takes place (for a schematic diagram of this model of brain development, see Fig. 10 in Redies, 2000).
Changes in radial glial fiber density at divisional borders
Our results demonstrate the presence of radial glial fibers at relatively late stages of diencephalic development (E11 and E15), when the mantle layer has attained to considerable size and approaches its final thickness and morphology. We could not follow individual fibers from the ventricular layer to the pial surface, probably because of the gross morphogenetic distortions that have taken place during development or because we did not cut sections in the exact plane of individual radial glial fibers. Nevertheless, the presence of radial glial fibers at the pial surface suggests that some (if not most) fibers span the entire thickness of the neural tube wall also at this relatively late stage. Radial glial fibers and divisional borders can be expected to be distorted roughly in parallel as they run from the ventricular surface to the pial surface.
Changes in radial glial fiber density at the boundaries of embryonic divisions have been described before, including at the corticostriatal border (Misson et al., 1988; Silver et al., 1993; Stoykova et al., 1997; Mai et al., 1998) and at the rhombomere boundaries (Heyman et al., 1995). Here, we show that such changes are associated more systematically with divisional boundaries than was believed previously, at least in the diencephalon of the chicken. Previous reports demonstrated raphes of radial glial fibers at the borders of some diencephalic brain nuclei (Geisert and Bidanset, 1993; Steindler, 1993; Kalman et al., 1998); however, it is unclear how these correlate with the divisional borders demonstrated in the current study. Some diencephalic glial boundaries shown by Silver et al. (1993) and Mai et al. (1998) are related more clearly to the interprosomeric boundaries underlined in our material.
How can the regional differences in radial glial density be explained? It has been shown that many of the divisional borders constitute borders of morphogenetic fields that display different spatial and temporal profiles of cell proliferation and migration (Rendahl, 1924; Bergquist, 1957; Puelles et al., 1987; Figdor and Stern, 1993). In the hindbrain, the rhombomeric boundaries contain molecularly distinct cell populations with relatively low mitotic activity compared with their surroundings (Guthrie et al., 1991). The presence of radial glial raphe at some of the divisional borders in the diencephalon suggests that these border regions have proliferative properties similar to those of the hindbrain. One way to explain differences in radial glial density is to assume that the overall number of radial glial cells becomes fixed early and changes little during further diencephalic morphogenesis; areas with lower proliferative activity (e.g., border regions) would then show a higher density of radial glial fibers. Alternatively, one could assume that differences in radial glial proliferation and differentiation between the embryonic divisions or between the divisions and their boundary regions account for the differences in radial glial density.
For some subdivisions, we also found considerable differences in the density of radial glial fibers at different levels of stratification. These differences may reflect the number of radially migrated neurons and the extent of their radial migration (Rakic, 1972; for hindbrain, see Marin and Puelles, 1995; Wingate and Lumsden, 1996). For example, in the dorsal thalamus, the density of radial glial fibers is high at the position of the superficially located prospective interstitial nucleus of the optic tract, low within the nucleus rotundus, and intermediate in the periventricular region (Fig. 9C). These changes are related inversely to the thickness of the dorsal thalamus at these different levels of radial stratification. Similarly, the areas in the pretectum containing densely packed neurons (e.g., PPT, SpL, and SpMcl in Fig. 10) display lower densities of radial glial fibers than the gray matter areas of lower cell density immediately surrounding these nuclei.
In conclusion, the current analysis supports the notion that the embryonic divisions of the diencephalon persist in the architecture of the mature diencephalon. In contrast to the hindbrain, in which cells originating in different neuromeres often merge into morphologically relatively homogeneous brain nuclei (Marin and Puelles, 1995), most of the boundaries in the diencephalon remain distinct histologically. With the exception of the nucleus of the basal optic root which seems to extend across several prosomeres (p1–p3; see footnote 3 in Table 2), all other diencephalic nuclei can be assigned to a single diencephalic (sub)division (Table 2, Fig. 18). The two-dimensional scheme of transverse and longitudinal divisions of the neural tube, thus, is transformed rather directly into the three-dimensional architecture of mature diencephalic gray matter. The third dimension emerges during mantle layer development and is represented by the radial stratifications of the neural tube wall.
Application of the prosomeric model to the developing diencephalon
The prosomeric model has been employed successfully as a paradigm in studies on brain development in several vertebrate species (Puelles and Rubenstein, 1993; Ekstrom and Ohlin, 1995; Puelles, 1995; Puelles et al., 1996; Milán and Puelles, 2000; Pombal and Puelles, 1999; Wullimann and Puelles, 1999). In the current work, this model is used to define the primary embryonic divisions in the chicken diencephalon in conjunction with earlier observations on the segmentation of the chicken diencephalon (Rendahl, 1924; Vaage, 1969; Martínez-de-la-Torre, 1985; Puelles et al., 1987, Puelles et al., 1991; Martínez et al., 1991; Puelles and Medina, 1994).
The following predictive properties of the prosomeric model also are applicable to the developing chicken diencephalon: 1) Each of the six prosomeres extends in a radial-glia-coherent fashion from the ventricular surface to the pial surface of the brain; 2) the alar domains forming the pretectum, dorsal thalamus/epithalamus, and ventral thalamus (p1–p3) extend in a coherent fashion from the basal plate to the roof of the diencephalon; in the ventral thalamus, the dorsalmost area (VTD) is relatively small and represents a previously unrecognized region of this diencephalic division [note that the three rostral prosomeres, p4–p6, extend from the basal midline (hypothalamus) into the telencephalic vesicle]; and 3) despite the morphogenetic distortions taking place during diencephalic development, the topologic relations between the different prosomeres remain invariant. For example, the area rostral to the pretectum is always dorsal thalamic territory that, in turn, is always followed rostrally by ventral thalamic territory.
Secondary subdivisions in the pretectum and dorsal thalamus
Rendahl (1924) suggested that the pretectal nuclei of chicken derive from two morphogenetically independent alar regions that were defined by their relation to the posterior commissure (commissural and precommissural pretectal regions). His pioneering data on the development of the spiriform nuclei suggested the existence of a third independent pretectal region, the juxtacommissural pretectum, found intercalated between the other two (see also Martínez-de-la-Torre, 1985; Martínez, 1987; Puelles et al., 1996; De Castro et al., 1998). The current study supports the usefulness of this general scheme for defining subdivisions in the chicken pretectum by mapping cadherin expression and radial glial topology. Evidence for the existence of transverse subdivisions within the pretectum also stems from studies on the early restriction of diencephalic cell intercalation (Figdor and Stern, 1993) and from mapping the expression of axonin-1, an adhesion molecule of the Ig superfamily (Redies et al., 1997), or other molecular markers (Sax-1, Pax-6, Ebf-1; Schubert et al., 1995; Garel et al., 1997). The same tripartite scheme also has been used in previous work on the mammalian, reptilian, amphibian, and lamprey pretectum (Martínez-de-la-Torre, 1985; Caballero-Bleda et al., 1992; Puelles and Medina, 1994; Puelles et al., 1996; Pombal and Puelles, 1999).
A novel, three-tiered subdivision scheme also was adopted for the dorsal thalamus, and it is presented here in detail for the first time, although precursor ideas and additional evidence can be found in Martínez-de-la-Torre's (1985) and Guillén's (1992) doctoral dissertations as well as in recent work on the reptilian dorsal thalamus (Diaz et al., 1994). In an analogy to the secondary subdivisions of the pretectum, the dorsal thalamic subdivisions (dorsal, intermediate, and ventral tiers plus an anteroventral subregion) are defined based on cytoarchitectonic evidence, cadherin expression data, and radial glial topology as well as on previously published indications for such subdivisions (see Rendahl, 1924; Kuhlenbeck, 1937; and other references cited above).
To understand possible developmental implications of our scheme, note that the rostral limit of the dorsal thalamus is formed by the zona limitans intrathalamica. Molecular expression data indicate that this structure coincides with a local dorsal extension of the basal/alar plate boundary into the alar plate (Macdonald et al., 1994; Puelles, 1995; Shimamura et al., 1995). The zona limitans possibly has inductive properties on neighboring diencephalic tissue, perhaps serving as an organizer for local secondary regionalization (for review, see Rubenstein et al., 1998). Note that the postulated anteroventral subregion of the dorsal thalamus represents a narrow stripe of tissue that is immediately adjacent to both the alar/basal plate boundary and the zona limitans (Fig. 18). On the molecular level, this domain clearly correlates with the early expression of the gene Nkx-2.2 at the caudal slope of the zona limitans, and all derivatives of the anteroventral region in the mantle continue to express this gene (Puelles et al., 1999). Anteroventral derivatives of alar p2 also are characterized by their selective RNA expression of the Sox family genes, Sox14 and Sox21, during and after their migration (Uchikawa et al., 1999), by their cad6B expression (Yoon et al., 2000), and by the absence of Gbx-2 and Sox2 expression that is found in the rest of the dorsal thalamus (Redies et al., 1997; Uchikawa et al., 1999).
In sagittal views (Figs. 12, 13, 18), the borders between the three tiers of the dorsal thalamus have an oblique orientation, intermediate between the longitudinal orientation of the alar/basal plate boundary in p2 and the transverse orientation of the pretectothalamic and zona limitans boundaries (p1/p2 and p2/p3 borders, respectively). It remains unclear to which extent this obliquity may be a consequence of the slanting topology of the caudal slope of the zona limitans. A peculiarity related to this obliquity may be that the epithalamic habenular complex is excluded from the caudalmost roof area of p2 (Fig. 18; Martínez-de-la-Torre, 1985; Guillén, 1992). Curiously, the boundaries between the three subregions of the pretectum also are slightly oblique relative to the prosomeric boundaries of p1, as reflected by the occupancy of the whole roof area by the commissural subregion (Fig. 18). The obliquity is one of the reasons why we do not regard these subdivisions as prosomeres by themselves, as proposed by Figdor and Stern (1993). Note that the secondary subdivisions in the ventral thalamus (i.e., see boundary between LA and GV) also seem to be oblique. Pending further analysis, they are not described here in detail.
Together, these data strongly suggest that oblique secondary limits appear at later stages of forebrain regionalization without the disappearance of previously established transverse interprosomeric (primary) boundaries. The oblique secondary limits introduce additional complexity in the dorsoventral and anteroposterior patterning of the neural tube wall. They establish immature intermediate primordia within the thalamic mantle zone. The cells in these primordia seem to share characteristic cellular and molecular properties (migration patterns, radial glial processes, cadherin expression, etc.). Like the primary divisions, the secondary subdivisions of the pretectum and dorsal thalamus each encompasses a complete radial unit. In the terms of Bergquist (1932) and Bergquist and Källén (1954), these radial units represent the structural product of one histogenetic migration area. The subdivision scheme proposed here is to be understood as a model that is a first approximation and will require changes and modifications in the future if additional independent areas are discovered.
The biologic significance of the secondary subdivisions, e.g., in partially restricting tangential cell migration among them (see, e.g., Figdor and Stern, 1993), remains to be established experimentally. Here and in the accompanying paper (Yoon et al., 2000), we have interpreted this aspect in terms of the best available information, suggesting that most tangential migration originates in the anteroventral region and takes place at the surface of the dorsal thalamus, largely respecting its segmental boundaries (Fig. 1A,B; Puelles et al., 1991, Puelles et al., 1999).
Later in development, additional molecular differentiation and probably connectivity-related specification divide the secondary primordia into tertiary subdivisions, leading to the development of brain nuclei, as proposed by Rose (1942) for his nuclear anlagen. Given nuclei still may undergo quaternary subdivisions among their constituent neuronal populations, as demonstrated here in an exemplary fashion by the complex cadherin expression profile of the nucleus rotundus (Fig. 16) and the ovoidal complex (Figs. 4, 5, 9). These subdivisions may correlate with functional specializations within the nuclei, as shown for cadherin expression in other brain regions, e.g., the parasagittal domains of the cerebellar cortex (Arndt et al., 1998).
Novel gray matter subdivisions of dorsal thalamus
By mapping cadherin expression, a number of gray matter areas were studied in more detail than had been done previously, leading to some novel terminology. We concentrate most of our comments below on the dorsal thalamus, in which the nuclear divisions in birds have been studied by Rendahl (1924), Huber and Crosby (1929), Kuhlenbeck (1937; for review, see Kuhlenbeck, 1973), and Puelles et al. (1991). Other more recent additions to our knowledge of distinct dorsal thalamic nuclei stem from hodologic analyses (for review, see Dubbeldam, 1998).
Anteroventral region.
A fundamental scheme of subdivisions for the dorsal thalamus into superficial and deeper cell masses was reported by Rendahl (1924). What we call the anteroventral region was identified as a source for superficial neurons: Cells migrate from the ventricular layer of this region first to the surface and then caudalward over the three main tiers (Fig. 1A, left pointing arrows; Fig. 1B; Puelles et al., 1991). This migration parallels the pattern seen in mammals that gives rise to the intergeniculate leaflet, a structure that is placed similarly adjacent to the zona limitans and under the dorsal geniculate nucleus (see Martínez et al., 1991; Puelles et al., 1991). In birds, the postmitotic neurons that come to cover the intermediate and ventral tiers constitute a characteristic population that, early on, is large in size relative to the surrounding immature grisea (nucleus superficialis magnocellularis of Rendahl, 1924). This population subsequently gives rise to the SRt, APR, ITO, and RR nuclei (possibly also to the PDSV nucleus). In the adult, these cells are of medium size, which has led to a number of confusions in the literature, as examined in detail by Puelles et al. (1991). Differences previously found between the ITO and the APR (Puelles et al., 1991) that also were detected in the current results (Table 2) suggest that it is convenient to retain these names for greater precision of description.
Finally, recent evidence (Puelles et al., 1999) suggests that cells originating in the dorsalmost aspects of the anteroventral subregion at least invade and possibly completely form the strongly cad7-positive dorsolateral lateral nucleus (DLL) and the less strongly cad7-positive parvicellular superficial nucleus (SPC), which cover the dorsal tier superficially (Figs. 3-6, 9, 11A,B, 12B, 13B, 17A). These cells are of relatively small size [nucleus superficialis parvocellularis (SPC) of Rendahl (1924); Puelles et al., 1999]. One part of this population was identified previously as the “DLL” nucleus by Huber and Crosby (1929); subsequent widespread usage of this terminology implicitly (and wrongly) assimilated it with the deeper lying dorsal tier nuclei (DLL was supposed to be a lateral part of the DLA formation). Guillén (1992) corroborated Rendahl's observations on the SPC but also identified an additional source of tangentially migrating, superficial thalamic neurons. These arise at a dorsocaudal neuroepithelial locus that is situated where the dorsal tier reaches the roof of p2, caudal to the epithalamus (Fig. 1A, right pointing arrow). Whereas the small SPC cells migrate caudalward over the dorsal tier with a resulting caudorostral gradient of neurogenesis, the dorsocaudally originated cells are very small (microcellular) and more basophilic, and they migrate rostralward, building the SMC, superficial to the SPC, with a final rostrocaudal neurogenetic gradient (Fig. 1B; Guillén, 1992). The current data further underline the differences between SPC and SMC by distinguishing their distinct profile of cadherin expression (Fig. 17). A cytoarchitectonic division of the dorsal superficial stratum into two laminae had been noted by both Rendahl (1924) and Huber and Crosby (1929) but was not reflected in their terminology, probably because they did not recognize the differential embryonic (migratory) origin. The SMC apparently contributes to the superficialmost part of what has been called the “DLL” nucleus (Fig. 9A,B) and to a laterorostral part of DLA and the superficial habenula, or nucleus superficialis epithalamicus of Rendahl (1924; see also Fig. 15D,F). The SMC cells are the last to be born in the chicken dorsal thalamus, and their migration has been followed autoradiographically (Guillén, 1992): Comparable observations were reported in a songbird (although without an interpretation) by Alvarez-Buylla et al. (1994). It is quite possible that a confusion in terminology has contributed to the poor anatomic understanding of this complex, because Kappers et al. (1936) seem to have applied the “SPC” term (or the alternative term of “nucleus of the septomesencephalic tract” of Edinger and Wallenberg, 1899) to the SMC (probably led by Rendahl's reference to its small cell size). It is of interest that the deep and superficial neuronal strata of DLL are known to have differential projections to the Wulst, which is the homolog of the primary visual cortex in birds (for review, see Medina and Reiner, 2000); the superficial strata project bilaterally, whereas the deep strata project only ipsilaterally (Bagnoli and Burkhalter, 1983). The mammalian lateral geniculate nucleus seems to be the homolog of the avian DLL, including some neighboring portions of the DLA that share a similar origin. In mammals, the dorsal thalamic structure homologous to the SMC may be the pulvinar, which also has a dorsocaudal topography and late appearance. However, a similar structure does not seem to be present in reptiles (Martínez-de-la-Torre, 1985; Puelles, unpublished observations), which also may indicate independent evolution in birds.
Dorsal and intermediate tiers.
Rostrally, the dorsal tier nuclei, as identified here by cadherin expression, comprise the DA, DMA, DLA, DIVA, and VIA nuclei. The DMA and DLA were defined by Huber and Crosby (1929), whereas DIVA and VIA were demonstrated hodologically (Medina and Reiner, 1997; Medina et al., 1997) essentially as subpopulations or subareas within DLA, because there are no clear-cut cytoarchitectonic limits. Accordingly, our identification of topographically corresponding Rcad-positive patches as DIVA and VIA is tentative and needs hodologic confirmation. We have distinguished the DA nucleus by its differential cadherin immunostaining pattern and its characteristic relation with the dorsalmost part of zona limitans (Fig. 3B, Table 2). Caudally, the dorsal tier comprises the DPo, DMP, DIP, and DLP nuclei plus the medial and lateral subhabenular nuclei (SHm and SHl, respectively). The DPo nucleus is introduced here as a caudal part of DA with which it shares a similar cadherin expression profile. The DMP, DIP, and DLP nuclei represent subdivisions originally introduced by Karten and collaborators (Karten and Revzin, 1966; Karten and Dubbeldam, 1973). The cadherin profile of DLP is comparable to that of DA and DPo, whereas DIP is similar to DLA, and DMP resembles DMA. There also are no strict cytoarchitectonic limits between the corresponding partners in each subgroup.
To distinguish the DLP from medial and lateral posterior derivatives of the intermediate tier, we have introduced the term “intermedioposterior nucleus” (IP) for the intermediate tier derivatives and, thus, reserve the prefix “dorsal” for the dorsal tier derivatives. It will be necessary to reexamine the connectivity of these caudal nuclei (DLP, IPm, and IPl) aided by cadherin markers before questions about possible homologies with the mammalian dorsal thalamus can be approached in a meaningful way. The other novelty introduced for a better understanding of the intermediate tier derivatives is the intermediomedial nucleus (IM), which can be distinguished both cytoarchitectonically and by its differential cadherin profile from neighboring periventricular cell populations (Table 2).
Ventral tier.
The separation of the ventral tier derivatives from those of the intermediate tier on the basis of cadherin staining agrees well with parallel observations in the reptilian thalamus (Martínez-de-la-Torre, 1985; Diaz et al., 1994) as well as with the ventralmost topology within the dorsal thalamus of the mammalian medial geniculate formation (Puelles et al., 1992). This tier can be divided into distinct deep, intermediate, and superficial portions that are unified by the radial course of the toroovoidal tract. Apart from the nucleus ovoidalis, previous literature has paid scarce attention to this area, particularly to its intermediate and superficial parts. In addition to the ovoidal complex (OV, SPO), we have distinguished the deep PM, POV, and ROV nuclei; the intermediate Po nucleus; and the superficial PI, PV, PDSV, and Z cell groups. The PM was identified by Rendahl (1924) and corresponds to the nucleus internus inferior posterior of Huber and Crosby (1929). The POV and ROV seem to represent a shell around the ovoidal complex. The Po nucleus is distinctly bilayered and may represent two nuclei (see Results). Although, in position, it roughly corresponds to the mammalian Po, we do not postulate any homology at present due to the lack of sufficient data. The PI and PV nuclei were defined by Huber and Crosby (1929) and also were demonstrated by AChE staining in a study by Martínez-de-la-Torre et al. (1990); the latter authors also characterized the PDSV as a strongly positive AChE nucleus interstitial to the AChE-positive component of the ventral supraoptic commissure. The nucleus zeta, a cell mass interstitial to the inflection point of the toroovoidal tract, is comparable topographically to the corresponding entity described in reptiles (Beccari, 1923).
Acknowledgements
This work was supported by the Deutsche Forschungsgemeinschaft (grant Re 616/4-1 to C.R.); the Land Baden-Württemberg Neurobiology Program (C.R.); the DGICYT Governmental Grant Agency, Madrid, Spain (grant PB98-397 to L.P.); the Spanish Ministerio de Relaciones Exteriores and the German Academic Exchange Service (grant HA1996-0149 to C.R. and L.P.); and the Human Frontier Science Program (grant GR-41-95 to L.P. and M.T.). The authors thank U. Dräger for the R5 antibody, R. Düchting for help with photography, and members of their laboratories for contributing to the Discussion.




